Abstract
Copolymers of N(-1,1-dimethyl-3-oxobutyl)acrylamide (DOBA) and Methyl methacrylate (MMA) were prepared in dimethylformamide using benzoylperoxide as the initiator at 70 °C. They were characterized by IR, 1H NMR, and 13C NMR. Copolymer compositions were determined by the analysis of 1H NMR spectra. The weight and number average molecular weights of the polymers were determined by size exclusion chromatography. Monomer reactivity ratios were found to be 1.52 for MMA and 0.43 for DOBA according to Kelen–Tüdos method. Glass transition temperatures of the copolymers were determined by differential scanning calorimetry. The initial decomposition temperatures of the polymers were about 215 °C as measured by thermogravimetric analysis under nitrogen atmosphere. Swelling properties of the polymers were measured in distilled water. Maximum water uptake of about 70% was observed for poly(DOBA). In case of copolymers, water uptake decreases with the increase of MMA content in the polymer chain.
Introduction
Polymeric biomaterials are widely used for a variety of medical and industrial applications. Recently, different types of polymers viz., biodegradable polymers, molecular-imprinted polymers, hydrogels and polymer composites, have been used in medical fields, implants, drug delivery systems, therapeutic devices, etc.[Citation1–Citation4] Industrially, they are employed in biotechnological and pharmaceutical bioprocesses which include bioseparations with hollow fibre and flat sheet membranes, immobilized enzyme and cell processes. A variety of polymers which include both natural and synthetic biodegradable polymers, swelling type polymers, inert polymers like polysulfones, etc. are used depending upon the nature of application.
Swelling type biocompatible polymers are used for a variety of biomedical applications such as controlled delivery systems, contact lenses, etc. because of their good biocompatible nature.[Citation5–Citation8] Non-ionic biocompatible polymers containing both hydrophilic and hydrophobic groups are proposed as means of enhancing the fixation characteristics of implants in bone through an expansion-fit mechanism.[Citation5] These polymers are usually prepared by copolymerization of monomers containing appropriate functional group, and the characteristics of the materials depends on the nature and composition of the comonomers and their distribution along the copolymer chain. Homo- and co-polymers based on hydroxyethyl methacrylate, glycidyl methacrylate, acrylic acid methacrylic acid, methyl methacrylate (MMA), etc. are extensively studied for various biomedical applications.[Citation9–Citation11] Copolymers of N(-1,1-dimethyl-3-oxobutyl)acrylamide (DOBA) with hydroxyethyl methacrylate and isobutyl/cyclohexyl methacrylate, and hydroxyethyl methacrylate/vinyl acetate were also proposed as useful materials for contact lenses and other biomedical applications.[Citation12,Citation13] Poly(MMA) is a well-known material which possesses excellent optical characteristics, resistance to discolouration, light weight, and has been used for a variety of applications.[Citation14,Citation15]
The present investigation describes the synthesis, characterization and water swelling properties of copolymers of hydrophilic DOBA with hydrophobic MMA.
Experimental
Materials
DOBA from Aldrich Co. USA was used as received. MMA was freed from inhibitor and distilled under vacuum. Benzoylperoxide (BPO) was recrystallized from chloroform-methanol mixture. All the other reagents and solvents were of analytical grade samples and used without further purification.
Copolymerization
Copolymers of different compositions of DOBA and MMA were prepared using 2 M solutions of the monomers in dimethylformamide with BPO (0.1 wt.% of monomers) as the free radical initiator at 70 °C. Required amounts of the monomers and initiator were dissolved in DMF in a polymerization tube, flushed with nitrogen gas and kept in a thermostat at 70 °C. After about 1 h, the polymerization solution was added to excess of methanol-water mixture, and the isolated polymer was washed with water and methanol. After purification by reprecipitation into water/methanol from DMF solution, the copolymers were dried at 45 °C (4 h) in an air circulating oven.
Instruments
IR spectra were recorded on a Perkin Elmer Spectrum GX FT-IR spectrophotometer as KBr pellet. 1H NMR and 13C NMR spectra of the polymers in CDCl3 solution were obtained with a DPX 200 MHz Bruker FT-NMR spectrometer using tetramethylsilane as the internal reference. Thermogravimetric analysis (TGA) were carried out with nitrogen in a Mettler 851e thermal analyser at a heating rate of 10 °C min−1. Glass transition temperatures of the polymers were determined with a Mettler DSC 822e at a heating rate of 10 °C min−1 in nitrogen. Waters 515 HPLC equipped with three ultrastyragel columns and RI-2410 detector was used for the determination of molecular weights of the polymers. The molecular weights were calibrated against polystyrene standards using tetrahydrofuran as the mobile phase.
Swelling properties
Pellets of about 1 cm diameter and 2.5 mm thickness of the polymers were prepared at a pressure of 10 bar, dried at 45 °C (2 h) and kept in room temperature for 1 h before the experiment. They were immersed in water at 30 °C, the swollen pellets were removed from water at various time intervals, surface-dried using tissue paper and weighed using a digital balance with an accuracy of ± 0.0001 g. The percentage weight gain of the swollen polymer samples was computed using the following expression:(1)
where, Wt and W0 are the weights of the polymer pellets at time ‘t’ and ‘zero’ (dry state), respectively.
Results and discussion
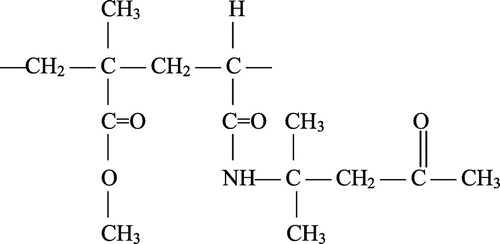
Several copolymers of DOBA with MMA were prepared by free radical polymerization in dimethylformamide solution using molar fractions of DOBA ranging from 0.10 to 0.90 in the monomer feed. The copolymerization reactions were proceeded in homogeneous solution for all the compositions of monomer feeds. The polymerization was restricted to reach conversions lower than 10 wt.% in order to utilize the differential copolymerization equation for the determination of monomer reactivity ratios. Table shows the molar compositions of the initial monomer feed and the resulting copolymer samples. The chemical nature of the copolymers is shown in Structure .
Table 1. Composition and molecular weight data for the copolymerization of DOBA with MMA.
Copolymer characterization
Solubility
Solubility is one of the important requirements of a polymer for its utilization for any application. It was observed that all the polymers were freely soluble in aprotic polar solvents like dimethylformamide, dimethylacetamide, N-methyl-2-pyrrolidone, dimethylsulfoxide, tetrahydrofuran, etc. and chlorinated solvents such as chloroform, dichloromethane, etc. They were insoluble in water, hydrocarbons like hexane, heptane, toluene, xylene, etc.
Molecular weights
Table gives the weight-average molecular weights and the polydispersity index values for poly(DOBA), poly(MMA) and copoly(DOBA–MMA) determined by gel permeation chromatography. The values of the polymers are in the range of 3.82–5.99 × 104 Da with polydispersity index values of 1.57–1.98, the normal molecular weight distribution expected for free radical polymerization of (meth)acrylate monomers.
IR spectra
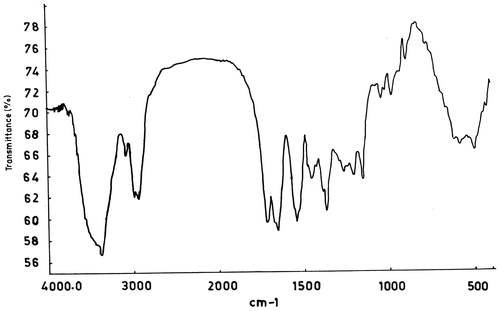
IR spectrum (Figure ) of poly(DOBA) shows a broad band around 3380 cm−1 corresponding to amide N–H stretchings. Peaks at 2973 and 2929 cm−1 are assignable to methyl and methylene groups of backbone and pendant unit. The two strong peaks at 1714 and 1667 cm−1 are due to carbonyl (C=O) stretchings of ketone and amide groups, respectively, present in the pendant unit. A strong peak at about 1542 cm−1 arises due to N–H bending vibrations. A doublet at 1386 and 1365 cm−1 may be assigned to the gem-dimethyl group present in the pendant unit.[Citation16]
The IR spectra of the copolymers exhibit more prominent features of DOBA unit since most of the absorptions due to MMA unit superimposes on those of DOBA. In addition to the peaks observed for poly(DOBA), the spectra of the copolymers show an additional strong band around 1730 cm−1 which is due to the esteric carbonyl stretchings of MMA unit.
1H NMR spectra
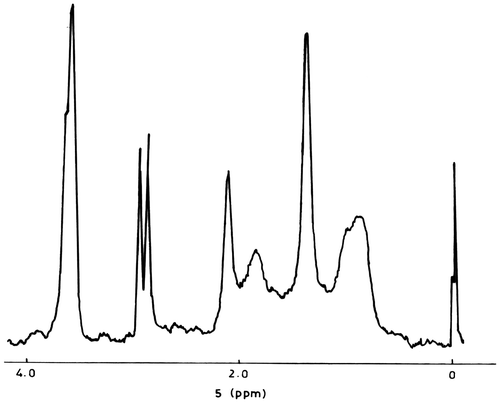
1H NMR spectrum of poly(DOBA) shows a doublet at 2.92 ppm due to CH2 protons in the pendant unit. The splitting of CH2 signals arises due to the presence of gem-dimethyl group on the neighbouring carbon. Signals at 2.14 and 1.40 ppm are assignable to methyl protons of acetyl (O=CCH3) and gem-dimethyl group, respectively, of the pendant unit. Resonance signals corresponding to amide (C=ONH) proton appear at about 8.03 ppm. Backbone CH2 and CH protons are observed as broad signals at 1.88 ppm.
1H NMR spectra of copolymers exhibit distinct resonance signals for both the monomeric units. The spectrum (Figure ) of copoly(DOBA–MMA) shows, in addition to the resonance signals observed for poly(DOBA), signals at 3.65 ppm corresponding to methoxy (–OCH3) protons of MMA unit. Two peaks with a more intense peak at 1.04 ppm and the less intense peak at 0.86 ppm due to α-methyl protons of MMA unit strongly suggest the presence of both isotactic and syndiotactic linkages in the copolymer backbone.
13C NMR spectra
13C NMR spectrum of poly(DOBA) shows resonance signals at 209.1 and 174.6 ppm which are assignable to ketone and amide carbonyl carbons, respectively, of the pendant N(-1,1-dimethyl-3-oxobutyl) amide unit. Signals at 51.4 ppm may be attributed to three different groups of carbons: (1) methylenic carbon flanked between gem-dimethyl and acyl group, (2) tertiary-substituted carbon of gem-dimethyl group and (3) the backbone methyne carbon. A peak at 42.1 ppm may be assigned to backbone methylene carbon. Signals at 31.7 and 27.4 ppm correspond to methyl carbons of acyl and gem-dimethyl groups, respectively. The spectra of copolymers show, in addition to the signals observed for poly(DOBA), signals at 60.71 and 18.06 ppm which are assignable to esteric methoxy (OCH3) and α-methyl carbons, respectively, of MMA unit. Resonance signals due to backbone –CH2 and tertiary (–C–) carbons appear at 50.40 and 45.12 ppm, respectively.
Copolymer compositions
1H NMR technique is well established as a simple and rapid method for the accurate determination of copolymer composition.[Citation17–Citation20] The assignment of resonance peaks in the 1H NMR spectrum allows to determine accurately the content of each type of monomeric units incorporated into the copolymer chain. Thus, the mole fractions of MMA in the copolymers were determined from the integrated intensities of methoxy protons at 3.65 ppm of MMA and the signals appearing at 3.65–0.88 ppm due to the aliphatic protons of both DOBA and MMA units.
The following expression was derived for the determination of copolymer compositions. Let ‘m1’ be the mole fraction of MMA, and (1−m1) that of DOBA. There are three methoxy protons in MMA and 22 aliphatic protons, i.e. 8 in MMA and 14 in DOBA, in both the monomers. Therefore,
on simplification it gives,(2)
Table gives the values of ‘C’ and the corresponding mole fractions of MMA and DOBA in the copolymers. They evidently display that for all the monomer feed compositions, copolymer contains more amount of MMA than the initial monomer feed mixture.
Monomer reactivity ratios
Monomer reactivity ratios are important quantitative values to predict the copolymer composition for any starting feed in batch, semi-batch or continuous reactors and to understand the kinetic and mechanistic aspects of the copolymerization. The reactivity ratios of DOBA and MMA were determined from the monomer feed and the resultant copolymer composition by the application of Kelen–Túdös method, the linear method of r1 and r2 determination in which all data are weighted equally and which is insensitive to transposition of data.[Citation21,Citation22] The reactivity ratio values obtained from the K–T plot are as follows:
The product of r1 and r2 (r1r2 = 0.65), which is less than 1, indicates a random distribution of comonomers in the polymer chain. The value of 1/r1 (= 0.66) suggests that the reactivity of growing radicals with MMA ends appears to be little higher towards its own monomer molecule. Conversely, the reactivity of growing radicals with DOBA ends, as observed by the value of 1/r2 (= 2.32), seems to be lesser towards its own molecule and higher towards the comonomer (MMA) molecule. These observations clearly suggest that the probability of MMA unit entering into the copolymer chain is somewhat high compared to that of DOBA.
Glass transition temperatures
The glass transition temperatures of the polymers were determined by differential scanning calorimetry and are presented in Table . The Tg value of poly(DOBA) and poly(MMA) are about 59 and 108 °C, respectively. All the copolymers did exhibit a single Tg, thus indicating the formation of random copolymer for all the monomer feed compositions. The Tg value of the copolymers depends on copolymer composition and increases with the increase of MMA content in the polymer chain. It is well known that the Tg of a polymer strongly depends on chain flexibility and bulkiness of the pendant unit. Poly(DOBA) contains a bulky N-(1,1-dimethyl-3-oxobutyl) pendant group compared to a small methyl group in poly(MMA). However, the Tg of poly(MMA) is about 49 °C higher than that of poly(DOBA). This clearly indicates that the presence of α-CH3 group in poly(MMA) exerts a larger effect on the Tg of the polymer when compared to the bulky pendant group in DOBA. It may be relevant to mention here that the Tg of poly(methyl acrylate) is about 10 °C, which is about 98 °C less than that of poly(MMA).
Table 2. Glass transition temperatures and TGA data for copolymers of DOBA with MMA system.
Thermogravimetric analysis
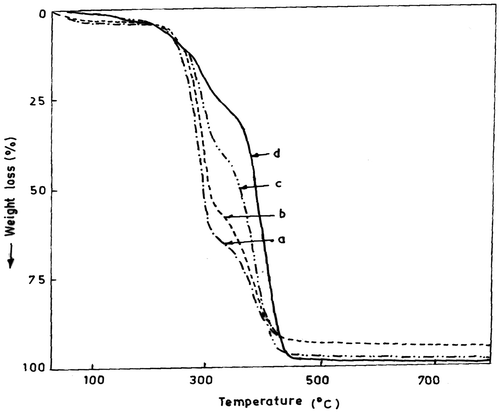
TGA is the most favoured technique for rapid evaluation of thermal stability of polymeric materials. It is especially useful in comparing the thermal stabilities of various copolymers. The TGA thermograms of polymers (Figure ) and differential TGA for two homopolymers and four copolymer samples are shown in Table . The initial decomposition temperatures (IDT) of poly(DOBA) and poly(MMA) are about 217 and 265 °C, respectively. Poly(MMA) decomposes in a single stage in the temperature range of 265–470 °C with a weight loss of about 96%. Poly(DOBA) undergoes decomposition in two stages, the first one at 217–317 °C with a weight loss of about 64%, and the second decomposition at 344–448 °C. The first stage decomposition of poly(DOBA) may be attributed to the cleavage and subsequent elimination of the pendant HNC(CH3)2CH2C(=O)CH3 group, which corresponds to about 66% of the total weight of the polymer, based on the formula weight of DOBA. The second stage decomposition may be due to the decomposition of polymer chain and subsequent loss of the decomposed products. The IDT of the copolymers depends on the composition of constituent monomers. The copolymers also decompose in two stages, similar to that of poly(DOBA). As the DOBA content in the copolymer was increased, the weight loss was decreased in the first stage (217–317 °C), and increased in the second stage in the temperature range of 345–448 °C. The total weight loss of the copolymers in both the decomposition stages was about 97%. The first stage decomposition corresponds to the elimination of HNC(CH3)2CH2C(=O)CH3 group of DOB unit and the second stage degradation may be attributed to cleavage of polymer chain.
Swelling properties
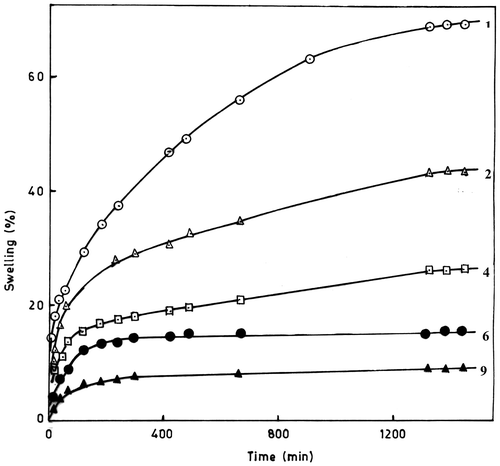
The primary objective of the swelling experiments is to study the influence of the hydrophilic/hydrophobic ratios on the swelling behaviour of the synthesized copolymers in pure water, saline water and phosphate buffer. The typical swelling curves obtained for a series of swelling measurements for poly(DOBA) and copolymer samples containing different amount of DOBA are presented in Figures . Generally, half of the equilibrium swelling (saturated weight gain) was reached in the first 10 h after immersion and maximum weight gain of about 70% was achieved for poly(DOBA) after about 24 h of immersion. Further swelling was very slow and stopped practically after about 24 h. In case of copolymers, the extent of swelling depends on copolymer composition and decreases with the increase of MMA content in the polymer chain. This may be attributed to the increase of the hydrophobic nature of the copolymers with the incorporation of more and more amount of MMA units in the polymer chain.
According to Flory’s swelling theory,[Citation23–Citation25] water swelling depends on ionic osmotic pressure, cross-linking density and the affinity of the polymer for water. To obtain a more quantitative understanding of the nature of water swelling of polymers, the initial swelling data were fitted to the exponential heuristic equation.(3)
Where Mt/Mα is the swelling of polymer in time ‘t’, k is a characteristic constant of the polymer and ‘n’ is a characteristic exponent of the transport mode of the penetrate. Mt and Mα is the amount of swelling at time ‘t’ and at equilibrium, respectively. For Fickian diffusion in which the rate of water diffusion is rate limiting, n = 0.5, whereas values of ‘n’ between 0.5 and 1 indicate the contribution of non-Fickian processes such as polymer relaxation.
We have evaluated the values of ‘n’ from the slopes and intercepts of the plot of log(Mt/Mα) against log(t) and were found to be in the range of 0.25–0.42 for all the polymer samples. The ‘n’ value of less than 0.5 strongly suggests that the mechanism of water swelling of poly(DOBA) and copoly(DOBA–MMA) pellets is a Fickian transport.
Conclusion
Copolymers of DOBA with MMA were synthesized and characterized by IR, 1H NMR, 13C NMR and gel permeation chromatography. The composition data and reactivity ratios of the monomers clearly suggest random distribution of comonomers in the copolymer chain. The copolymers show a single Tg; thus, indicating the formation of random copolymers. The copolymers possess good thermal stabilities with IDT of above 215 °C, as observed by thermogravimetric analysis in nitrogen. Water uptake of the copolymers depends on the composition of the monomers and decreases with the increase of MMA content in the polymer chain. This is attributed to the increase of the hydrophobic nature of the copolymers with the incorporation of more and more amount of MMA units in the polymer chain.
Acknowledgement
The result was developed within the CENTEM project, reg. no. CZ.1.05/2.1.00/03.0088 that is co-funded from the ERDF within the OP RDI programme of the Ministry of Education, Youth and Sports.
References
- Halliday AJ, Campbell TE, Nelson TS, McLean KJ, Wallace GG, Cook MJ. Levetiracetam-loaded biodegradable polymer implants in the tetanus toxin model of temporal lobe epilepsy in rats. J. Clin. Neurosci. 2013;20:148–152.
- Linxian L, Zewei B, Levkin PA. Boronate–dextran: an acid-responsive biodegradable polymer for drug delivery. Biomaterials. 2013;34:8504–8510.
- Rostamizadeh K, Vahedpour M, Bozorgi S. Synthesis, characterization and evaluation of computationally designed nanoparticles of molecular imprinted polymers as drug delivery systems. Int. J. Pharm. 2012;424:67–75.
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 2012;64:49–60.
- Prabhakar PK, Sai R, Anuradha PR, Sawant SN, Doble M. Biocompatibility studies on polyaniline and polyanilinesilver nanoparticle coated polyurethane composite. Colloids Surf., B. 2011;86:146–153.
- Gesine W, Carsten S, Stefan F, Thomas K. Biodegradable polymers and their potential use in parenteral veterinary drug delivery systems. Adv. Drug Delivery Rev. 2004;56:1453–1466.
- Hong C, Lin Y, Wei S, Zhongkui W, Dan L. Biocompatible polymer materials: role of protein–surface interactions. Prog. Polym. Sci. 2008;33:1059–1087.
- Darren S, Jadranka TS, Anthony R, Sanjay G. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Controlled Release. 2010;146:6–15.
- Tomić SLJ, Mićić MM, Filipović JM, Suljovrujić EH. Synthesis, characterization and controlled release of cephalexin drug from smart poly(2-hydroxyethyl methacrylate/poly(alkylene glycol)(meth)acrylates hydrogels. Chem. Eng. J. 2010;160:801–809.
- Casas M, Ferrero C, Paz MV, Castellanos MRJ. Synthesis and characterization of new copolymers of ethyl methacrylate grafted on tapioca starch as novel excipients for direct compression matrix tablets. Eur. Polym. J. 2009;45:1765–1766.
- López P, Argemí A, Andanson JM, Fernández V, García-González CA, Kazarian SG, Saurina J, Domingo C. Impregnation of a biocompatible polymer aided by supercritical CO2: evaluation of drug stability and drug–matrix interactions. J. Supercrit. Fluids. 2009;48:56–63.
- Ormsby R, McNally T, Hare PO, Burke G, Mitchell C, Dunne N. Fatigue and biocompatibility properties of a poly(methyl methacrylate) bone cement with multi-walled carbon nanotubes. Acta Biomater. 2012;8:1201–1212.
- Dalton PD, Flynn L, Shoichet MS. Manufacture of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterials. 2002;23:3843–3851.
- Otaibi AGA, Qahtani ESA. Polymethyl methacrylate intraocular lens opacification 20 years after cataract surgery: a case report in a tertiary eye hospital in Saudi Arabia. Saudi J. Ophthalmol. 2012;26:105–107.
- Ergün Y, Dirier C, Tanoğlu M. Polymethyl methacrylate based open-cell porous plastics for high-pressure ceramic casting. Mater. Sci. Eng. A. 2004;385:279–285.
- Moore J. Spectroscopy of organic molecules. New York: Academic Press; 1990.
- Zhou Z, Kümmerle R, Qiu X, Redwine D, Cong R, Taha A, Baugh D, Winniford B. A new decoupling method for accurate quantification of polyethylene copolymer composition and triad sequence distribution with 13C NMR. J. Magn. Reson. 2007;187:225–233.
- Viel S, Mazarin M, Giordanengo R, Trang NTP, Charles L, Caldarelli S, Bertin D. Improved compositional analysis of block copolymers using diffusion ordered NMR spectroscopy. Anal. Chim. Acta. 2009;654:45–48.
- Kim Y, James HH. Analysis of sequence distribution in methyl methacrylate-methyl acrylate copolymers by 13C NMR spectroscopy. Polymer. 2002;43:3229–3237.
- Rami Reddy AV, Sambasiva PR, Reddy GH. Synthesis and characterization of copolymers of 3-hydroxy-4-benzoylphenyl methacrylate with methyl methacrylate and their metal chelates. Eur. Polym. J. 1999;35:965–971.
- Kelen T, Tudos F. Copolymerization and determination r1, r2 for monomers. J. Macromol. Sci. Chem. 1975;A9:1–27.
- Kennedy JP, Kelen T, Tudos F. Analysis of the linear methods for determining copolymerization reactivity ratios – 2. A critical reexamination of cationic monomer reactivity ratios. J. Polym. Sci. 1975;A1:2277–2289.
- Flory PJ. Principles of polymer chemistry. New York, NY: Cornell University Press; 1953. Chapter XIII.
- Jin S, Bian F, Lin M, Chen S, Liu H. Swelling mechanism of porous P(VP-co-MAA)/PNIPAM semi-IPN hydrogels with various pore sizes prepared by a freeze treatment. Polym. Int. 2009;58:142–148.
- Xu SM, Cao LQ, Wu RL, Wang JD. Salt and pH responsive property of a starch-based amphoteric superabsorbent hydrogel with quaternary ammonium and carboxyl groups (II). J. Appl. Polym. Sci. 2006;101:1995–1999.