Abstract
A photoactive highly fluorinated diamine was synthesized, characterized, and used in preparation of structurally confirmed poly(imidazole-ether-imide)s (PIEIs). These polymers showed excellent solubility, moderate viscosities, good thermal stability (Tg = 230–280 °C, Ti = 390–435 °C, T10% = 480–510 °C), low dielectric constant (2.60–2.76), and water absorptions. The PIEIs films showed highly optical transparency with light transmittance at 550 nm of as high as 80% and cutoff wavelength of as low as 390 nm. These polymers exhibited fluorescence emission peaks around 410–490 nm with the quantum yields measured in solution varied from 12 to 32%.
1. Introduction
High-performance polymers, such as polyimides, with high glass transition temperature (Tg), high thermal stability, and good mechanical properties have been identified for a variety of applications, for example, as engineering plastics for aerospace industries, in optical and electronic devices and also as films or membranes.Citation1Citation2Citation3 However, in spite of having excellent combination of properties, most of these high-performance polymers have some serious drawbacks, for example, insolubility in common organic solvents, intractability, infusibility, and strong color that often limit their utility in various advanced technological applications. In order to meet these requirements, structural modifications of these polymers often become essential. Therefore, considerable efforts have been made in recent years to develop highly optical transparent, low dielectric constant, and low refractive index aromatic polyimides with good processability by the incorporation of flexible linkages,Citation4Citation5 bulky pendant groups Citation6Citation7Citation8, and noncoplanar biphenylene moieties Citation9Citation10 into the polymer backbones. It has been observed that introduction of fluorinated substituents can impart many of these desirable properties to the polymers, making them suitable for a much wider range of applications.Citation[11] Among the above-mentioned strategies, introducing of fluorinated substituents, such as hexafluoroisopropylidene linkages, perfluoroalkyl groups, pendent trifluoromethyl groups, have been considered as one of the most promising methods. As well known, the high electronegativity and low molar polarization of the fluorinated groups could endow aromatic polyimides with many attractive features, such as good organo-solubility, high optical transparency, low dielectric constant, as well as low water uptake.Citation12Citation13Citation14Citation15Citation16Citation17Citation18Citation19Citation20 It is also well known that the incorporation of fluorinated substituents into polymers decreases the dielectric constant, due to the low electronic polarizability of the C–F bond, as well as the increase in fractional free volume which accompanies the replacement of methyl groups by trifluoromethyl (–CF3) groups. Thus, many fluorinated polyimides have been developed in order to reduce the dielectric constant.Citation[21] An additional positive effect of fluorinated substituents is to enhance the solubility and optical transparency of polyimides. However, the fluorinated groups attached in the chemical structures of aromatic diamine monomers could usually reduce the condensing reactivity of the diamine with aromatic dianhydride, resulting in aromatic polyimides with poor mechanical properties caused by the relatively low molecular weights. Hence, how to design and synthesize fluorinated aromatic diamines as the monomers of polyimides has still been a scientific and technological challenge. Recent studies demonstrated that polyimides derived from ether-bridged aromatic diamines with trifluoromethyl (–CF3) groups are soluble high-temperature polymeric materials with low-moisture uptake, low dielectric constant, high optical transparency and low birefringence.Citation22Citation23Citation24Citation25Citation26Citation27Citation28Citation29 Imidazole ring is a useful n-type building block with high-electron affinity and good thermal stability and has been successfully incorporated in small molecules and polymers as the electron-transport component of OLEDs. Moreover, lophine, 2,4,5-triphenylimidazole, and its derivatives are well-known potential chemiluminescence compounds and have significant analytical applications using their fluorescence and chemiluminescence properties.Citation30Citation31Citation32Citation33Citation34Citation35Citation36 In this study, the newly prepared fluorinated diamine, 2-(4-trifluoromethylphenyl)-4,5-bis(4-(4-amino-2-trifluoromethylphenoxy)phenyl)imidazole (TFIA), was used to synthesis fluorinated polyimides poly(imidazole-ether-imide)s (PIEI)s with three commercially available aromatic dianhydrides. TFIA and the intermediates were characterized by mass, FT-IR, 1H, and 13C NMR spectroscopy, and elemental analysis. The prepared PIEIs having imidazol, trifluoromethyl, and aryl ether units in their backbones were characterized by FT-IR, 1H NMR, elemental analysis and viscosity measurements and their properties, such as solubility, thermal, photophysical, optical transparency, dielectric constant and water absorption, were investigated.
2 Experimental
2.1 Materials
All materials and solvents were purchased either from Merck or from Fluka Chemical Co. 4-(trifluoromethyl)benzaldehyde, 2-chloro-5-nitrobenzotrifluoride, 4,4′-dimethoxy benzil, ammonium acetate, hydrazine monohydrate, Pd/C, potassium carbonate, acetic acid, acetic dianhydride, reagent-grade dianhydrids, such as pyromellitic dianhydride (PMDA), 4,4′-(hexaflouro isopropylidene) diphthalic anhydride (6FDA), 3,3′,4,4′-benzophenone tetracarboxylic dianhydride (BTDA), and solvents, such as DMF, DMSO, ethanol, methanol, acetone, and tetrahydrofuran (THF), were used without further purification. N-methyl-2-pyrrolidone (NMP), N, N-dimethyl acetamide (DMAc), and pyridine were purified by distillation over calcium hydride under reduced pressure.
2.2 Characterization
FT-IR measurements were performed on a Bruker-IFS48 spectrometer (Ettlingen, Germany). The spectra of solids were obtained using KBr pellets. 1H NMR spectra were recorded on a Bruker Avance 400 MHz instrument using dimethylsulfoxide-d6 (DMSO-d6) solvent and tetramethylsilane as an internal standard. Differential scanning calorimetry (DSC) and thermogravimetric (TGA) analyses were recorded on a Stanton Redcraft STA-780 (London, UK) at heating rate of 10 °C/min under N2 atmosphere. Tgs were read at the middle of the transition in the heat capacity and were taken from the second heating scan after quick cooling from 350 °C at a cooling rate of 200 °C/min. Inherent viscosities were measured with an Ubbelohde viscometer with 0.5 g/dL NMP solution at 30 °C. UV–visible absorption and fluorescence emission spectra were recorded in NMP solution and in polymer films on a Cecil 5503 and Perkin–Elmer LS-3B spectrophotometers, respectively. Cutoff wavelength (absorption edge) values (λ0) of the prepared thin films were determined with a PERKIN ELMER PTP-1 Peltier System Lambda 25 UV-vIS Spectrometer. Water uptakes were determined by weighing the changes of polyimide film before and after immersion in water using two methods; (I) at 25 °C for 24 h and (II) in boiling water at 100 °C for 30 min. The dielectric constant (ϵr) was measured by the LCR meter (4284A: Hewlet Packard) at 1 MHz frequency after coating gold on two surfaces of polyimide films with 3000 Å thick and 1.5 cm diameter.
2.3 Monomer synthesis
The synthetic procedures for the synthesis of target diamine, 2-(4-trifluoromethylphenyl)-4,5-bis(4-(4-amino-2-TFIA are reported in the previous paper Citation[37].
2.4 PIEIs synthesis
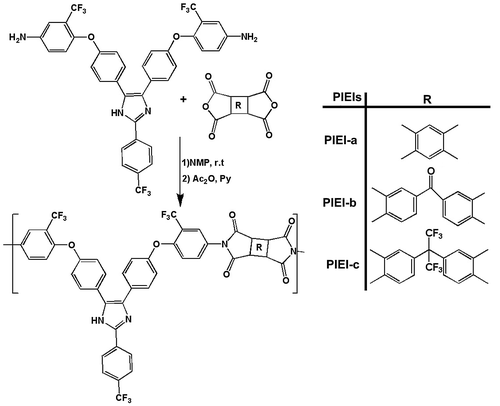
General procedure for the preparation of PIEIs is illustrated in Scheme . A typical example of polymerization is as follows. A 100-mL two-necked, round-bottomed flask equipped with a magnetic stirrer bar, nitrogen gas inlet tube, and calcium chloride drying tube was charged with 1 mmol of the diamine (TFIA) and 10 mL of dry NMP. The mixture was stirred at room temperature for 0.5 h. Then, 1 mmol of a dianhydride was added, and the mixture was stirred at room temperature for 24 h, forming a viscous solution of poly(amic acid) (PAA) precursor in NMP. The PAA was converted into polyimide by chemical imidization process. The chemical imidization was carried out by adding 3 mL of a mixture of acetic anhydride/pyridine (6/4, v/v) into the PAA solution with stirring at room temperature for 1 h. Then, the mixture was stirred at 130 °C for 10 h to yield a homogeneous solution. The polymer solution was poured slowly into methanol to form a precipitate. The precipitate was collected by filtration, washed thoroughly with hot methanol and dried at 80 °C in vacuum overnight.
2.4.1 PIEI-a
This polymer was obtained by using diamine TFIA and dianhydride PMDA. Yield = 91% and ηinh = 0.81 dL/g. FT-IR (KBr, cm−1): 3550 (N–H, imidazole), 1780, 1730 (C=O imide), 1374 (C=N stretching) and 1227 (C–O–C). 1H NMR (400 MHz, DMSO-d6): δ = 13.40 (1H, imidazole ring), 6.90–7.72 (22H, aromatic protons) ppm. [C46H21F9N4O6]n: Calcd C, 61.74%; H, 2.12%; N, 6.26%; found: C, 61.69%; H, 2.25%; N, 6.32%.
2.4.2 PIEI-b
This polymer was obtained by using diamine TFIA and dianhydride BTDA. Yield = 93% and ηinh = 0.72 dL/g. FTIR (KBr, cm−1): 3546 (N–H, imidazole), 1776, 1729(C=O imide), 1378 (C=N stretching) and 1231 (C–O–C). 1H NMR (400 MHz, DMSO-d6): δ = 13.48 (1H, imidazole ring), 6.85–8.10 (24H, aromatic protons) ppm. [C53H25F9N4O7]n: Calculated C, 63.60%; H, 2.50%; N, 5.60%; found: C, 63.51%; H, 2.59%; N, 5.66%.
2.4.3 PIEI-c
This polymer was obtained by using diamine TFIA and dianhydride 6FDA. Yield = 90% and ηinh = 0.71 dL/g. FT-IR (KBr, cm−1): 3543 (N–H, imidazole), 1782, 1731 (C=O imide), 1398 (C=N stretching) and 1233 (C–O–C). 1H NMR (400 MHz, DMSO-d6): δ = 13.50(1H, imidazole ring), 6.70–8.40 (24 H, aromatic protons) ppm. [C55H25F15N4O6]n: Calculated C, 57.99%; H, 2.91%; N, 4.92%; found: C, 57.90%; H, 3.02%; N, 4.87%.
2.5 Polyimide film preparation
To prepare crack-free and homogeneous thin films for testing the optical properties, solutions of PIEIs were made by dissolving about 0.5 g of the PIEI in 5 mL of NMP to afford an approximate 10 wt.% solution. The homogeneous solution was poured into a 9-cm-diameter glass culture dish, which was heated under vacuum at 50 °C for 1 h, 100 °C for 2 h, and 150 °C for 5 h to evaporate the solvent slowly. Polymer films were self-stripped off from the glass surface by soaking in water. The polymer films were further dried in vacuum oven at 170 °C for 10 h.
3 Results and discussion
3.1 PIEIs synthesis and characterization
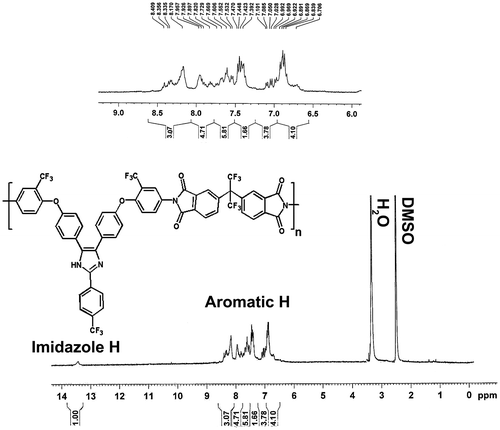
In general, aromatic polyimide is synthesized from the condensation of aromatic diamine and aromatic dianhydride by either a two-step condensation polymerization method, that is, the formation of PAA followed by a thermal or chemical imidization to give polyimide, or a one-step thermal polycondensation in solution at elevated temperature. In the present study, the fluorinated polyimides were prepared via two-step condensation using chemical imidization. A new aromatic diamine (TFIA) was synthesized according to the synthetic procedure reported in the previously published paper Citation[37]. Three new polyimides containing imidazol-triflouromethyl pendent groups were prepared in good yields (90–95%) by polycondensation of equal molar amounts of the diamine with commercially available aromatic dianhydrides, such as PMDA, BTDA, and 6FDA as shown in Scheme . The polycondensation was carried out in NMP at room temperature for 24 h to form poly(amic acid)s, followed by chemical imidization with acetic anhydride and pyridine. Inherent viscosity of the polymers was measured at a concentration of 0.5 g/dL in NMP at 25 °C. The inherent viscosities of the polyimides were in the range of 0.71–0.81 dL/g indicating that the polymers have reasonable high molecular weights. All the polymers were characterized by elemental analysis, FT-IR, and 1H NMR techniques. The elemental analysis data of the fluorinated polyimides were in good accordance with the calculated ones. The FT-IR spectra of the resulting polymers exhibited the characteristic absorption bands of the five-membered imide ring at 1780 and 1730 cm−1 (typical of imide carbonyl asymmetric and symmetric stretching), 1375 cm−1 (C–N stretching), and at 1087 and 780 cm−1 (imide ring deformation), together with a strong absorption band at 1233 cm−1 due to the C–O stretching. As expected, C–F multiple stretching absorptions were also measured at 1250 and 1145 cm−1, respectively. Figure shows a representative 1H NMR spectrum of the fluorinated aromatic polyimide (PIEI-c) in DMSO-d6, in which it can be seen that all of the aromatic protons appeared in the region of 6.70–8.40 ppm and of the N–H protons of imidazol ring at 13.50 ppm.
3.2 Solubility and morphology of PIEIs
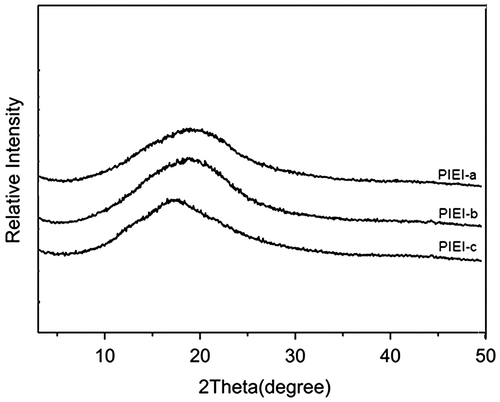
The solubility behavior of the prepared polyimides was tested qualitatively in various organic solvents at 5% (w/v), and the results are summarized in Table . All the polymers exhibited excellent solubility in polar aprotic solvents, such as NMP, DMAc, DMF, and DMSO, at room temperature and in less polar solvent, such as THF and pyridine, on heating at 60 °C. The good solubility of these polymers should be the result of introduction of the bulky imidazole pendant with three –CF3 groups in the backbone. When the CF3-substituted diamine (TFIA) is polymerized with various dianhydrides, the corresponding polymers with ortho- and para-oriented –CF3 moieties per repeat unit would be an exceptional material from the solubility point of view. Dense packing of the polymer chains was probably disturbed by the bulky –CF3 groups and phenyl- CF3 containing imidazole ring which led to the increased chain distances and decreased chain interactions; consequently, the solvent molecules were able to penetrate more easily into the polymer chains and interact with the polar linkages of the polymer backbone. Comparison of solubility of the previously reported polyimides with these polyimides indicate that the presence of polar –CF3 pendants and ether and imidazole functional units in the main chains have improved the solubility of these polymers significantly.Citation38Citation39Citation20 It was also found that the solubility of the fluorinated polyimides depended, to some extent, on the polymer backbone structures. For instance, PIEI-c (6FDA–TFIA) showed fast solubility that can be due to the high loadings of the –CF3 groups in polymer backbones. These polymers can be processed into thin low colored films by casting from their solutions. Figure shows the wide-angle X-ray diffraction patterns of the fluorinated polyimides, in which it can be seen that all of the synthesized polyimides are completely amorphous in morphologic structure. The polymer backbones with multiple trifluoromethyl groups and bulky aromatic side moieties would increase the disorder of the polymer chains, resulting in decreasing of the chain to chain interactions and reducing of chain packaging efficiency to hinder the polymer crystallization.
Table 1. Solubility behavior of fluorinated polyimides.
3.3 Optical transparency and photophysical properties of PIEIs
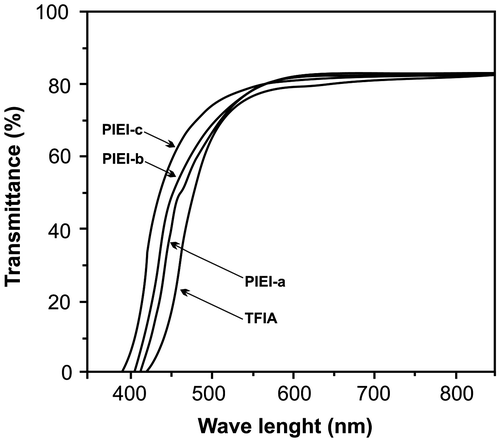
The photophysical properties of the diamine (10−5 M) and polyimides in dilute NMP solutions (0.2 g/dL) and in thin films were tested by UV–vis and fluorescence spectroscopy and the optical data are listed in Table . TFIA showed strong UV absorption with maximum at λa.max = 300 nm. The absorption spectra of these polymers in solutions and in films were nearly identical in the range of λab = 280–310 nm, which shows a relatively small energy band gap for π → π∗ transition. The transmittance spectra of the fluorinated polyimide films (7–10 μm in thickness) are shown in Figure . These polymers showed good optical transparency with light transmittance at 550 nm of as high as 80% and cutoff wavelength of as low as 390 nm. The 6FDA is the most popular fluorinated dianhydride, the quaternary carbon brings very unique properties for this dianhydride compared with the other fluorinated dianhydrides.Citation[40] As can be seen in Figure , the transparency of the polymer increased when the number of CF3 groups increased. The PIEI-c produced from TFIA and 6FDA showed more transparency than PIEI-a and PIEI-b due to higher content of –CF3 groups in the polymer backbone. This could be interpreted by the high fluorine loadings in the polymer backbones, which resulted in the weakened intermolecular cohesive force due to the lower polarizability of the C–F bond,Citation[20] which would also resulted in the improvements in polyimide solubility in organic solvents.Citation[41] In other words, these results can be attributed to the reduction of the intermolecular charge-transfer complex (CTC) between alternating electron-donor (diamine) and electron-acceptor (dianhydride) moieties. The bulky and electron withdrawing –CF3 and –C(CF3)2− groups in diamine and 6FDA, respectively, was effective in decreasing CTC formation between polymer chains in PIEI-c through steric hindrance and the inductive effect (by decreasing the electron-donating property of diamine moieties). The decrease in intermolecular CTC formation is understandable also from the significant solubility of these polyimides. The light transmittance of these fluorinated polymers are not only affected by the fluorine loading but also by the film thickness and, to some extent, by the polyimide backbones. However, these fluorinated polymers showed higher cutoff wavelength values in comparison with other reported fluorinated polyimides.Citation14Citation23 This can be interpreted by the presence of phenyl-substituted imidazole rings in the polymer backbones.
Table 2. Photophysical properties of fluorinated polyimides.
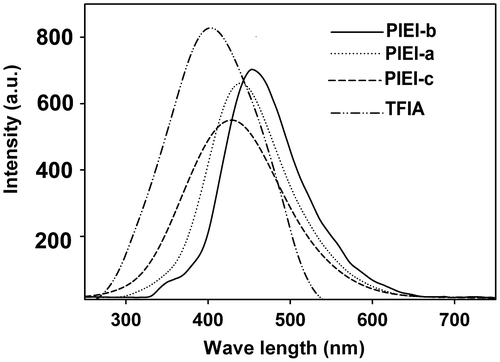
To investigate the emission properties of TFIA and PIEIs, an excitation wavelength of 320 nm was used in all cases. The fluorescence emission spectra of all PIEIs and TFIA in dilute NMP solutions (0.2 g/dL and 10−5 M, respectively) are shown in Figure . TFIA showed strong blue fluorescent light with λem = 415 nm. To measure the photoluminescence (PL) quantum yields (Φf), dilute polymer solutions (0.2 g/dL) in NMP were prepared. A 0.1 N solution of quinine in H2SO4 (Φf = 0.55) was used as reference according to the literature.Citation[33] The fluorescence spectra of these PIEIs in NMP solutions exhibited broad emission peaks with the maxima in the range of 410–490 nm and quantum yields in the range of Φf = 12–32%. In comparison with PIEI-b and PIEI-c which has flexible –C=O and –C(CF3)2, respectively, between phenyl rings, PIEI-a showed the highest quantum yield (32%) which can be due to its rigid structure with high restriction in internal rotation and as a result of that less energy is dissipated as heat.
3.4 Thermal properties of PIEIs
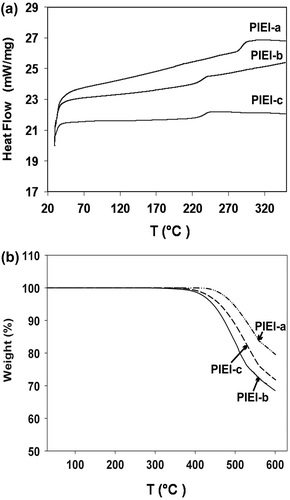
DSC and TGA methods were applied to evaluate the thermal properties of polyimides. In the DSC curves of the fluorinated polyimides, as illustrated in Figure , there is no melting endothermic behavior was observed except the Tgs. The Tg values of these polymers were taken as the midpoint of the change in slope of the baseline in DSC curve obtained from second scan, quenching from temperature of 350 °C to room temperature yielded amorphous samples so that in most cases the Tgs could easily be observed in the heating traces of DSC. The Tg values were measured in the range of 230–280 °C depending on the stiffness of the dianhydride in the polymer backbone., as listed in Table , of which PIEI-a has the highest Tg value (280 °C) implying that this polymer backbones were more rigid than PIEI-b and PIEI-c, probably due to presence of flexible carbonyl and aliphatic units between phenyl rings in the backbones of PIEI-b and PIEI-c respectively.
Table 3. Thermal properties of fluorinated polyimides.
The thermal stability of polyimides was evaluated by TGA at 10 °C/min in nitrogen atmosphere. The TGA curves are shown in Figure , and the thermal stability data determined from the original TGA curves are listed in Table . No weight loss was detected until the temperature was scanned up to 380 °C. The initial decomposition temperatures (Ti) for these polyimides were in the range of 385–435 °C, and temperature of 10% weight loss were in the range of 480–510 °C with the char yields at 700 °C were up to 83% in nitrogen. PIEI-a showed the highest thermal stability which can be due to rigid structure of the dianhydride in the polymer backbones, while –C(CF3)2− group in the dianhydride unit in the backbones of PIEI-c increased thermal stability of this polymer in comparison with PIEI-b. The experimental results indicated that the fluorinated polyimides possessed great thermal stability to withstand the harsh environments of many high-tech applications such as microelectronic manufacturing and packaging industry.
3.5 Dielectric properties and water absorption of PIEIs
Lower dielectric constant is one of the most desirable properties for electronic devices. A dielectric is a material that is an electrical insulator or in which an electric field can be sustained with a minimum dissipation of power. Traditionally, dielectric materials are made from inorganic substances such as mica and silicon dioxide. However, polymers have also gained wide use as dielectric materials. This is due to the easier processing, flexibility, able to tailor made for specific uses and better resistance to chemical attack.Citation[42] Many dielectric polyimides have been created, and the original motivation for the development of polyimide dielectrics was as a low-cost alternative to on-chip silicon dioxide with high Tg, high modulus, and low dielectric constant as compared with silicon dioxide.Citation[43] The dielectric constants (ϵr) of the prepared fluorinated polyimides were determined in the range of 2.60–2.76, as shown in Table , which are lower than the non-fluorinated polyimides such as PMDA–ODA (3.16),Citation[44] PI–POSS (2.65–3.22) Citation[45], and PI/PSSQ (2.79–3.61) Citation[46] nanocomposites, but comparable with other fluorinated polyimides.Citation18Citation19Citation23 The high electrical insulating and low dielectric constants of the fluorinated polyimides are mainly attributed to the high fluorine loadings in the polyimide backbones. It can be seen in Table that the dielectric constant of PIEI-c prepared from diamine TFIA and dianhydride 6FDA is lower than that of PIEI-a (2.76) and PIEI-b (2.68) due to higher loading of –CF3 groups in the backbone of PIEI-c. The strong electronegativity of fluorine atoms would result in very low C–F polarizability, and the trifluoromethyl groups, combined with the high free volumes of bulky side phenyl substituents, could endow the polyimides with low dielectric constants and high electrical insulating properties. The presence of voids, its content and volume, which are filled with air (ϵ = 1.0) is also responsible for the reduction in dielectric constant.Citation[42] Meanwhile, the phenylene ether units thatcould induce the dilution effect of the polar imide ring have also contributed to the reducing in the dielectric constants.Citation[47] These results suggest that the –CF3 groups in the structure of polyimides play an important role in their dielectric performance.
Table 4. Dielectric constants and water absorption (%) of fluorinated polyimides.
Polyimide materials usually show higher moisture uptakes than the hydrocarbon polymers because of the presence of imide groups. The moisture absorption of aromatic polyimides can be up to 3.0–3.5% depending on the chemical structures of polymer and the relative humidity of the surrounding environments, which have a significant influence on the dielectric properties of polymers. The dried polymer films (50 mm in diameter and 3.10 mm thick) were immersed in water at room temperature for 24 h (method I) and in boiling water for 30 min (method II). The weight differences were then determined, and the results are shown in Table . The higher temperature could accelerate the diffusion of water molecules into the polymer. In comparison, as can be seen in the table, the polyimide PIEI-c based on 6FDA dianhydride showed lower value of water uptake (1.45%) compared with the PIEI-a (2.38%) and PIEI-b (2.96%) which are higher than those fluorinated polyimides without bulky side group which were reported by Yang et al. Citation[23]. The moisture absorption of polyimides might be related to several factors including the chemical structures, the introduction of fluorinated groups and of other functional groups, the geometrical packing of the polymer chains as well as film-processing parameters, and so forth in which the chemical structure and the presence of functional groups in polymer might be major concern factors. The trifluoromethyl groups inhibit the absorption of moisture molecules on the surface of the fluorinated polymers. On the other hand, the bulky groups could loosen the packing of the polyimide backbone chains, resulting in the increase of free volume in the polymers that should ensure the polymer to entrap some of the water molecules. Clearly, these two opposing factors affect the water absorption values of the fluorinated polyimides, and the low water uptakes are attributed to the high hydrophobicity derived from the presence of multiple trifluoromethyl groups in the polymer backbones.
4 Conclusions
Multi trifluoromethyl-substituted aromatic diamine bearing collective functional groups of flexible ether linkages and bulky derivative of imidazole heterocyclic ring was synthesized and used in polymerization with commercial dianhydrides to yield polyimides. Experimental results indicated that these fluorinated aromatic polyimides with inherent viscosities of 0.65–0.71 dL/g showed great solubility in common polar solvents. Polyimide films prepared by solution-casting method exhibited good thermal stability with the Tg of 230–280 °C and initial decomposition temperature (Ti) varied from 390 to 435 °C in N2. Dielectric constants of as low as 2.60 and low moisture absorptions (1.45–2.96%) were measured. The fluorinated polyimide films also showed highly optical transparency with transmittance at 550 nm of as high as 80.0% and cutoff wavelength of as low as 398 nm.
References
- Cassidy PE. Thermally stable polymers. New York: Marcel Dekker; 1980.
- Rabilloud G. High performance polymers 3: polyimides in electronics; chemistry and applications. Paris: Editions Technip; 2000.
- Jung MS, Lee TW, Lee HH, Sohn BH. Synthesis and characterizations of a polyimide containing a triphenylamine derivative as an interlayer in polymer light-emitting diode. Polymer. 2006;47:2670–2676.
- Spiliopoulos IK, Mikroyannidis JA. Rigid-rod polyamides and polyimides derived from 4,3″-diamino-2′,6′-diphenyl- or di(4-biphenylyl)-p-terphenyl and 4-amino-4″-carboxy-2′,6′-diphenyl-p-terphenyl. Macromolecules. 1998;31:522–529.
- Liaw DJ, Liaw BY, Tseng JM. Synthesis and characterization of novel poly (amide-imide)s containing hexafluoroisopropylidene linkage. J. Polym. Sci., Part A: Polym. Chem. 1999;37:2629–2635.
- Liaw DJ, Hsu PN, Chen PN, Lin SL. High glass transitions of new polyamides, polyimides, and poly(amide-imide)s containing a triphenylamine group: synthesis and characterization. Macromolecules. 2002;35:4669–4676.
- Liaw DJ, Liaw BY, Hsu PN, Hwang CY. Synthesis and characterization of new highly organosoluble poly(ether imide)s bearing a noncoplanar 2,2′-dimethyl-4,4′-biphenyl unit and kink diphenylmethylene linkage. Chem. Mater. 2001;13:1811–1816.
- Ghaemy M, Bazzar M. Synthesis of soluble and thermally stable polyimides from 3,5-diamino-N-(4-(8-quinolinoxy) phenyl) aniline and various dianhydrides. J. Appl. Polym. Sci. 2011;119:983–988.
- Liaw DJ, Chang FC, Leung MK, Chou MY, Muellen K. High thermal stability and rigid rod of novel organosoluble polyimides and polyamides based on bulky and noncoplanar naphthalene−biphenyldiamine. Macromolecules. 2005;39:4024–4029.
- Liaw DJ, Liaw BY, Jeng MQ. Synthesis and properties of new polyamides and polyimides derived from 2,2′-dimethyl-4,4′-bis(4-aminophenoxy)biphenyl. Polymer. 1998;39:1597–1607.
- Dhara MG, Banerjee S. Fluorinated high-performance polymers: poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010;35:1022–1077.
- Yang SY, Ge ZY, Yin DX, Liu JG, Li YF, Fan L. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3-trifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. J. Polym. Sci., Part A: Polym. Chem. 2004;42:4143–4152.
- Yin DX, Li YF, Yang HX, Yang SY, Fan L, Liu JG. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3,5-ditrifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. Polymer. 2006;46:3119–3127.
- Li HS, Liu JG, Wang K, Fan L, Yang SY. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3,5-ditrifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. Polymer. 2006;47:1443–1450.
- Li HS, Liu JG, Rui JM, Fan L, Yang SY. Synthesis and characterization of novel fluorinated aromatic polyimides derived from 1,1-bis(4-amino-3,5-dimethylphenyl)-1-(3,5-ditrifluoromethylphenyl)-2,2,2-trifluoroethane and various aromatic dianhydrides. J. Polym. Sci., Part A: Polym. Chem. 2006;44:2665–2674.
- Ge ZY, Fan L, Yang SY. Synthesis and characterization of novel fluorinated polyimides derived from 1,1′-bis(4-aminophenyl)-1-(3-trifluoromethylphenyl)-2,2,2-trifluoroethane and aromatic dianhydrides. Eur. Polym. J. 2008;44:1252–1260.
- Xie K, Zhang SY, Liu JG, He MH, Yang SY. Synthesis and characterization of soluble fluorine-containing polyimides based on 1,4-bis(4-amino-2 trifluoromethylphenoxy)benzene. J. Polym. Sci., Part A: Polym. Chem. 2001;39:2581–2590.
- Zhao XJ, Liu JG, Rui JM, Fan L, Yang SY. Synthesis and characterization of organosoluble polyfluorinated polyimides derived from 3,3′,5,5′-tetrafluoro-4,4′-diaminodiphenylmethane and various aromatic dianhydrides. J. Appl. Polym. Sci. 2007;103:1442–1449.
- Zhao XJ, Liu JG, Yang HX, Fan LS, Yang Y. Novel polyfluorinated polyimides derived from α,α-bis(4-amino-3,5-difluorophenyl)phenylmethane and aromatic dianhydrides: synthesis and characterization. Eur. Polym. J. 2008;44:808–820.
- Yang CP, Hsiao SH, Chung CL. Organosoluble, low-dielectric-constant fluorinated polyimides based on 2,6-bis(4-amino-2-trifluoromethylphenoxy)naphthalene. Polym. Int. 2005;54:716–724.
- Hougham G, Cassidy PE, Johns K, Davidson T. Fluoropolymers: synthesis and properties. New York: Kluwer Academic/Plenum; 1999 . 2: 233–370.
- Xie K, Liu JG, Zhou HW, Zhang SY, He MH, Yang SY. Soluble fluoro-polyimides derived from 1,3-bis(4-amino-2-trifluoromethyl- phenoxy) benzene and dianhydrides. Polymer. 2001;42:7267–7274.
- Tao L, Yang H, Liu J, Fan L, Yang S. Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer. 2009;50:6009–6018.
- Zhou HW, Liu JG, Qian ZG, Zhang SY, Yang SY. Soluble fluorinated polyimides derived from 1,4- (4′-aminophenoxy)-2-(3′-trifluoromethylphenyl)benzene and aromatic dianhydrides. J. Polym. Sci., Part A: Polym. Chem. 2001;39:2404–2413.
- Liu JG, He MH, Li ZX, Qian ZG, Wang FS, Yang SY. Synthesis and characterization of organosoluble polyimides with trifluoromethyl-substituted benzene in the side chain. J. Polym. Sci., Part A: Polym. Chem. 2002;40:1572–1582.
- Banerjee S, Madhra MK, Salunke AK, Jaiswal DK. Synthesis and properties of fluorinated polyimides. 3. Derived from novel 1,3-bis[3′-trifluoromethyl-4′(4″-amino benzoxy)benzyl]benzene and 4,4-bis[3′-trifluoromethyl-4′(4-amino benzoxy)benzyl]biphenyl. Polymer. 2003;44:613–622.
- Kute K, Banerjee S. Novel semi-fluorinated poly(ether imide)s derived from 4-(p-aminophenoxy)-3-trifluoromethyl-4′-aminobiphenyl. Macromol. Chem. Phys. 2003;204:2105–2112.
- Yang CP, Hsiao SH, Wu KL. Organosoluble and light-colored fluorinated polyimides derived from 2,3-bis(4-amino-2-trifluoromethylphenoxy)naphthalene and aromatic dianhydrides. Polymer. 2003;44:7067–7078.
- Hsiao SH, Yang CP, Huang SC. Polyimides from 1,5-bis(4-amino-2 trifluoromethylphenoxy)naphthalene and aromatic tetracarboxylic dianhydrides. Eur. Polym. J. 2004;40:1063–1074.
- Mikroyannidis JA, Panayiotis D, Panayiotis VI, Spiliopoulos K. Synthesis, characterization and photophysics of poly (p-phenylenevinylene)-type polymers bearing substituted 2H-imidazole rings in the backbone. Synth. Met. 2004;145:87–93.
- Zhou XH, Yan JC, Pei J. Exploiting an imidazole-functionalized polyfluorene derivative as a chemosensory material. Macromolecules. 2004;37:7078–7080.
- Pan Y, Tang X, Zhu L, Huang Y. Synthesis and characterization of a new high-Tg photorefractive material. Eur. Polym. J. 2007;43:1091–1095.
- Feng K, Hsu FL, DerVeer DV, Bota K, Bu XR. Tuning fluorescence properties of imidazole derivatives with thiophene and thiazole. J. PhotoChem. Photobiol., A. 2004;165:223–228.
- Hadjikallis G, Hadjiyannakou SC, Vamvakaki M, Patrickios CS. Synthesis and aqueous solution characterization of novel diblock polyampholytes containing imidazole. Polymer. 2002;43:7269–7273.
- Pan Y, Tang X. Synthesis and characterization of azobenzene-containing side-chain photorefractive polymers. Eur. Polym. J. 2008;44:408–414.
- Fridman N, Kaftory M, Speiser S. Structures and photophysics of lophine and double lophine derivatives. Sens. Actuators, B. 2007;126:107–115.
- Ghaemy M, Rahimi Berenjestanaki F. Novel para-linked and CF3-substituted poly(amide-ether-imidazole)s: solubility, optical and thermal properties. J. Fluorine Chem. 2012;144:86–93.
- Akutsu F, Inoki M, Sawano M. Preparation and characterization of novel aromatic polyimides having 4,5-di(1,3phenylene)imidazole structure. Polymer. 1998;39:6093–6098.
- Mathews AS, Kim D, Kim Y. Synthesis and characterization of soluble polyimides functionalized with carbazole moieties. J. Polym. Sci., Part A: Polym. Chem. 2008;46:8117–8130.
- Ando S, Matsuura T, Sasaki S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997;29:69–76.
- Yang CP, Hsiao SH, Chen KH. Organosoluble and optically transparent fluorine-containing polyimides based on 4,4′-bis(4-amino-2-trifluoromethylphenoxy)-3,3′,5,5′-tetramethylbiphenyl. Polymer. 2002;43:5095–5104.
- Silaghi MA, editor. Dielectric material. Rijeka: InTech; 2012. ISBN 978-953-51-0764-4.
- Kohl PA. Low-dielectric constant insulators for future integrated circuits and packages. Annu. Rev. Chem. Biomol. Eng. 2011;2:379–401.
- Russell TP, Gugger H, Swalen JD. In-plane orientation of polyimide. J. Polym. Sci., Part B: Polym. Phys. 1983;21:1745–1756.
- Lee YJ, Huang JM, Kuo SW, Lu JS, Chang FC. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer. 2005;46:173–181.
- Tsai MH, Whang WT. Low dielectric polyimide/poly (silsesquioxane)-like nanocomposite material. Polymer. 2001;42:4197–4207.
- Watanabe Y, Shibasaki Y, Ando S, Ueda M. Synthesis and characterization of polyimides with low dielectric constants from aromatic dianhydrides and aromatic diamine containing phenylene ether unit. Polymer. 2005;45:5903–5908.