Abstract
In the search for new materials to separate 2,4-dinitrophenol (DNP) from 2,4-dinitroanisole (DNAN), we have focused on the polymer design and synthesis of novel porous poly(amide-sulfonamide) (PASA) containing both repeated amide and sulfonamide functional groups from the reaction of m-chlorosulfonylbenzoyl chloride with 4,4′-diamino diphenyl sulfone in DMAc, using low-temperature solution polymerization. The structure and thermal property of the PASA were characterized by BET, SEM, FT-IR, 1H NMR, XRD, TGA; the selective removal of 2,4-DNP from DNAN solution was analysed by adsorption experiments. The BET, SEM and XRD results showed that the PASA with a porous morphology is nearly amorphous; the molecular weight of the polymer was investigated by GPC. Thermogravimetric analysis indicated that the reasonably high temperature recorded (>380 °C) before a 5% weight loss occurred. The removal rate of 2,4-DNP solution was 54.11% at 30 °C.
1. Introduction
2,4-Dinitroanisole (DNAN) has been widely used in the fine chemical field, such as dye intermediate and insecticide, and also is the most promising alternative of trinitrotoluene.[Citation1] The traditional production scheme is produced in solutions with alkali as catalytic,[Citation2–Citation4] although having a high transformation rate, the influence of 2,4-dinitrophenol (DNP) as an impurity is unavoidable. 2,4-DNP is one of nitro-aromatic compounds being highly toxic to living organisms [Citation5,Citation6] and its determination is of great significance in environmental protection.[Citation7] It is also a classic oxidative phosphorylation uncoupler and extensively detected in industrial wastewater of textiles, dyes, pesticide and pharmaceutical industries.[Citation8] DNP has been listed in the priority pollutants list by United States Environmental Protection Agency.[Citation9]
The adsorption method [Citation10] is one of the most commonly used method for dealing with nitro-phenol. Recently, the polyamide has emerged as a new reinforcing material with the advantages of being cheap and plentiful.[Citation11] Zhang et al. [Citation12] has achieved the preparative separation of total flavonoids from Ginkgo extract on polyamide resin and the results showed that it can be a good adsorbent. Kirupha et al. [Citation13] has developed novel polyamides bearing pyridyl and azomethine groups binding sites to remove Cu2+ and Pb2+ ions from aqueous solution. Polyamide adsorption resin is also used for disposing nitro phenol in the wastewater, and has achieved good results.[Citation14] However, its most serious drawback is its proneness to organic fouling which arises primarily from the hydrophobicity of the polyamide active layer, into which many natural organic compounds easily adsorb, eventually leading to irreversible deterioration of its performance.[Citation15]
The objective of this work was the design, synthesis and characterization of novel poly(amide-sulfonamide) (PASA) polymer. Introduction of the m-chlorosulfonylbenzoyl chloride monomer into the backbone of the highly symmetrical polymer polyamide did not alter the close packing of the polymer chain in such a way that many properties were improved. Due to the existence of the sulfone (–SO2–) group structure in its molecular main chain, the polymer PASA has a better hydrophilicity [Citation16] than polyamides, it enhances the adsorption ability of the polyamide for 2,4-DNP, thus will improve the yield of 2,4-DNAN.
2. Experimental
2.1. General characterization
Infrared spectra of the monomers and polymers (KBr pellets) were recorded on Spectrum One-B (Perkin Elmer®) FT-IR spectrometer. 1H NMR spectra were measured in CHCl3-d1 for monomers and in dimethyl sulfoxide (DMSO)-d6 for polymers using TMS as internal standard with a Varian® INOVA spectrometer (400 MHz for 1H). Nitrogen adsorption was determined on a Tristar® 3000 Sorptometer. The morphological analysis of PASA polymer was characterized by JSM-6460 LV scanning electron microscopy (JEOL®). Relative molecular weight was measured by gel permeation chromatography (GPC) (Waters® Corporation) equipped with Styrogel® THF columns. GPC measurements were carried out using THF as eluent with a 1.0 mL/min flow rate. A wide-angle X-ray diffraction pattern of PASA was obtained at room temperature on a Japan D/Max-3B X-ray diffractometer using Cu-Kα X-radiation. Thermogravimetric analysis was performed with a TG209 (NETZSCH®, Germany) under the nitrogen atmosphere at a heating rate of 10 °C min−1 in the range of 25–700 °C.
2.2. Materials
The chlorosulfonic acid was purchased from Shenyang third chemical reagent factory (China), benzotrichloride was obtained from Sinopharm® (China), and both were used for monomer synthesis as received. 4,4′-diaminodiphenylsulfone (Aladdin®, China) and dimethylacetamide DMAc (Sinopharm®, China) were used as received. 2-Methylpyridine (Sinopharm®, China) was purified with distillation under reduced pressure.
2.3. Synthesis of monomers
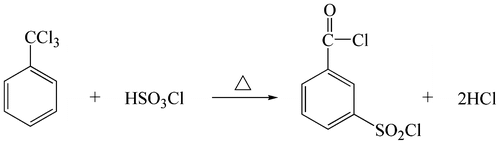
Thirty-four millilitres of (0.166 mol) chlorosulfonic acid was heated to a temperature of about 100 °C in a flask equipped with mechanical stirrer, reflux condenser, thermometer and addition funnel, and 40 mL (0.149 mol) benzotrichloride was added over a 2 h period at 120–130 °C with agitation. After the addition, the mixture was further reacted at 120 °C for about 2 h until little or no further evolution of hydrogen chloride was observed. The mixture was then distilled, and the product m-chlorosulfonylbenzoyl chloride of boiling point 146 °C at 9 mm-Hg was collected.
IR υmax (KBr, cm−1): m-chlorosulfonylbenzoyl chloride: 3 077 (Ar-H), 1 759 (C=O), 1 382, 1 173 (–SO2–).
1HNMR δH (400 MHz, CDCl3): m-chlorosulfonylbenzoyl chloride: 7.83 (t, J = 7.97 Hz, 1H, ArH), 8.32 (d, J = 7.98 Hz, 1H, ArH), 8.45 (d, J = 7.93 Hz, 1H, ArH), 8.70 (s, 1H, ArH).
2.4. Solution polymerization
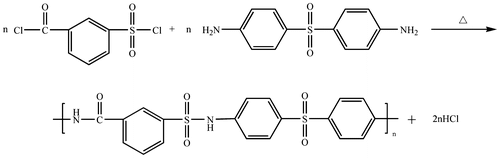
4,4′-Diaminodiphenylsulfone (0.01 mol) and 20 ml of DMAc were added into a 100 ml three-necked round-bottom flask equipped with a mechanical stirrer and a nitrogen gas inlet tube. The mixture was stirred to form homogenous solution and cooled to 5–10 °C with an ice-water bath. 2-Methylpyridine (0.02 mol) was added to the solution in order to neutralize the acid produced. Then m-chlorosulfonylbenzoyl chloride (0.01 mol) was added dropwise to the solution while stirring. The mixture was stirred for further 1 h at low temperature, the cooling bath was changed to an oil bath and the mixture was stirred at 60 °C for another 24 h. The resulting mixture was poured into water and washed thoroughly with hot water and 95% alcohol. Finally, the polymer precipitate was collected by filtration and dried under vacuum for over 12 h.
2.5. Adsorption experiments
The adsorption studies of 2,4-DNP on PASA were performed by shaking 0.1 g PASA with 20 ml 2,4-DNAN (10 mg/L) which contains 2,4-DNP (10.68 mg/L) at 200 rpm for 24 h at 30 °C. Then, the 2,4-DNP concentration in the filtrate was determined by a Cray 50 Scan UV spectrophotometer (Varian®). Data are representative of at least three experiments and standard deviations are less than 5.0%.
3. Results and discussion
3.1. Monomer synthesis
The m-chlorosulfonylbenzoyl chloride monomers were synthesized by trichlorotoluene with chlorosulfonic acid in our laboratory under mild condition as reported.[Citation17] The reaction may be illustrated in Scheme .
3.2. Solution polymerization
Solution polymerization is preferable for industry production, due to its simple operation. In this paper, the novel polymer PASA was prepared at equimolar monomer of m-chlorosulfonylbenzoyl chloride with 4,4′-diamino diphenyl sulfone in DMAc by the formula in Scheme . The molecular weights (Mn and Mw) of the PASA which were determined by GPC were 10,472 and 15,365, respectively. The low molecular weight dispersity (Mw/Mn = 1.46729) of the polymer indicates that the polymer consists of chains with narrowly distributed lengths.
3.3. Spectroscopic characterization
The chemical structure of PASA polymer was characterized by the combined methods of IR, 1H NMR spectroscopy.
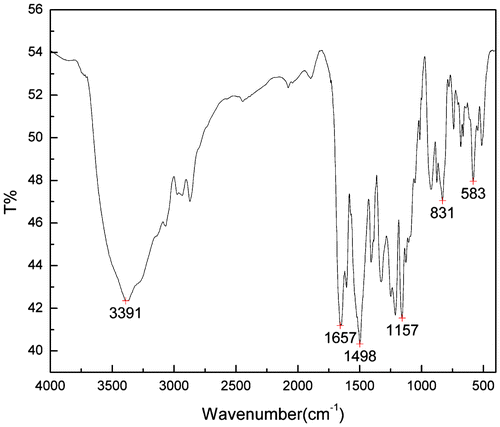
As is shown in Figure , the band at 3391 cm−1 is assigned to the N–H vibration; the band at 1657 cm−1 is assigned to the C=O stretching vibration of the amide group (amide I), the amide II appears at 1498 cm−1; the characteristic peaks for the symmetric and asymmetric sulfonamide stretching modes were observed at 1157 and 1322 cm−1, respectively.[Citation18] As expected, the IR spectra demonstrated that the polycondensation reaction occurred.
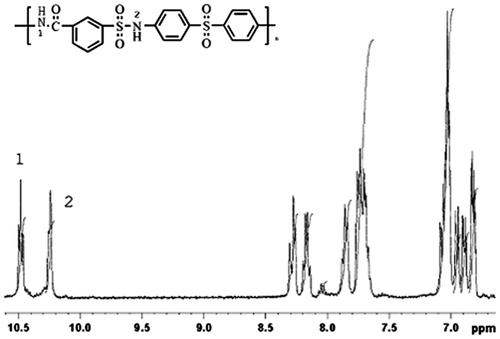
The polymer PASA which is soluble in dimethyl sulfoxide(DMSO) was adequately characterized using 1H NMR spectroscopy. Typical spectra of the polymer together with selected signal assignments are shown in Figure . As can be seen, the absorption peak at 10.5 ppm was assigned to be the N–H protons in carbonyl group, the peak at 10.2 ppm was attributed to the N–H protons in sulfonyl amine group, and the absorption peaks appearing in the region of 7.5–8.5 ppm were assigned to the aromatic protons.[Citation19] It can be clearly seen that all the protons are in agreement with the expected or proposed structure.
3.4. Physical properties
3.4.1. Morphology characterization
The morphology of the PASA was characterized by a scanning electron microscope (Figure ). The pores can be distributed homogeneously in the PASA and the size is about 30–50 μm. In order to measure the total surface area and the pore size distribution, a N2 adsorption test was conducted. BET surface areas were calculated from the linear part of the BET plot. The BET surface area of the polymer PASA was found to be 20.270 m2/g. Pore volume of the polymer was 0.028 cc/g. Thus, it demonstrated that the PASA has certain surface area, which is a crucial factor for adsorption materials. This also proved the potential of PASA to be used in the adsorption field.
3.4.2. Crystallinity
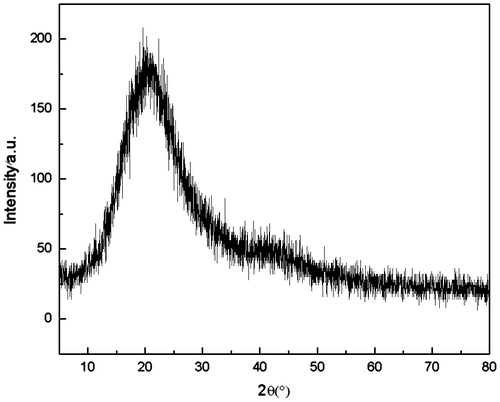
As physical properties of the polymer are basically influenced by degree of polymerization, orientation and crystallinity,[Citation20] it is of great significance to assess the degree of crystallinity in the polymer system. The degree of crystallinity for the PASA was investigated by X-ray (2θ = 5–80°), the result obtained is shown in Figure . The PASA presented a little degree of crystallinity, which exhibited a widened peak at 20°.
3.4.3. Thermal characterization
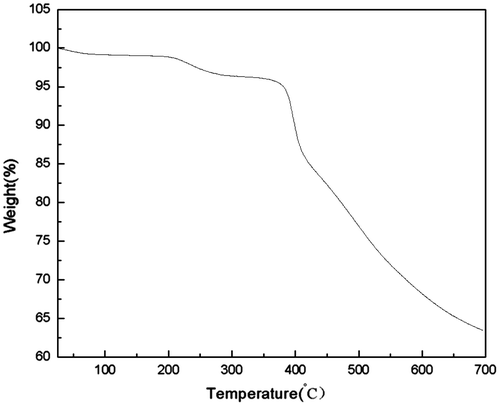
The thermal behaviour of the PASA was evaluated by means of thermogravimetric analysis (Figure ). Since the polymer sample was dried at 70 °C for 24 h in a vacuum oven prior to the measurements, these percentage weight losses below 120 °C were due to loss of water (including both absorbed and weakly bonded species). With the temperature increased to 200 °C, the decomposition of oligomers with a small molecular weight led to the mass loss of materials. The polymer has a good thermal stability, as indicated by the reasonably high temperatures recorded (>380 °C) before a 5% weight loss occurs.[Citation21] Due to the existence of the conjugated aromatic rings and the additional sulfone (–SO2–) group structure in its molecular main chain, the PASA with such molecular structure cannot be easily destroyed at high temperature.
3.4.4. Adsorption ability
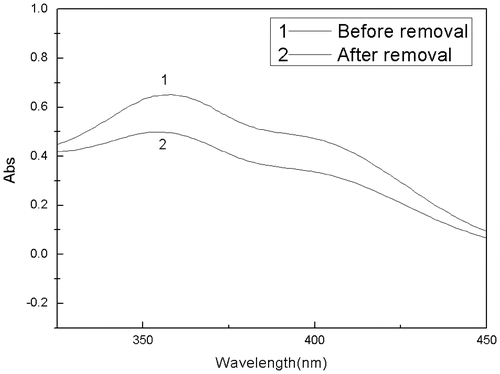
As the 2,4-DNP has an absorption peak at 359 nm wavelength,[Citation5] therefore, the 2,4-DNP concentration in the filtrate was determined by a UV spectrophotometer. The final 2,4-DNP concentration was 4.902 mg/L, thus the removal efficiency of the 2,4-DNP from 2,4-DNAN solution reached 54.11% (Figure ).
Another two types of PASAs from the reaction of m-chlorosulfonylbenzoyl chloride with 4,4′-diamino diphenyl ether and 4,4′-diaminodiphenylmethane, respectively, using low temperature solution polymerization were also prepared in our laboratory. Compared with the polymer made from 4,4′-diamino diphenyl sulfone, these two polymers have a lower adsorption ability due to the less sulphone groups in the main chain. Sulphones make great contribution to the hydrophilicity of the polymer, thus, the adsorption ability of the polymer has been improved. Both the high specific surface area and the precipitates which are easy to be separated due to their settling characteristics suggested that the PASA is an effective sorbent to selectively remove 2,4-DNP from the aqueous solution of 2,4-DNAN, so that the problem of superfluous 2,4-DNP in the hydrolysate should be solved. Hence, a study of the PASA modified by the pore-forming reagent is now underway and the detail of the kinetic and thermodynamic of the adsorption process will be investigated in the further paper.
4. Conclusions
In this study, we aimed to obtain a polymer material with better adsorption properties to separate 2,4-DNP from the aqueous solution of 2,4-DNAN. In response to this research challenge, we have synthesized and characterized the PASA from the reaction of m-chlorosulfonylbenzoyl chloride with 4,4′-diamino diphenyl sulfone in DMAc, using low-temperature solution polymerization reactions. The polymer was nearly amorphous with a porous morphology and a rather high thermostability. The removal rate of 2,4-DNP solution was 54.11% at 30 °C. Therefore, there appears to be a potential functional monomer for synthesis of polymers and the novel polymer PASA shows good potential for use as an absorbent.
Acknowledgements
The authors are grateful for the financial support from Liaoning Provincial Department of Education Science general project (L2011079) and Liaoning Province Natural Science Fund Project (201202012).
References
- Zhang G-Q, Dong H-S. Review on melt-castable explosives based on 2,4-dinitroanisole. Chin. J. Energetic Mat. 2010;18:604–609.
- Cao R-J, Mei D. The study of nucleophilic aromatic substitution reaction-study of the etherify about 2,4-dinitro-chlorobenzene. J. Xi’an Jiaotong Univ. 1994;28:7–11.
- Highsmith TK, Johnston HE. Continuous process for preparing alkoxynitroarenes. Google Patents. 2003.
- Schechter MS, Haller HL. Colorimetric determination of I-chloro-2,4-dinitrobenzene as impurity in 2, 4-dinitroanisole. Ind. Eng. Chem., Anal. Ed. 1944;16:326–327.
- Shukla SS, Dorris KL, Chikkaveeraiah BV. Photocatalytic degradation of 2,4-dinitrophenol. J. Hazard. Mater. 2009;164:310–314.
- Wang X-G, Wu Q-S, Liu W-Z, Ding Y-P. Simultaneous determination of dinitrophenol isomers with electrochemical method enhanced by surfactant and their mechanisms research. Electrochim. Acta 2006;52:589–594.
- Liu Y, Zhu L, Zhang Y, Tang H. Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer. Sens. Actuators, B Chem. 2012;8:171–172, 1151–1158.
- Oh S-Y, Kang S-G, Kim D-W, Chiu PC. Degradation of 2,4-dinitrotoluene by persulfate activated with iron sulfides. Chem. Eng. J. 2011;172:641–646.
- Podeh MRH, Bhattacharya SK, Qu M. Effects of nitrophenols on acetate utilizing methanogenic systems. Water Res. 1995;29:391–399.
- Sabio E, Zamora F, Ganan J, González-García C, González J. Adsorption of p-nitrophenol on activated carbon fixed-bed. Water Res. 2006;40:3053–3060.
- Liu H-L, Liu S-S. Enrichment and separation of chromium(VI) and chromium (III) in environmental water with polyamide resin. Chin. J. Anal. Chem. 1998;26:963–966.
- Zhang J, Hayat K, Zhang X, Tong J, Xia S. Separation and purification of flavonoid from ginkgo extract by polyamide resin. Sep. Sci. Technol. 2010;45:2413–2419.
- Kirupha SD, Murugesan A, Vidhyadevi T, Baskaralingam P, Sivanesan S, Ravikumar L. Novel polymeric adsorbents bearing amide, pyridyl, azomethine and thiourea binding sites for the removal of Cu(II) and Pb(II) ions from aqueous solution. Sep. Sci. Technol. 2012;48:254–262.
- Sun Y-W, Wang B. Preparation of phthalimidomethyl polysulfone affinity membrane and its removal properties of p-nitrophenol. J. Tianjin Polytechnic Univ. 2010;1:14–18.
- Freger V, Gilron J, Belfer S. TFC polyamide membranes modified by grafting of hydrophilic polymers: an FT-IR/AFM/TEM study. J. Membr. Sci. 2002;209:283–292.
- Chan W-H, Ng C-F, Lam-Leung S-Y, He X. Pervaporation of aqueous ethanol solution through poly(amidesulfonamide)s (PASAs) membranes. Polymer. 1998;39:2461–2467.
- Zhang C-L, Wang H-X. Synthesis of m-chlorosulfonylbenzoyl chloride. Chin. J. Synth. Chem. 2007;15:378–379.
- Cavus S, Gurdag G. Noncompetitive removal of heavy metal ions from aqueous solutions by poly[2-(acrylamido)-2-methyl-1-propanesulfonic acid-co-itaconic acid] hydrogel. Ind. Eng. Chem. Res. 2009;48:2652–2658.
- Faghihi K, Shabanian M, Valikhani N. New poly(amide-imide)s based on 1,3-bis[4,4′-(trimellitimido) phenoxy] propane and hydantoin derivatives: synthesis and properties. Des. Monomers Polym. 2011;14:109–119.
- Chan WH, Ng CF, Lam-Leung SY, He X, Cheung OC. Water–alcohol separation by pervaporation through poly (amide-sulfonamide)s (PASAs) membranes. J. Appl. Polym. Sci. 1997;65:1113–1119.
- Mallakpour S, Dinari M. High-speed microwave-promoted direct poly-amidation reactions of bulky chiral dicarboxylic acide with different aromatic diamines in imidazolium types ionic liquid as a reaction medium. Des. Monomers Polym. 2010;13:51–64.