Abstract
An efficient route is used to prepare poly(3,4-ethylenedioxythiophene)/silver nanocomposite by chemical oxidative polymerization of 3,4-ethylenedioxythiophene monomer in Ag colloidal medium through emulsion technique using iron (III) p-toluenesulfonate hexahydrate (Fe(OTs)3.6H2O) oxidant. Sodium dodecyl sulfate plays a dual role involving both as emulsifier of the polymerization medium and stabilizer of the simultaneously reduced Ag nanoparticles. To compare the results obtained, an Ag-unloaded PEDOT is also prepared analogously. The resulted samples are fully characterized by fourier transform infrared, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, UV/vis, and thermogravimetric analyses. No remarkable aggregation of Ag nanoparticles occurs during the polymerization reaction. Moreover, average particle size of the incorporated Ag is found to be <10 nm.
1. Introduction
Nanotechnology is rapidly growing with nanoparticles produced and utilized in a wide range of commercial products throughout the world.[Citation1] Polymer-based nanocomposites are undoubtedly accounted as one of the most important members of these products. Among all nanoparticles used, silver nanoparticles (Ag NPs) have exceptional position due to their extensive applications in the field of nanotechnology. Ag NPs are used in electronics,[Citation2] bio-sensing,[Citation3] antibacterial materials,[Citation4] clothing,[Citation5] food industry,[Citation6] paints,[Citation7] sunscreens,[Citation8] cosmetics,[Citation9] and medical devices.[Citation10] Like other nanosized particles, Ag NPs also show extremely better behavior relative to their bulk samples. However, nanoparticles have very large surface areas and hence, very high surface energies that drive spontaneous room temperature agglomeration and sintering via secondary recrystallization onto larger particles.[Citation11] Therefore, under unprotected conditions, Ag NPs have high tendency of size growth via particles agglomeration. Deposition of Ag NPs within a polymeric material gives them an excellent opportunity to remain further in nanoscale. This situation allows them to show their desirable effects for a long period of time. So far, these nanoparticles have been widely incorporated into various types of advanced polymers such as high-temperature polymers [Citation12–14] and electrically conducting polymers.[Citation15–17]
Poly(3,4-ethylenedioxythiophene) (PEDOT) as one of the most successful conducting polymers seems to be a good choice for the deposition of Ag NP’s. PEDOT polymer has a number of significant characteristics such as excellent environmental stability, high conductivity, and transparency in thin oxidized films.[Citation18] So far, PEDOT has been used as a key component in many kinds of advanced devices.[Citation19,20] It seems that each development in the preparation area of PEDOT and its nanocomposites can also move their application area. So many reports have been devoted to the synthesis techniques of PEDOT polymer yet.[Citation21,22] Among them, chemical oxidative polymerization of 3,4-ethylenedioxythiophene monomer by emulsion technique can lead to a nanosized PEDOT polymer with spherical,[Citation23] cylindrical [Citation24], or flake morphology.[Citation25] In chemical preparation of PEDOT by an oil-in-water technique, conventionally used oxidants including iron(III) salts are completely dissolved in the continuous phase of the medium. Over critical micelle concentration (CMC), the emulsifier molecules can produce numerous nanosized spaces namely micelles, which the polymerization reaction mainly takes place inside them.
Alongside our recent studies related to PEDOT-containing nanocomposites,[Citation26,27] in this work, a facile and efficient route was used chemically to synthesis poly(3,4-ethylenedioxythiophene)/silver (PEDOT/Ag) nanocomposite starting from EDOT monomer in the presence of sodium dodecyl sulfate (SDS) emulsifier in silver colloid. Herein, SDS plays a key role during the polymerization reaction course, which will be discussed in depth. Moreover, some important characteristics of PEDOT/Ag nanocomposite involving optical behavior, morphology, and thermo-stability were fully investigated. In order to study the role of Ag NPs incorporated into the polymer matrix, a pure sample of PEDOT was also synthesized by a similar manner to that of used to prepare PEDOT/Ag nanocomposite. The samples obtained were thoroughly characterized by fourier transform infrared (FT-IR), X-ray diffraction (XRD), near-ultraviolet/visible (UV/vis) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and thermogravimetric analysis (TGA/DTG).
2. Experimental
2.1. Materials
3,4-Ethylenedioxythiophene (EDOT) monomer (≥98%) and iron (III) p-toluenesulfonate hexahydrate (Fe(OTs)3.6H2O) oxidant and SDS emulsifier were purchased from Aldrich and used without further purification. Sodium borohydride (NaBH4), sodium iodide (NaI), silver nitrate (AgNO3) were supplied from Merck and used as received. All aqueous solutions were prepared using deionized water.
2.2. Characterization
FT-IR spectra of the samples were recorded on a PERKIN ELMER RX I FT-IR spectrometer. The spectra of solids were obtained using KBr pellets. UV/vis spectra were taken with a PERKIN ELMER PTP-1 Peltier System Lambda 25 UV/VIS Spectrometer. Wide-angle XRD patterns were performed at room temperatures with film specimens on a Bruker Advance D5 X-ray diffractometer with Ni-filtered Cu/Ka radiation (30 kV, 25 mA). Morphology studies of the samples were determined with a HITACHI S-360 SEM and a Philips CM120 TEM. TGA was performed on a Mettler TA 5000 system (Columbus, OH) under nitrogen atmosphere at a heating rate of 15 °C min−1.
2.3. Synthesis of silver colloid
Colloid of Ag NP’s was prepared as follows: 2.5 mL of AgNO3 solution (0.01 M) was added to a solution of SDS (0.231 g, 0.8 mmol) in deionized water (75 mL), while stirring. After 10 min, 2.5 mL of NaI solution (0.01 M) was poured into the previous mixture under vigorous stirring. A light yellow solution of AgI nanoparticles was obtained after nearly 20 min at room temperature. Then, NaBH4 (0.015 g, 0.4 mmol) was slowly added to AgI colloid and the mixture was stirred for another 30 min until AgI was thoroughly reduced, and a reddish brown colloid of Ag NP’s was eventually prepared.
2.4. Synthesis of PEDOT/Ag nanocomposite
EDOT monomer (0.84 mL, 8 mmol) was added to the above nanofluiod of Ag NP’s (80 mL). The mixture was gently stirred for 1 h at room temperature. Then, 20 mL solution of Fe(OTs)3.6H2O oxidant (13.550 g, 20 mmol) was slowly poured into the reaction flask, while stirring. The mixture was allowed to stir for another 24 h at room temperature. The reaction components were poured into acetonitrile (200 mL) in one portion, and then stirred for 15 min. The resulting precipitate was filtered and washed several times with deionized water, methanol, and acetone, then dried under vacuum for 48 h at 40 °C.
3. Results and discussion
3.1. Synthesis
On the preparation line of silver colloid, in the first step, the reaction between AgNO3 and NaI results in AgI in the form of dispersed nanosized particles, which could be homogeneously dispersed by SDS stabilizer. Then, uniformly dispersed Ag NP’s are produced from AgI reduction using NaBH4 as reducing agent. Ag NP’s are prevented from aggregation since SDS molecules are adsorbed on initial Ag NP’s. This adsorption could be originated from the electrostatic interaction between Ag NP’s and sulfur atom of SDS. Into the resulting Ag colloid, a chemical oxidative polymerization of EDOT monomer by an emulsion technique could lead to PEDOT/Ag nanocomposite. SDS as the stabilizer of Ag colloid (dispersant of the nano-Ag particles) in the first step played the role of an emulsifier in the second step. Therefore, employing an emulsion technique for in situ synthesis of polymer-based nanocomposites has an exceptional advantage since the emulsifier used in this technique plays simultaneously the role of an efficient dispersant of the present nanoparticles as well. In the water-based medium to create spherical micelles, the concentration of SDS was selected slightly over CMC.[Citation28] Prior to the addition of Fe(OTs)3.6H2O oxidant to the reaction mixture, EDOT monomer was allowed to penetrate the created micelles by stirring for 1 h. When the orange solution of the oxidant was added to the reaction flask containing EDOT monomer dissolved in the Ag colloid, the reddish brown color of the medium turned gradually into dark blue. It should be noted that, iron(III) chloride (FeCl3) could not be successfully used instead of Fe(OTs)3 because in addition to EDOT monomer, the metallic silver present in the medium in the presence of chloride ion is readily oxidized to silver ion, leading to AgCl along with PEDOT polymer. In other words, in this condition a PEDOT/AgCl nanocomposite will be also resulted along with PEDOT/Ag nanocomposite. Except the oxidation of EDOT monomer to PEDOT polymer, not any oxidation of metallic Ag to silver ion was observed when Fe(OTs)3.6H2O oxidant is used, and this is attributed to the absence of chloride ion in the polymerization medium.
3.2. FT-IR spectroscopy
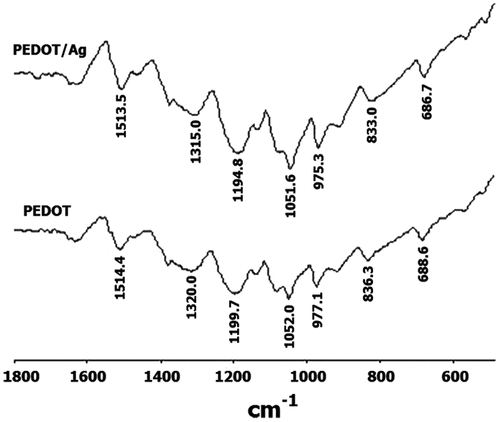
Figure shows the FT-IR spectra of PEDOT/Ag nanocomposite (top) and pure PEDOT (bottom). A distinguished band corresponding to the C–H bending, which generally appears at about 890 cm−1 in the FT-IR spectrum of monomer,[Citation29] has been disappeared in these two spectra, demonstrating the formation of PEDOT chains with 2,2′-coupling. Vibrations at 1352 and 1510 cm−1 are attributed to the stretching modes of C–C and C = C in the thiophene ring. The vibration modes of the C–S bond in the thiophene ring can be seen at 684, 838, and 978 cm−1. The bands at about 1120 and 1200 and cm−1 are assigned to the stretching modes of the ethylenedioxy group, and the band around 920 cm−1 is due to the ethylenedioxy ring deformation mode.
3.3. X-ray diffraction
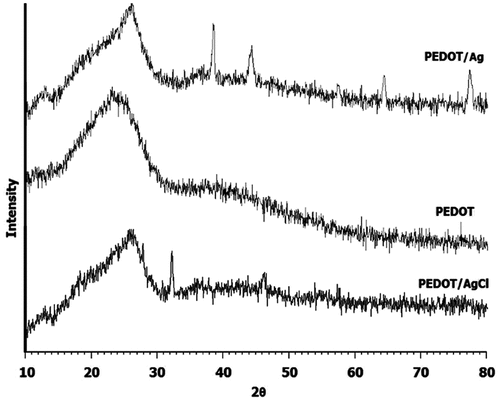
XRD patterns of both samples prepared, i.e. Ag-incorporated PEDOT nanocomposite and pure PEDOT, are displayed in Figure . As stated earlier, when FeCl3 was used as the chemical oxidative polymerization oxidant, due to Cl− ions, which facilitate the oxidation of metallic silver, the nano-Ag particles present in the reaction medium in an undesirable process were mainly converted to nano-AgCl particles incorporated into the polymer matrix. This situation was clearly demonstrated by XRD, and the characteristic peaks of AgCl nanoparticles are detectable in the diffractogram of AgCl-loaded PEDOT (bottom). All diffractograms show that there is a broad amorphous modest intensity peak centered near 2θ value of 25°, which can be assigned to (0 2 0) reflection.[Citation30] The PEDOT–Ag diffractogram (top) shows the diffraction features appearing 2θ as 38.6°, 44.5°, 64.6° and 77.5° that correspond to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of Ag nanoparticles with cubic symmetry, respectively. In order to estimate the mean particle size of Ag after incorporation within the polymer matrix, Scherrer’s equation was used. For PEDOT/Ag nanocomposite, the mean size of Ag particles was measured about 9 nm in size. This case obviously shows that in the present facile technique to prepare PEDOT/Ag nanocomposite, no remarkable agglomeration happened during the polymerization course.
3.4. SEM and TEM analyses
Figure displays scanning electron micrographs of PEDOT/Ag nanocomposite (a, b) along with the pure PEDOT (c) prepared by the same procedure. By a brief look at these images it can be concluded that for both samples the particles exhibit agglomerated spheres with cauliflower-like morphology. This high similarity in morphologies originates from the equivalent conditions, which applied during course of the polymerization reaction. Indeed, such conditions include the type and concentration of the surfactant used, and polymerization time and temperature. In addition, according to the literature, the degree of nanoparticles incorporation is not high when the nanoparticles are not simultaneously the same oxidant.[Citation31,32] Therefore, it is thoroughly reasonable that the micrographs of both samples to be equivalent. Figure (d) shows the two transmission electron micrographs of PEDOT/Ag nanocomposite. Nano-Ag agglomerates are detectable as dark spherical dots within the cloud-shape matrix of the macromolecules. TEM images show almost a uniform distribution of the nano-Ag particles around the PEDOT matrix with the approximate mean size of 10 nm, which this is nearly in agreement with the results obtained from XRD analyses.
3.5. UV/vis absorption spectroscopy
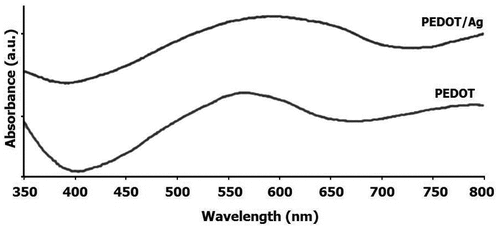
Near-UV/vis absorption spectra of the resulting samples are presented in Figure . In both spectra, the absorption maximum located at about 600 nm is the characteristic of PEDOT, which is associated to the π→π* transition.[Citation33,34] For the pure PEDOT prepared through a conventional emulsion technique, this broad band centered around 563 nm. For PEDOT/Ag nanocomposite, however, a slight red shift was observed. This red shift observed in the absorption spectrum of PEDOT/Ag nanocomposite should be related to the presence of nano-Ag particles within the polymer matrix. The energy gap between π and π* levels diminishes when the nano-Ag particles are incorporated within the polymer matrix. This in turn can demonstrate a strong interaction between PEDOT macromolecules and nano-Ag particles. In addition to the above peak, a weaker and wider band beyond 700 nm was also seen in the absorption spectrum of both samples. As stated elsewhere, this band usually appears due to the bipolaron subgap transition in the expanded coil conjugated macromolecules.[Citation35] In addition, the characteristic peak of the Ag nanoparticles, which should be appeared at around 400 nm, is not observed after combining with PEDOT polymer, because the surface plasmon resonance bands of Ag nanoparticles are sensitive to their surrounding environment.[Citation36]
3.6. Thermo-stability
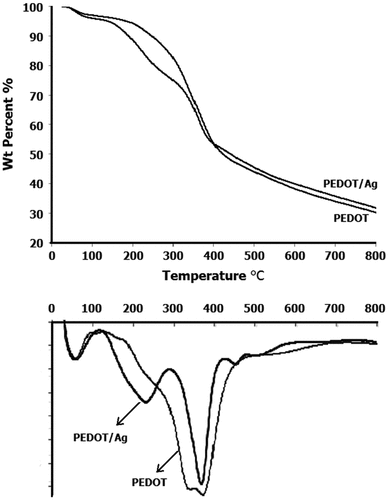
TG analysis as a dynamic technique is generally employed to detect the thermo- degradability of synthesized samples. Accordingly, to achieve the thermograms of the Ag-incorporated PEDOT nanocomposite (PEDOT/Ag) and pure PEDOT, TGA/DTG analysis was performed and the profiles are presented in Figure . While a one-step decomposition only takes place for pure PEDOT, a distinct two-step thermal decomposition was observed for PEDOT/Ag nanocomposite, ranging at 160–325 °C and 325–400 °C. The first intense drop happened at below 325 °C can be corresponded to the degradation of short-size macromolecules and oligomers, and the second one, which appears at above 325 °C deals with the long-size macromolecules.[Citation37,38] Hence, from comparing the thermograms, it can be clearly deduced that the short-size macromolecules and oligomers are present further in the PEDOT/Ag sample composition relative to the pure PEDOT. Above 400 °C no significant weight loss could be observed in the thermograms of both samples. The slight thermo-stability of PEDOT/Ag nanocomposite vs. the pure PEDOT at elevated temperatures should be reasonably related to the nano-Ag particles present in the polymerization medium. Therefore, the char yield of PEDOT/Ag nanocomposite at 800 °C was found to be slightly greater (%1.6) than that of the pure PEDOT. This should be attributed to the presence of nano-Ag particles entrapped into the polymer chains while precipitating at the work-up stage.
4. Conclusion
A facile route was successfully utilized using emulsion technique to synthesize PEDOT/Ag nanocomposite. Two steps were consecutively accomplished to prepare Ag-incorporated PEDOT nanocomposite. (1) A uniformly dispersed water-based nanofluid of metallic silver was prepared. (2) A chemical oxidative polymerization of EDOT monomer in SDS micellar medium within the pre-prepared Ag colloid was eventually resulted in the final nanocomposite. In addition to the facility of the employed technique to reach PEDOT/Ag nanocomposite, the approximate mean size of Ag nanoparticles was found to be about 10 nm, which this in turn can account as another point of this technique. SEM micrograph of PEDOT/Ag nanocomposite exhibited agglomerated spheres with cauliflower-like morphology. A homogeneous dispersion of nano-Ag particles into the polymer body could be observed from TEM images. Study on the photo-absorption spectra showed that the absorption maximum was red-shifted nearly 30 nm when the nano-Ag particles were incorporated within the PEDOT polymer. Furthermore, from TGA/DTG analysis, it was concluded that no thermo-stability occurred in the presence of nano-Ag particles at least below 400 °C.
Acknowledgment
The authors wish to express their gratitude to the Faculty of Chemistry and Research Council of Damghan University for financial support of this research.
References
- Parodi A, Quattrocchi N, Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013;8:61–68.
- Sahoo PK, Kamal SSK, Shankar B, Sreedhar B, Durai L. Facile chemical synthesis of nano-silver powders for printable electronics applications. J. Exp. Nanosci. 2012;7:520–528.10.1080/17458080.2010.536588
- Graham D, Faulds K, Smith WE. Biosensing using silver nanoparticles and surface enhanced resonance Raman scattering. Chem. Commun. 2006;42:4363–4371.10.1039/b607904k
- Singh G, Patankar RB, Gupta VK. The preparation of polymer/silver nanocomposites and application as an antibacterial material. Polym.-Plast. Technol. Eng. 2010;49:1329–1333.10.1080/03602559.2010.496428
- Zhang D, Chen L, Zang C, Chen Y, Lin H. Antibacterial cotton fabric grafted with silver nanoparticles and its excellent laundering durability. Carbohydr. Polym. 2013;92:2088–2094.10.1016/j.carbpol.2012.11.100
- Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011;363:1–24.10.1016/j.jcis.2011.07.017
- Kumar A, Vemula PK, Ajayan PM, John G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008;7:236–241.10.1038/nmat2099
- Shi L, Shan J, Ju Y, Aikens P, Prud’homme RK. Nanoparticles as delivery vehicles for sunscreen agents. Colloids Surf., A. 2012;396:122–129.10.1016/j.colsurfa.2011.12.053
- Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed. Nanotechnol. Biol. Med. 2010;6:570–574.10.1016/j.nano.2009.12.002
- Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents. 2009;34:103–110.10.1016/j.ijantimicag.2009.01.017
- Vasiliev AN, Gulliver EA, Khinast JG, Riman RE. Highly dispersible polymer-coated silver nanoparticles. Surf. Coat. Technol. 2009;203:2841–2844.10.1016/j.surfcoat.2009.02.019
- Lin ZW, Qi SL, Wu DZ. Formation of double-surface-silvered polyimide films via a direct ion-exchange self-metallization technique: the case of BPADA/ODA and [Ag(NH3)2]+. J. Appl. Polym. Sci. 2012;125:3552–3559.10.1002/app.v125.5
- Youssef AM. Polymer nanocomposites as a new trend for packaging applications. Polym.-Plast. Technol. Eng. 2013;52:635–660.10.1080/03602559.2012.762673
- Liu Q, Seveyrat L, Belhora F, Guyomar D. Investigation of polymer-coated nano silver/polyurethane nanocomposites for electromechanical applications. J. Polym. Res. 2013;20:309.10.1007/s10965-013-0309-z
- Mishra S, Shimpi NG, Sen T. The effect of PEG encapsulated silver nanoparticles on the thermal and electrical property of sonochemically synthesized polyaniline/silver nanocomposite. J. Polym. Res. 2012;20:49.
- Borole DD, Kapadi UR, Mahulikar PP, Hundiwale DG. Conducting polymers: an emerging field of biosensors. Des. Monomers Polym. 2006;9:1–11.10.1163/156855506775526205
- Das TK, Prusty S. Review on conducting polymers and their applications. Polym.-Plast. Technol. Eng. 2012;51:1487–1500.10.1080/03602559.2012.710697
- Taggart DK, Yang Y, Kung SC, McIntire TM, Penner RM. Enhanced thermoelectric metrics in ultra-long electrodeposited PEDOT nanowires. Nano Lett. 2011;11:2192–2193.10.1021/nl201142j
- Weickert J, Sun H, Palumbiny C, Hesse HC, Schmidt-Mende L. Spray-deposited PEDOT:PSS for inverted organic solar cells. Sol. Energy Mater. Sol. Cells. 2010;94:2371–2374.10.1016/j.solmat.2010.08.018
- DeLongchamp D, Hammond PT. Layer-by-layer assembly of PEDOT/polyaniline electrochromic devices. Adv. Mater. 2001;13:1455–1459.10.1002/1521-4095(200110)13:19<>1.0.CO;2-S
- Elschner A, Kirchmeyer S, Lovenich W, Merker U, Reuter K. PEDOT: principles and applications of an intrinsically conductive polymer. London: Taylor and Francis; 2011.
- Long YZ, Li MM, Gu C, Wan M, Duvail JL, Liu Z, Fan Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Progr. Polym. Sci. 2011;36:1415–1442.10.1016/j.progpolymsci.2011.04.001
- Xia Y, Wei M, Lu Y. One-step fabrication of conductive poly(3,4-ethylenedioxythiophene) hollow spheres in the presence of poly(vinylpyrrolidone). Synth. Met. 2009;159:372–376.10.1016/j.synthmet.2008.10.004
- Zhang X, Lee J, Lee GS, Cha D, Kim MJ, Yang DJ, Manohar SK. Chemical synthesis of PEDOT nanotubes. Macromolecules. 2006;39:470–472.10.1021/ma051975c
- Lei Y, Oohata H, Kuroda S, Sasaki S, Yamamoto T. Highly electrically conductive poly(3,4-ethylenedioxythiophene) prepared via high-concentration emulsion polymerization. Synth. Met. 2005;149:211–217.10.1016/j.synthmet.2005.01.004
- Behniafar H, Moaref H. Poly(3,4-ethylenedioxythiophene)/TiO2 nanocomposites prepared in the presence of surfactants in binary solvents. J. Polym. Res. 2013;20:132.10.1007/s10965-013-0132-6
- Behniafar H, Moaref H. Modified solution technique for preparing poly(3,4-ethylenedioxythiophene) and a poly(3,4-ethylenedioxythiophene)/silver nanocomposite: optical and thermal behavior. J. Appl. Polym. Sci. 2013;130:2707–2712.10.1002/app.39370
- Dominguez A, Fernandez A, Gonzalez N, Iglesias E, Montenegro L. Determination of critical micelle concentration of some surfactants by three techniques. J. Chem. Edu. 1997;74:1227–1231.10.1021/ed074p1227
- Selvaganesh SV, Mathiyarasu J, Phani KLN, Yegnaraman V. Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res. Lett. 2007;2:546–549.10.1007/s11671-007-9100-6
- Wu J, Li Y, Feng W. A novel method to form hollow spheres of poly(3,4-ethylenedioxythiophene): growth from a self-assemble membrane synthesized by aqueous chemical polymerization. Synth. Met. 2007;157:1013–1018.10.1016/j.synthmet.2007.10.011
- Zou H, Wu S, Shen J. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem. Rev. 2008;108:3893–3957.10.1021/cr068035q
- Folarin OM, Sadiku ER, Maity A. Polymer-noble metal nanocomposites: review. Int. J. Phys. Sci. 2011;6:4869–4882.
- Huajing Z, Yadong J, Xu J, Yajie Y. Analysis on the characteristic properties of PEDOT nano-particle based on reversed micelle method. J. Wuhan Univ. Technol. 2011;26:422–428.
- Han MG, Foulger SH. Facile synthesis of poly(3,4-ethylenedioxythiophene) nanofibers from an aqueous surfactant solution. Mater. Sci. Eng. 2006;10:1164–1169.
- Ouyang J, Xu Q, Chu C, Yang Y, Li G, Shinar J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer. 2004;45:8443–8450.10.1016/j.polymer.2004.10.001
- Jing S, Xing S, Yu L, Wu Y, Zhao C. Synthesis and characterization of Ag/polyaniline core–shell nanocomposites based on silver nanoparticles colloid. Mater. Lett. 2007;61:2794–2797.10.1016/j.matlet.2006.10.032
- Kiebooms R, Aleshin A, Hutchison K, Wudl F, Heeger A. Doped poly(3,4-ethylenedioxythiophene) films: thermal, electromagnetical and morphological analysis. Synth. Met. 1999;101:436–437.10.1016/S0379-6779(98)01121-7
- Vitoratos E, Sakkopoulos S, Dalas E, Paliatsas N, Karageorgopoulos D, Petraki F, Kennou S, Choulis SA. Thermal degradation mechanisms of PEDOT:PSS. Org. Electron. 2009;10:61–66.10.1016/j.orgel.2008.10.008