Abstract
In the present work, a new diimide-diacid monomer bearing pyridine rings was prepared. A series of novel nanostructured, organosoluble, thermally stable and wholly aromatic poly(amide-imide)s (PAIs) were synthesized from diimide-diacid, by direct polycondensation with various aromatic diamines in N-methyl-2-pyrrolidone using triphenyl phosphite and pyridine as a condensing agent. The nanostructured PAIs were achieved in good yields and possessed moderate inherent viscosities in the range of ηinh = 0.37–0.51 dL g−1. The synthesized polymers were characterized with FT-IR, 1H-NMR, UV–vis spectroscopy, elemental analysis, X-ray diffraction, field emission scanning electron microscopy (FE-SEM) and thermogravimetric analysis (TGA) techniques. All of the PAIs were amorphous in nature, were readily soluble in many organic solvents and could be solution cast into tough and flexible films. These polymers exhibited strong UV–vis absorption in the range of 294–311 nm in solution and in films. According to TGA data, these polymers exhibited initial decomposition temperature in the range of 396–433 °C, temperature of 10% weight loss from 446 to 537 °C, char yields more than 75% at 700 °C in nitrogen atmosphere and can be classified as self-extinguishing polymers. FE-SEM results show that, these nanostructured PAIs have spherical and cylindrical beads and the particle size is around 35–90 nm.
1. Introduction
Wholly aromatic polyimides (PIs) or their copolymers are well known as high performance polymers due to their excellent thermal and thermooxidative stabilities, an extremely high purity, non-flammability, outstanding mechanical and electrical properties, good balance between chemical, physical and mechanical properties. Therefore, all kinds of PIs have been widely used in the fields of high temperature adhesives, composite matrices, fibers, films, foams, moldings, wire-coating enamels, membranes, as well as microelectronic materials.[Citation1–8] However, wholly aromatic PIs, though thermally stable, do not always provide the optimal properties for many specialty applications because of deficiencies in solubility, processability and transparency.[Citation9,10] Therefore, much effort has been made to increase the solubility and processability of PIs. Many monomers have been specially designed using different approaches, such as introducing of flexible linkages or bridging functional groups,[Citation11,12] bulky pendant groups into the polymer backbones,[Citation13–15] heterocyclic rings [Citation16] and meta-catenated as a less symmetric aromatic units [Citation17–19] to afford the success in preparation of organosoluble polyamides.
For the PIs materials, synthesis of newly heteroaromatic monomers and corresponding PIs that have both good processability and maintained thermal stability would be very interesting. In general, the heteroaromatic structures in the main chain of a polymer would impart certain properties to it, while pyridine with heteroaromatic structure would have excellent stabilities derived from its aromaticity and molecular symmetry, as well as polarizability resulting from nitrogen atom in pyridine ring. So new kinds of heteroaromatic diacide, diamine, dianhydride or other monomers holding pyridine unit should have contributions for the thermal stability, chemical stability, retention of mechanical property of the resulting polymer at elevated temperature and the resulting polymer holding pyridine ring should also exhibit a good solubility during processing.[Citation20–22]
Replacement of PIs by copolyimides such as poly(amide-imide)s (PAIs) may be useful in modifying the intractable character of PIs.[Citation23,24] Because of containing both imide and amide linkages in the main chain of polymer repeating units, PAIs have properties between PIs and polyamides; therefore, this kind of polymers offers a good compromise between high thermal property and processability. Aromatic PAIs possess desirable characteristic for a variety of applications as they retain exceptional thermomechanical properties at high temperature with good dimensional stability, excellent creep, chemical resistance as well as solvent resistance and have found extensive use in industries.[Citation25–28]
The designing of new nanostructure materials from organic molecules has opened up new fundamental and practical frontiers to develop physical properties of materials significantly for different applications. Different nanostructure polymers have been developed for a wide range of advanced uses such as drug delivery devices, conducting wires and optoelectronic devices.[Citation29–32]
In this work, a new kind of heteroaromatic diimide-diacid monomer containing imide ring and pyridine moiety, 2,6-Bis[3(1,3-dioxoisoindoline-5-carboxylic acid) benzoyl] pyridine, has been synthesized successfully, and a series of novel nanostructured, organosoluble, thermally stable and wholly aromatic PAI have been also prepared by the direct polycondensation in N-methyl-2-pyrrolidone (NMP), triphenyl phosphite/pyridine (TPP/Py), lithium chloride (LiCl) and calcium chloride (CaCl2). This new diimide-diacid was characterized by Fourier transform infrared (FT-IR) and 1H-NMR spectroscopy and elemental analysis. The obtained PAIs were fully characterized by FT-IR, 1H-NMR and UV–vis spectroscopy, elemental analysis, viscosity measurement, quantitative solubility, X-ray diffraction (XRD), thermo gravimetric analysis (TGA) and field emission scanning electron microscopy (FE-SEM).
2. Experimental
2.1. Materials
All chemicals and solvents used were of analytical reagent grade purchased from Fluka Chemical Co. (Buchs, Switzerland), Aldrich Chemical Co. (Milwaukee, WI) and Merck (Darmstadt, Germany). 1-methylpyrrolidin-2-one (NMP) as solvent was distilled over barium oxide under reduced pressure. Other reagents were used without further purification.
2.2. Instrument and measurement
Furrier transform infrared (FT-IR) spectra of the samples were recorded with a Jasco-680 (Japan) spectrometer at a resolution of 4 cm−1. The spectra of solids were obtained using KBr pellets. The pressed disk containing 2 mg of the sample and 200 mg of fine grade KBr was scanned at wavenumber range of 400–4000 cm−1. Proton nuclear magnetic resonance (1H-NMR, 500 MHz) spectra were recorded in DMSO-d6 solution using a Bruker (Germany) Avance 500 instrument. Inherent viscosities (ηinh) were measured by a standard procedure using a Cannon-Fenske Routine Viscometer (Cannon, Mainz, Germany) at a concentration of 0.5 g/dL at 25 °C. Elemental analyses were performed by Leco, CHNS-932. Quantitative solubility of PAIs in various solvents was determined using 5 mg of the polymer in 1 mL of solvent. The XRD patterns were recorded by employing a Philips X’PERT MPD diffractometer (Cu Kα radiation: λ = 0.154056 nm at 40 kV and 30 mA) over the 2θ range of 10–80° at a scan rate of 0.05°/min. The samples for UV–visible measurements were prepared by cutting pieces of the cast films. These films have thicknesses 0.1–0.2 mm. UV–visible transmission spectra of PAI films were obtained using a JASCO V-750 UV/Vis/NIR spectrophotometer in the spectra range from 200 to 800 nm at a wavelength scan rate of 60 nm/min. Derivative thermal gravimetric (TGA/DTG) data for polymers were taken on Perkin Elmer in nitrogen atmosphere at a heating rate of 10 °C.min−1 from room temperature to 800 °C. Surface morphology of polymers was characterized using FE-SEM [HITACHI: S-4160].
2.3. Synthesis of diimide-diacid (6)
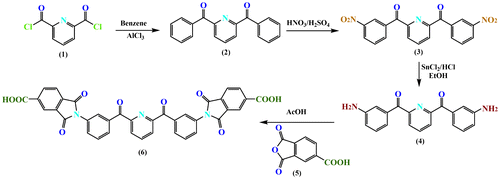
For synthesis of heteroaromatic diimide-diacid (6), 2,6-Bis (3-aminobenzoyl) pyridine (4) was prepared and characterized based on literature.[Citation33] A flask was charged with (1 g, 3.15 mmol) diamine (4), (1.21 g, 6.30 mmol) trimellitic anhydride (5), and 10 mL of acetic acid. The mixture was heated to reflux at 80 °C for about 12 h. The mixture was then cooled and poured into water. The white precipitate was filtered and dried in a vacuum oven at 70 °C overnight. The yield of the reaction was about 88% and the melting point was 243–245 °C. Elemental Analysis: Calculated. C, 66.77%; H, 2.88%; N, 6.31%; O, 24.04%, Found. C, 66.51%; H, 2.73%; N, 6.49%; O, 24.27%.
2.4. Synthesis of PAIs
The PAIs were prepared by the following general procedure: in a 50 mL two necked round bottomed flask equipped with a reflux condenser, a magnetic stirrer bar and a nitrogen gas inlet tube, a mixture of 0.50 mmol diamine, diacid (6) (0.33 g, 0.50 mmol), TPP (1.20 mL, 5 mmol), LiCl (0.60 g, 14.15 mmol), CaCl2 (0.60 g, 6.21 mmol), 1 mL pyridine and 5.0 mL NMP was refluxed at 100 °C for 8 h under nitrogen atmosphere. After cooling, the reaction mixture was poured into a large volume of methanol and the precipitate was collected and washed thoroughly with hot water, then dried in a vacuum oven at 80 °C for 24 h. The yield of the polymerization reaction after extraction of polymers in hot methanol and removing low molecular weight oligomers were in the range of 74–91%. The inherent viscosity of the polymers were in the range of ηinh = 0.37–0.51 dL/g.
2.5. PAIs film preparation
The PAI films were fabricated by casting from solution. A solution of polymer was made by dissolving about 0.5 g of the PAI sample in 5 mL of NMP to afford an approximately 10 wt% solution. The homogeneous solution cast in glass Petri dishes. (bubbles were removed by shaking and blowing air), which was placed in a 90 °C oven overnight to evaporate most of the solvent; then the semidried film was further dried in vacuum at 160 °C for 5 h, for the removal of residue solvent and form solid films. The thickness of the films was controlled by using the same amount of total materials and same sized glass Petri dish. Freestanding PAI films were then peeled out from the glass plate and were used for transmission UV–vis absorption, XRD measurements, solubility tests and thermal analyses.
3. Result and discussion
3.1. Synthesis and characterization of monomer (6)
A new hetroaromatic diimide-diacid compound, 2,6-Bis[3(1,3-dioxoisoindoline-5-carboxylic acid) benzoyl] pyridine, was synthesized by a four step procedure, respectively, as shown in Scheme . Firstly, 2,6-dibenzoylpyridine was formed by Friedel–Crafts acylation of benzene with 2,6-pyridinedicarbonyl chloride using anhydrous aluminum chloride as a catalyst, then dinitro compound, 2,6-bis(3-nitrobenzoyl) pyridine, was synthesized by the nitrification of 2,6-dibenzoylpyridine with nitric acid (99%), and 2,6-bis(3-nitrobenzoyl) pyridine was converted to 2,6-Bis (3-aminobenzoyl) pyridine by the reduction of the nitro groups into NH2 groups by reaction with SnCl2 and HCl in ethanol. Finally, the hetroaromatic diacid (6) was prepared by the condensation of 2,6-Bis (3-aminobenzoyl) pyridine with two mole equivalents of trimellitic anhydride (5) in acetic acid.
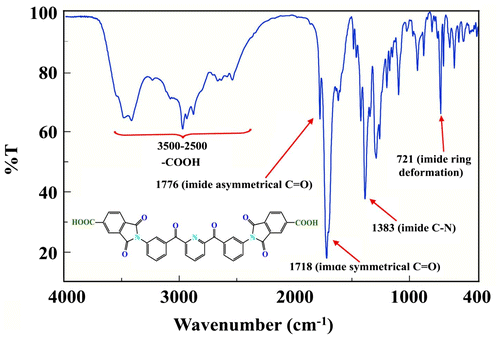
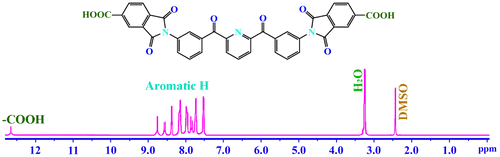
The structure of diimide-diacid monomer (6) was confirmed by FT-IR spectrum, 1H-NMR spectroscopy and elemental analysis. The FT-IR spectrum (Figure ) of monomer (6) showed absorption bands around 2900–3500 cm−1 (COOH group), 1750 and 1732 cm−1 (typical of imide carbonyl asymmetric and symmetric stretching) confirming the presence of carboxylic acid groups and imide ring in the structure. As shown in Figure , in the 1H-NMR spectrum of diimide-diacid (6), the resonance signal at 7.52–8.46 ppm is ascribed to the aromatic protons. The signal at 12.82 ppm is related to the proton of carboxylic acid. These results clearly confirmed that diacid prepared herein is consistent with the proposed structure.
3.2. Polymers synthesis
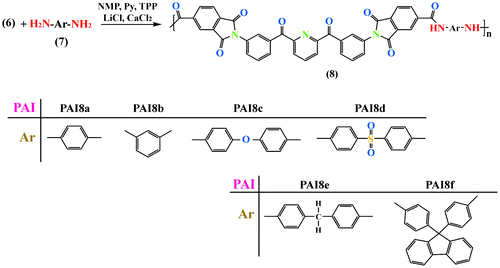
According to the phosphorylation polyamidation technique first described by Yamazaki and co-workers,[Citation34] a series of novel PAIs containing imide ring and heteroaromatic pyridine group was prepared by the direct polycondensation reaction between the diimide-diacid compound (6) and the commercially available aromatic diamines (7a–7f), as shown in Scheme . The polymerization was carried out via solution polycondensation using TPP and Py as condensing agents. The reaction readily proceeded within a homogeneous solution in NMP and in the presence of LiCl and CaCl2. The synthesis and some physical properties of these new PAIs (8a–8f) are given in Table . All the polymers were obtained in high yields (83–91%) and the inherent viscosities were in the range of ηinh = 0.37–0.51 dL/g, indicative of the formation of moderate molecular weights polymers.
Table 1. Synthesis and some physical properties of PAIs.
3.3. PAIs structural identification
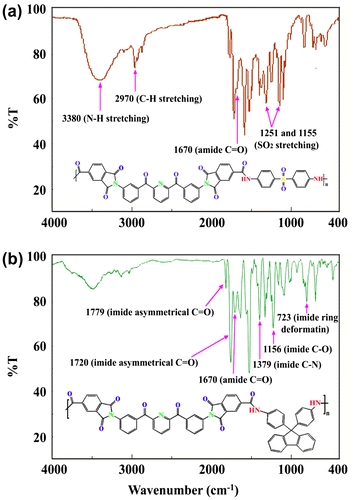
The polymer structures were confirmed by FT-IR, 1H-NMR spectroscopy and elemental analysis techniques. In general, in the FT-IR spectrum of PAIs, the characteristic absorption bands for the five membered imide ring appears at 1773–1779 (C=O asymmetrical stretching), 1714–1720 cm−1 (C=O symmetrical stretching), 1377–1389 cm−1 (C–N stretching), 721–728 cm−1 (imide ring deformation). The absorptions of amide groups appeared at 3333–3367 cm−1 (N–H stretching) and 1663–1674 cm−1 (amide C=O stretching). The FT-IR spectra of PAI8d and PAI8f are shown in Figure .
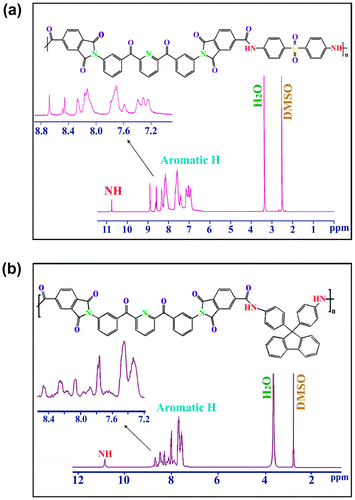
1H-NMR spectra of the PAI8d and PAI8f are illustrated in Figure . All the protons in these spectra are in good agreement with the proposed structures. The resonance peaks appearing in the region of 10.09–10.94 ppm also support the formation of amide linkages. The signals in the range of 7.25–8.21 ppm are attributed to the aromatic groups in the polymer’s main chain. The spectral data of PAIs are listed in Table and elemental analysis values for the obtained PAIs are also listed in Table . The elemental analysis values generally agreed with the calculated values for the proposed structures of PAIs.
Table 2. FT-IR and 1H NMR data of the PAIs.
Table 3. Elemental analysis of PAIs.
3.4. PAIs solubility
It is known that conventional wholly aromatic PIs are insoluble in common organic solvents. One of the major objectives of this study was to produce nano structure PAIs containing imide ring and hetroaromatic pyridine group with improved solubility. The solubility behavior of the new resulting PAIs was investigated quantitatively in different organic solvents at 10% (w/v), and the results were summarized in Table . These polymers exhibited excellent solubility in the polar aprotic solvents such as NMP, N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) at room temperature, and even in less polar solvents like m-cresol and pyridine by heating, but showed poorer solubility in dioxane (DO) and chloroform (CHCl3) due to the low value of dielectric constant of these solvents. The good solubility of these PAIs might be attributed to their nanostructure, the presence of the polar amide linkage in the main chain and the polarizability resulting from the nitrogen atom in pyridine ring. The PAI8a derived from the more rigid p-phenylenediamine toward PAI8b containing m-phenylenediamine showed a decreased solubility in m-cresol and pyridine.[Citation35] Also the unique enhanced solubility of the meta-related polymer (PAI8b) might be attributed to the higher entropy state of the polymer chains resulting from the meta orientation. The greater disorder should facilitate solvation of the polymer chains. For PAI8c and PAI8d the presence of sulfone and ether linkages can also contribute effectively in the solubility of these polymers by interacting with the polar molecules of solvents. The presence of methylene groups in the backbone of PAI8e increased flexibility and also disturbed the planarity of aromatic units, resulting in a reduction of the close packing and increased the solubility in a less polar solvent like pyridine. The solubility of PAI8f should be the result of the introduction of the bulky pendent group in PAI backbone which disturbed dense packing of the polymer chains and led to the increased chain distances and decreased chain interactions; finally, the solvent molecules were able to penetrate more easily to solubilize the polymer chains. Therefore, the good solubility makes these PAIs potential candidates for practical applications in spin or dip coating and ink jet printing processes. All the polymers could afford flexible and tough films by solvent casting, and these films are essentially amorphous as evidenced by the XRD patterns.
Table 4. Quantitative solubilityTable Footnotea of the PAIs.
3.5. XRD analysis of PAIs
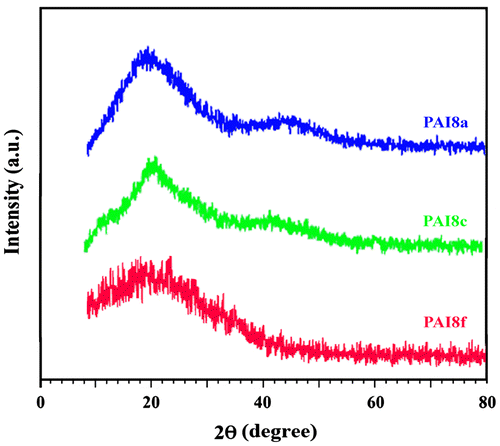
XRD is the most commonly used method to study the structure of nanostructure materials. As indicated by X-ray diffractograms of PAIs in Figure , the weak reflection centered at a 2θ value around 20° was characteristic of the amorphous polymers with only a low degree of molecular ordering. This might have been caused by the presence of heterocyclic imide ring and wholly aromatic structures in the polymer chain, which limit the molecular mobility, decreased the intermolecular forces between the polymer chains and constrict the formation of crystals.
The PAI8f due to the presence of bulky pendent group of diamine is less crystalline than other PAIs. Furthermore, the amorphous character is also supported by the reasonable solubility of PAIs, which is in agreement with the general rule that the solubility decreases with increasing crystallinity.
3.6. Nano structure of PAIs
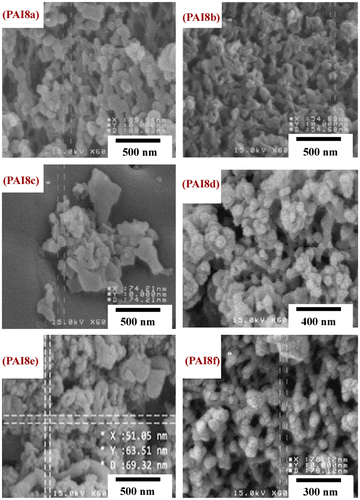
The development of nanostructured polymers has opened up new fundamental and rapidly growing field of research, which has attracted notable attention in recent years. Nano structured polymers have been developed for a wide range of advanced uses such as conducting wires, optoelectronic devices, material science, biotechnology, biomedicine, biochemical sensor design and drug delivery systems.[Citation36,37] Morphological characterization of PAIs was studied by FE-SEM. In this study it is very interesting to mention that the FE-SEM images of PAIs showed that the obtained macromolecules have nano structure morphology (Figure ). As can be seen in Figure , the average diameter of polymeric particles is in the range of 35–90 nm. The PAIs offered a homogeneous nanostructure, dispersed as spherical and cylindrical beads, and also have amorphous and spongy morphology.
3.7. Optical properties of PAIs
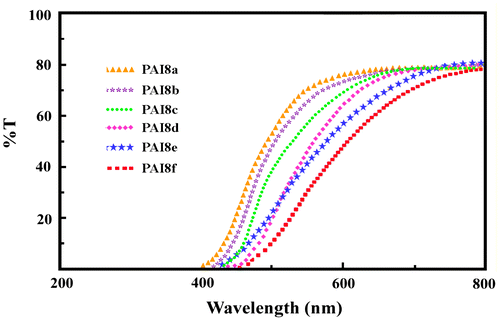
The optical properties of the PAIs are investigated by UV–vis spectroscopy. Table lists some important optical data obtained from dilute solutions (0.2 g/dL) of the PAIs in NMP and from thin films. The absorption spectra of the resulting PAIs in solutions and in films were nearly identical with each other. The PAIs showed strong UV absorption with maximum at range of λmax = 294–311 nm, which are attributed to the π–π* transitions. Figure depicts the UV–vis transmittance spectra of PAI films, the λ0 values (absorption edge or cut off wavelength) of the resulting polymers were found to be in the range of 380–390 nm and the optical transmittance values at 700 nm are higher than 80%, indicating high degree of the film transparency.
Table 5. Optical properties of the PAIs.
3.8. Thermal stability of PAIs
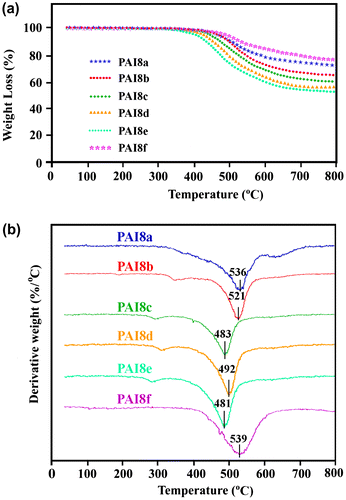
Thermal stability plays an important role in determining both technological applications and processing conditions of polymers and generally studied by TGA and derivative of thermaogravimetric (DTG). TGA and DTG analysis in a nitrogen atmosphere at a rate of 10 °C min−1 were used to study the thermal properties of these PAIs. TGA and DTG curves of PAIs are shown in Figure and the obtained results are given in Table . The initial decomposition temperature (Ti), the temperature of 5% weight loss (T5) and 10% weight loss (T10), that is an important criterion for evaluation of thermal stability, are ranged between 396–433 °C, 413–476 °C and 446–537 °C, respectively. There was a small weight loss at the temperature range from 80 to 200 °C in the TGA curves of PAIs, which can be attributed to the loss of absorbed moisture hydrogen bonded with amide linkages that was not removed in the drying process. The amount of carbonized residue (char yield or CR) of PAIs was from 53.4 to 75.9 at 800 °C under a nitrogen gas flow, depending on the structure of diamine component. The high char yields of these polymers can be attributed to their high aromatic content. An examination of data reveals that that the T10 of PAI8b was slightly higher than that of PAI8a, which may be attributed to the fact that the meta-oriented architecture has less dense polymer chain packing than para-oriented, due to the smaller conformational entropy of para-oriented chain. So, the former has better thermal stability. From TGA curves it can be detected that the most stable polymer is PAI8f with Ti of 439 °C, T5 of 510 °C, T10 of 520 °C, which can be due to absence of flexible linkage such as ether, methylene, or sulfone group in the polymer backbone and also due to bulky rigid side group of diamine. As can be seen from the DTG, all PAIs decomposed in one stage weight loss process. The temperature of the maximum speed of decomposition (Tmax) is situated in the range of 435–590 °C and it is due to the degradation of the amidic linkages.
Table 6. Thermal characteristic data of PAIs.
Interesting relationships have been found between the limiting oxygen index (LOI), CR and heat of combustion as the parameters of combustion process. The amount of CR can be applied as decisive factor for evaluating of the halogen free polymers in agreement with Van Krevelen and Hoftyzer equation: LOI = 17.5 + 0.4 CR.[Citation38] For all PAIs the LOI values calculated based on their char yield at 800 °C and were in the range of 38.9–47.9. From this equation, a higher char yield will enhance flame retardancy of polymers and based on the LOI values, these PAIs can be classified as self-extinguishing polymers.
According to Johnson [Citation39] the LOI values of many common materials can be logically well predicted by the expression, provided the atomic C/O ratio is larger than 6: ∆Hcomb = 8000/LOI, where ∆Hcomb is the specific heat of combustion in kJ g−1. The ∆Hcomb values of the PAIs were in the range 16.7–20.5 kJ g−1. It is concluded from Table data that these nano structure PAIs established high thermal stability that could be ascribed to the incorporation of high rigid imide ring, heteroaromatic pyridine moieties and amide linkages.
4. Conclusions
A series of novel nano structure PAIs containing hetroaromatic pyridine ring and different linkages in the main chains were successfully synthesized from a new symmetrical diimide-dicarboxylic acids monomer and various commercial aromatic diamine via direct phosphorylation polycondensation reaction. The synthesized PAIs, with inherent viscosities of ηinh = 0.37–0.51 dL g−1, were obtained in high yields. The structures of monomer and PAIs were confirmed by different techniques. Due to the presence of amide and imide linkages and pyridine groups in main chain of polymers, these macromolecules exhibited interesting properties such as thermal stability, solubility in many polar aprotic organic solvents and capability of forming colorless flexible thin films through solution casting. The thin films of PAIs showed high optical transparency in the UV–vis light region. With XRD, it was found that all of these polymers were amorphous. Morphological studies showed that the synthesized macromolecules have nano structure and according to FE-SEM results, the particle size of PAIs is around 35–90 nm. On the basis of TGA/DTG data, these PAIs are thermally stable and can be classified as self-extinguishing polymers. In sum, these nano structure PAIs displayed excellent solubility, good film forming capability and reasonable thermal stability suitable for thermoforming processing.
Acknowledgements
We wish to express our gratitude to the Payame Noor University of Estahban, for partial financial support.
References
- Hajibeygi M, Shabanian M, Khodaei-Tehrani M. Synthesis and characterization of new organosoluble poly(amide-imide)s with good thermal properties containing photosensitive and bicyclo segments in the main chain. Des. Monomers Polym. 2011;14:617–633.
- Bessonov MI, Zubkov VA, editors. Polyamic acid and polyimides: synthesis, transformation and structure. Boca Raton (FL): CRC Press; 1993.
- Ghosh MK, Mittal KL, editors. Polyimides: fundamentals and applications. New York (NY): Marcel Dekker; 1996.
- Sachdev HS, Khojasteh MM, Feger C, editors. Advances in polyimides and low dielectric polymers. New York (NY): Society of Plastic Engineers; 1999.
- Mallakpour S, Zeraatpisheh F. Novel heat resistant nanostructure poly(amide–imide)s containing new TMA-based diacid via conventional polycondensation reaction in an ionic green medium: synthesis, morphology, and thermal properties. Des. Monomers Polym. 2013;16:313–322.
- Ghaemy M, Alizadeh R, Behmadi H. Synthesis of soluble and thermally stable polyimide from new diamine bearing N-[4-(9H-carbazol-9-yl)phenyl] formamide pendent group. Eur. Polym. J. 2009;45:3108–3115.
- Yang CP, Lin JH. Synthesis and properties of aromatic polyamides derived from 1,7-bis(4-aminophenoxy)naphthalene and various aromatic dicarboxylic acids. J. Polym. Sci. Part A: Polym. Chem. 1996;34:341–348.
- Akbarian-Feizi L, Mehdipour-Ataei S, Yeganeh H. Investigation on the preparation of new sulfonated polyimide fuel cell membranes in organic and ionic liquid media. Int. J. Polym. Mater. 2014;63:149–160.10.1080/00914037.2013.812086
- Bruma M, Hamciuc E, Sava I, Hamciuc C, Iosip MD, Robison J. Compared properties of polyimides based on benzophenonetetracarboxylic dianhydride. Rev. Roum. Chim. 2003;48:629–638.
- Imai Y. Recent advances in synthesis of high-temperature aromatic polymers. React. Funct. Polym. 1996;30:3–15.
- Wang XL, Li YF, Gong CL, Ma T, Yang FC. Synthesis and properties of new pyridine-bridged poly(ether-imide)s based on 4-(4-trifluoromethylphenyl)-2,6-bis[4-(4-aminophenoxy)phenyl]pyridine. J. Fluorine Chem. 2008;129:56–63.
- Calderón V, García FC, De La Peña JL, Maya EM, García JM. Synthesis and characterization of new aromatic polyamides bearing crown ethers or their dipodal counterparts in the pendant structure. I. Benzo-12-crown-4 and ortho-bis(2-ethoxyethoxy)benzene. J. Polym. Sci. Part A: Polym. Chem. 2006;44:2270–2281.
- Maya EM, Lozano AE, de la Campa JG, de Abajo J. Soluble polyamides and polyimides functionalized with benzo-15-crown-5-pendant groups. Macromol. Rapid Commun. 2004;25:592–597.
- Hsiao SH, Chen CW, Liou GS. Novel aromatic polyamides bearing pendent diphenylamino or carbazolyl groups. J. Polym. Sci. Part A: Polym. Chem. 2004;42:3302–3313.
- San-José N, Gómez-Valdemoro A, García FC, Serna F, García JM. Aromatic polyamides with pendant urea moieties. J. Polym. Sci. Part A: Polym. Chem. 2007;45:4026–4036.
- Pal RR, Patil PS, Salunkhe MM, Maldar NN, Wadgaonkar PP. Synthesis and characterization of aromatic polyamides containing an s-triazine ring with thiophenoxy linkages. Polym. Int. 2005;54:569–575.
- Haba O, Seino H, Aoki K, Iguchi K, Ueda M. Synthesis of ordered polymer by direct polycondensation. VIII. Ordered polymer from two nonsymmetric monomers. J. Polym. Sci. Part A: Polym. Chem. 1998;36:2309–2314.10.1002/(ISSN)1099-0518
- Sava I, Bruma M. Kinetics of Temperature-induced and Reaction-induced phase separation studied by modulated temperature DSC. Macromol. Symp. 2006;239:36–41.
- Sava I, Bruma M, Ronova I. The influence of conformational parameters on some physical properties of polyamides containing pendant acetoxybenzamide groups. High Perform. Polym. 2004;16:435–446.
- Jung Y, Lee J, Lee S, Cho H, Shim H. Fluorene copolymers containing bithiophene/2,5- or 2,6-pyridine units: A study of their optical, electrochemical, and electroluminescence properties. J. Polym. Sci. Part A: Polym. Chem. 2006;44:4611–4620.10.1002/pola.v44:15
- Mehdipour-Ataei S, Heidari H. Synthesis and characterization of novel soluble and thermally stable polyamides based on pyridine monomer. Macromol. Symp. 2003;193:159–168.
- Li L, Kikuchi R, Kakimoto M, Jikei M, Takahashi A. Synthesis and characterization of new polyimides containing nitrile groups. High Perform. Polym. 2005;17:135–147.
- Tian XY, Yao J, Zhang X, Li M, Liu W, Qiao C, Yu D. New optically active poly(amide-imide)s based on N, N'-(pyromellitoyl)-bis-L-amino acid and methylene diphenyl-4,4'-diisocyanate: synthesis and characterization. Des. Monomers Polym. 2014;17:201–207.
- Sarkar A, Honkhambe PN, Avadhani CV, Wadgaonkar PP. Synthesis and characterization of poly(amide-imide)s containing pendent flexible alkoxy chains. High Perform. Polym. 2005;17:3646–3654.
- Dezern JF. Proc. Am. Chem. Soc. 1990;63:361.
- Beltramo M. Eng. Plast. 1993;6:40.
- Liaw DJ, Chen WH. High glass transitions of novel organosoluble polyamide-imides based on noncoplanar and rigid diimide-dicarboxylic acid. Polym. Degrad. Stabil. 2006;91:1731–1739.
- Yang CP, Su YY. Fluorinated aromatic polyamides and poly(amide-imide)s: synthesis and properties. Macromol. Chem. Phys. 2005;206:1947–1958.
- Dai L. Polymer nanostructures. In: Nalwa HS, editor. Encyclopedia of nanoscience and nanotechnology. Stevenson Ranch (CA): American Scientific Publishers; 2004. p. 763–790.
- Livi S, Duchet-Rumeau J, Gerard JF. Application of supercritical CO2 and ionic liquids for the preparation of fluorinated nanocomposites. J. Colloid Interface Sci. 2011;369:111–116.
- Mallakpour S, Zadehnazari A. Synergic effects of molten ionic liquid and microwave irradiation in preparation of optically active nanostructured poly(amide-imide)s containing amino acid and dopamine moiety. Polym. Plast. Technol. Eng. 2012;51:1090–1096.
- Mallakpour S, Dinari M. Novel nanostructure amino acid-based poly(amide–imide)s enclosing benzimidazole pendant group in green medium: fabrication and characterization. Amino Acids. 2012;43:1605–1613.
- Shujiang Z, Yanfeng L, Daxue Y, Xiaolong W. Study on synthesis and characterization of novel polyimides derived from 2,6-Bis(3-aminobenzoyl) pyridine. Eur. Polym. J. 2005;41:1097–1107.
- Yamazaki N, Matsumoto M, Higashi F. Studies on reactions of the N-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J. Polym. Sci. Polym. Chem. Ed. 1975;13:1373–1380.10.1002/pol.1975.170130609
- Zhang M, Wang Z, Gao L, Ding M. Polyimides from isomeric diphenylthioether dianhydrides. J. Polym. Sci. Part A: Polym. Chem. 2006;44:959–967.
- Ania F, Baltá-Calleja FJ, Flores A, Michler GH, Schol- tyssek S, Khariwala D, Hiltner A, Baer E, Rong L, Hsiao BS. Nanostructure and crystallization phenomena in multilayered films of alternating iPP and PA6 semicrystalline polymers. Eur. Polym. J. 2012;48:86–96.
- Curiel MMLG. (Polymer-inorganic nanocomposites [dissertation]. Netherlands: University of Twente; 2004.
- Van Krevelen DW. Some basic aspects of flame resistance of polymeric materials. Polymer. 1975;16:615–620.
- Johnson PR. A general correlation of the flammability of natural and synthetic polymers. J. Appl. Polym. Sci. 1974;18:491–504.