Abstract
The two-dimensional classification was applied to the estimation of hydrophilic–hydrophobic properties of four amine-containing (meth)acrylic monomers, 2-hydroxyethyl methacrylate, and four oligo(ethylene glycol) methacrylates of various degrees of the ethoxylation. For the monomers, the standard free energy of partition between water and organic phase and standard free energy of adsorption of monomers at the interface were determined in hexane/water systems. Based on the standard free energy of partition and standard free energy of adsorption amphiphilic properties of the monomers were evaluated in terms of the two-dimensional diagram. The monomers were divided into three groups with respect to the position of the monomers in the diagram. These positions are connected with the nature of the substances. The classification allows estimating of monomers partition between interfaces or bulk phases in systems with developed interfaces.
1. Introduction
Functional water-soluble (meth)acrylic polymers are widely used in industry. For example, (co)polymers of amine-containing (meth)acrylic monomers are applied on a large scale as flocculants and paper additives.[Citation1,2] Starting from the 1990s, the production of water-soluble polymeric plasticizers for concretes and dispersants on the basis of oligo(ethylene glycol) methacrylates (OEGMA) intensively increases.[Citation3,4] Furthermore, in recent years it has been shown that some amine-containing and ethoxylated amphiphilic (meth)acrylic polymers exhibit thermosensitive properties in aqueous solutions. In particular, lower critical solution temperature is demonstrated by homopolymers of 2-(dimethylamino)ethyl methacrylate (DMAEMA),[Citation5] 2-(diethylamino)ethyl methacrylate (DEAEMA),[Citation6] and their copolymers with other monomers.[Citation7–9] Temperature-responsive copolymers have been prepared on the basis of OEGMA.[Citation10,11] A number of papers have been published on synthesis and study of the thermosensitive properties of copolymers of amine-containing methacrylates and OEGMA.[Citation12,13] The temperature-responsive amphiphilic polymers possess considerable prospects for the broad application in medicine and technology,[Citation14,15] therefore, an interest of researchers in the polymers based on the above-mentioned monomers constantly grows. In particular, an important issue is the prediction and explanation of the thermosensitive properties of the water-soluble (meth)acrylic polymers.
One of the factors that determine stimuli-sensitive properties of amphiphilic polymers in addition to the general ratio and distribution of comonomer units along a macromolecular chain, macromolecular architecture and molecular weight are the hydrophilic–hydrophobic properties of monomer units in polymers.[Citation15] For example, it has been shown that thermo-responsive polymers containing amphiphilic monomer units are capable to demonstrate protein-like behavior and undergo temperature-induced conformational transitions from coils to globules of individual macromolecules.[Citation16,17] This is due to the ability of amphiphilic units of macromolecules to locate at the interface between the aqueous phase and globule core formed by hydrophobic units. The degree of the amphiphilicity of an individual polymer unit is possible to evaluate by hydrophilic–hydrophobic properties of the initial monomer. Therefore, the estimation of the amphiphilic properties of amine-containing methacrylates and OEGMA of different structures can help in the prediction and description of the thermosensitive properties of their homopolymers and block copolymers.
Moreover, the estimation of the hydrophilic–hydrophobic properties of monomers is important for studying their behavior in water and heterogeneous water–oil systems. Solution (co)polymerization in water and emulsion polymerization in aqueous–organic medium are the main methods for the production of the polymers based on the water-soluble amine- and oligo(ethylene glycol)-containing (meth)acrylic monomers.[Citation18,19] In the course of the study of emulsion polymerization, it is necessary to have data on the distribution of monomers between water and organic phase as well as on the local concentration of monomers at the interface. The correct assessment of the amphiphilic properties of monomers also allows explaining features of their behavior in the course of polymerization in aqueous solutions – e.g. the tendency to diffuse from the bulk of the solvent into the more hydrophobic region of a growing macroradical. Earlier, it has been shown that there is the relation between the diffusion and the unusual effect of the permanence of the instantaneous copolymer composition (although the change of monomer composition) during copolymerization of amine-containing and other (meth)acrylic monomers with acrylamide in aqueous solutions.[Citation20–22]
There are various methods for the experimental estimation of the hydrophilic–hydrophobic properties of organic compounds. The hydrophilic–lipophilic balance of nonionic substances has been correlated with many of their physical properties such as dielectric constants, heats of hydration, critical micelle concentrations, cloud points, phase inversion temperatures of emulsions, spreading coefficients of oil in water or reversed and interfacial tensions at oil–water interfaces.[Citation23] One of the widely used methods is the determination of distribution coefficients for the substances in systems non-polar solvent/water, such as octanol–water [Citation24] and heptane–water.[Citation25] A few years ago, Okhapkin and coauthors proposed a two-dimensional classification which was applied for the estimation of the amphiphilic properties of the water-soluble monomers.[Citation26] In accordance with this method, standard free energy of partition between water and organic phase (Δfpart) and standard free energy of adsorption of monomers at the interface (Δfads) are determined in hexane–water-monomer systems. For hydrophilic monomers, the value of the Δfpart is postulated to be positive, for hydrophobic monomers – negative; for monomers with the high interfacial activity Δfads > 0, for ones with low activity Δfads < 0.
The experimental determination of the above parameters allows performing quantitative comparison of the distribution of different monomers between hydrophilic and hydrophobic regions of the system, as well as performing the distribution of monomers between the interface and the bulk phase. In the work,[Citation26] the two-dimensional classification was applied for the comparative evaluation of the amphiphilic properties of four vinyl monomers – N-isopropylacrylamide, N-vinylpyrrolidone, 1-vinylimidazole, and N-vinylcaprolactam. The choice of these monomers associated with the fact that on their basis amphiphilic synthetic thermoresponsive polymers are able to be produced,[Citation27] including protein-like polymers, which are perspective enzyme-like catalysts.[Citation28,29] Later, the offered two-dimensional scale of the amphiphilicity was successfully used by Okhapkin and coauthors for the estimation of the properties of amino acid residues of proteins.[Citation30] These experiments confirmed the universality of the method for the evaluation of the amphiphilicity of various organic substances, and confirmed prospects for the offered approach for the understanding of the relation of the composition of natural proteins with their unique thermosensitive properties.
The purpose of our work is to use the two-dimensional classification, described by Okhapkin and coauthors, for the comparative evaluation of the amphiphilic properties of amine-containing (meth)acrylic monomers and ethoxylated monomers of various degrees of the ethoxylation. Structures and abbreviations of the monomers used in this work are given in Table .
Table 1. Structures and abbreviations of the studied amine- and oligo(ethylene glycol)-containing (meth)acrylic monomers.
2. Experimental
2.1. Materials
DMAPMA, DMAEMA, DEAEMA, 2-hydroxyethyl methacrylate (HEMA) were purchased from Sigma-Aldrich. DMAPA was synthesized by Schotten–Baumann reaction.[Citation31] Amine-containing monomers and HEMA were distilled twice under reduced pressure. HOEGMA-6 (Bisomer PEM 6 LD), MOEGMA-8 (Bisomer MPEG 350 MA), MOEGMA-12 (Bisomer MPEG 550 MA), MOEGMA-23 (Bisomer S 10 W) were purchased from Cognis and used as received. Hexane (p.a. grade) was distilled at normal pressure.
2.2. Methods
Before the determination of the distribution coefficients, the solutions of the monomers in the water–hexane system (1:1 vol., 25 °C) had been kept during 2 days for reaching the equilibrium concentration of the monomers in the aqueous and organic phases. UV spectrophotometry (SF-46 instrument) was used for the determination of the amine-containing monomers concentration. The measurement was performed at the following wavelengths, nm: 200 (DMAEMA), 212 (DEAEMA), 205 (DMAPMA), 198 (DMAPA). High-performance liquid chromatography was used for determination of the concentration of the OEGMA: Chromos LC-301 instrument with Alpha-10 isocratic pump and Sapphire UV detector, Phenomenex Luna 5u column, the mobile phase consists of acetonitrile and water (20:80, v/v), UV-detector (wavelength −210 nm).
The values of Δfpart were calculated by the equation. [Citation26]
where the partition coefficient P is the ratio of the equilibrium concentrations of a monomer in water (cw) and hexane (ch):
Drop-weight method was applied to determine the interfacial tension.[Citation32] The determination was carried out at 25 °C. The outer diameter of the glass tube was equal to 0.3.
For the estimation of the standard free energy of adsorption at the interface the following expression was used.[Citation26]
where:
R – is the gas constant;
T – is the temperature of the experiment, K;
τ – is the film thickness (accepted equal to 0.6 nm [Citation26]);
α – is the derivative of interfacial pressure (γ0 − γ) with respect to concentration
where
γ0 – is the interfacial tension at hexane/water interface in the absence of a monomer, m N/m,
γ – is the interfacial tension at hexane/water interface in the presence of a monomer, m N/m.
3. Results and discussion
As it was stated above, for the comparative evaluation of the amphiphilic properties of the monomers in accordance with the chosen two-dimensional classification it is necessary to determine Δfads and Δfpart values. Isotherms of interfacial tension in hexane–water-monomer systems were obtained to determine the standard free energy of adsorption for the monomers studied. The isotherms showed that all of the monomers exhibit interfacial activity because they reduce the interfacial tension. The data for the amine-containing monomers, which are divided into two pairs for illustration of the molecular structure influence, are presented in Figures and . Figure shows the isotherms for the two amine-containing amides that differ in the presence or absence of the methyl substituent in the vinyl group (respectively, DMAPMA and DMAPA). The isotherms of the two amine-containing methacrylic esters with various structures of the amino group (DMAEMA and DEAEMA) are presented in Figure . The interfacial tension isotherms in systems of hexane–water-ethoxylated methacrylic esters with hydroxyl end groups are shown in Figure and with methoxy end groups in Figure .
Figure 1. Dependence of the interfacial tension (γ) on the concentration of the monomers (C) for aqueous solutions of DMAPMA (1) and DMAPA (2).
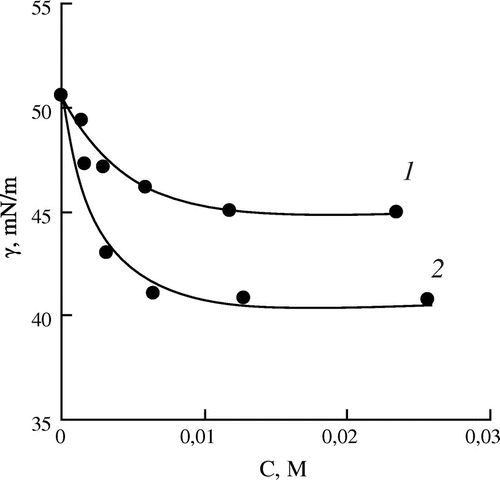
Figure 2. Dependence of the interfacial tension (γ) on the concentration of the monomers (C) for aqueous solutions of DMAEMA (1) and DEAEMA (2).
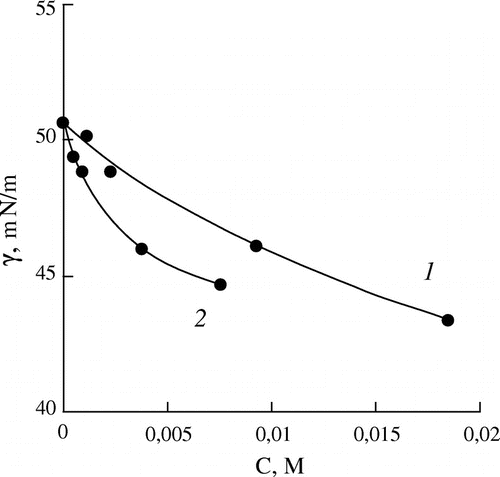
Figure 3. Dependence of the interfacial tension (γ) on the concentration of the monomers (C) for aqueous solutions of HEMA (1) and HOEGMA (2).
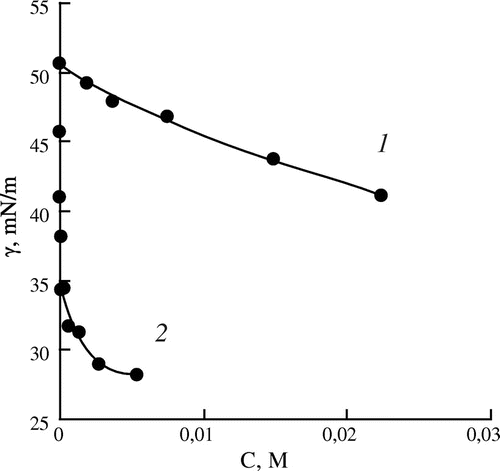
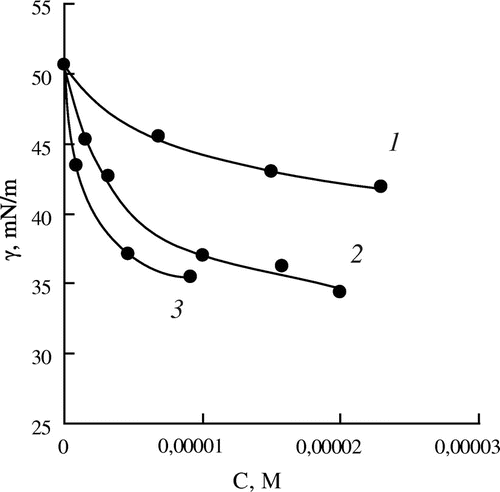
Figure 4. Dependence of the interfacial tension (γ) on the concentration of the monomers (C) for aqueous solutions of MOEGMA -12 (1), MOEGMA -8 (2), MOEGMA -23 (3).
The analysis of the obtained experimental data reveals a number of features connected with the influence of the monomers structure on their amphiphilic properties. Thus, for the acrylic and methacrylic amides (DMAPA and DMAPMA), the interfacial tension isotherms show similar behavior (the curves reach a plateau). However, in the case of DMAPA there is more significant decrease in the interfacial tension (20% more), and the reaching of constant values of the interfacial tension occurs at lower monomer concentration (about 0.007 M). In the presence of DMAPMA, the interfacial tension was reduced by only 11% and the decreasing stopped at concentration of the monomer equal to about 0.012 M. Consequently, the introduction of the methyl substituent in the vinyl group reduces the interfacial activity of the aminoamides.
The interfacial activity of the two methacrylic esters, containing methyl (DMAEMA) or ethyl (DEAEMA) substituents in the amino group, at concentration below 0.012 M is sufficiently close to that of methacrylic amide – DMAPMA. However, in the case of the amine-containing esters the interfacial tension continues to decrease considerably at the monomer concentration above 0.012 M. Among the two esters, DEAEMA containing more hydrophobic (ethyl) substituents in the amino group possesses a somewhat higher activity.
The replacement of the amino group by the hydroxyl group for the methacrylic esters almost does not change the interfacial activity of the monomers – the interfacial tension isotherms of HEMA and DMAEMA are practically the same. Finally, the interfacial activity of OEGMA increases sharply in comparison with the amine-containing monomers and HEMA, and significantly increases with the increase in the ethoxylation degree.
The values of α and Δfads were calculated on the basis of the interfacial tension isotherms (Table ). Besides, the distribution coefficients of the monomers between water and hexane (P) and values of Δfpart calculated on their basis are shown in Table .
Table 2. The calculated values of ∆fpart, ∆fads, and of the intermediate coefficients P and α.
In accordance with,[Citation26] the values of Δfads and Δfpart obtained for the amine-containing and ethoxylated (meth)acrylic monomers were plotted on the two-dimensional diagram (Figure ). There are four regions in the diagram: quadrant of hydrophilic interfacially active substances (I), quadrant of hydrophilic interfacially inactive substances (II), quadrant of hydrophobic interfacially active substances (III), and hydrophobic interfacially inactive substances (IV).
Figure 5. Two-dimensional diagram of the interfacial activity and distribution of substance in water–hexane mixture for the monomers 1–9 (numbers correspond to Table ). (●) ethoxylated metha crylates; (○) amine-containing metha crylates and (meth)acrylamides.
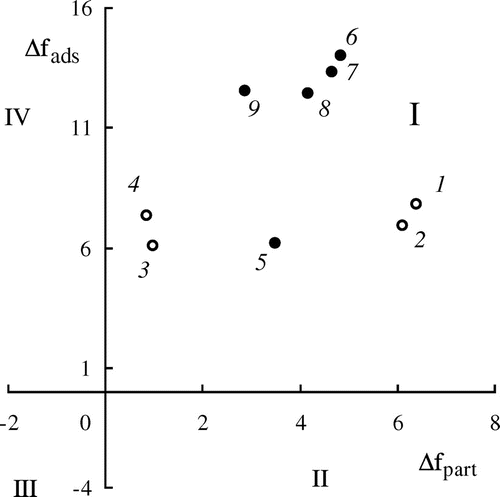
The obtained results show that all the monomers are hydrophilic and interfacially active (quadrant I). According to the degree of the hydrophilicity (Δfpart), the monomers form the following row:
The sequence is completely consistent with general approaches to the influence of the structure and content of hydrophilic fragments in monomer molecules: an amide group is more hydrophilic in comparison with an ester one; a hydroxyl group is more hydrophilic than a methoxy one. As it was expected, the hydrophilicity of the molecules increases with the increase in the number of ethylene glycol units in molecules of OEGMA, and the introduction of the methyl substituent into the vinyl group as well as the methylene group into the amino group increases the hydrophobicity of the monomers.
The ratio of the Δfads and Δfpart allows estimating the distribution of monomers both between organic and aqueous phases and between the interface and the bulk phases. In accordance with the ratio of Δfads and Δfpart values, the amine-containing and ethoxylated (meth)acrylic monomers can be divided into three groups. The first group includes the amides DMAPA and DMAPMA (No. 1, 2 in the diagram), as they have approximately identical and rather high Δfads and Δfpart values. Such monomers in biphasic water–organic systems should have the high concentration in the bulk of aqueous phase and in the region of phases boundary (especially in the case of the developed interface of phases). They provide the low concentration in the bulk of an organic phase. The second group consists of OEGMA (No. 6–9 in the diagram). The values of Δfpart for these monomers are 1.5–2 times lower, and the values of Δfads are 2 times higher in comparison with the monomers of the first group. Due to this fact, their concentrations in the bulk of the aqueous phase are reduced on account of increasing concentration at the interface. The amine-containing esters DMAEMA and DEAEMA (Nos. 3, 4 in the diagram) constitute the third group. They are similar to the first group in the values of Δfads, and approach the border of hydrophilic and hydrophobic compounds in the values of Δfpart. Consequently, the concentrations of the monomers of the third group in the aqueous and organic phases become comparable, and significant part of the monomer molecules locates at the interface.
Monoethoxylated methacrylate (HEMA, No. 5 in the diagram) takes an intermediate position between the three monomer groups in the diagram. The value of Δfads for HEMA is close to those for amine-containing monomers of the first and third groups, and the value of Δfpart corresponds to those for ethoxylated esters of the second group.
4. Conclusion
The obtained results show that the ratio of the hydrophilic and hydrophobic properties of the monomers shifts to the hydrophobic field in the following order: aminoalkyl (meth)acrylamides > ethoxylated methacrylates > aminoalkyl methacrylates. The all investigated monomers possess the high interfacial activity. OEGMA are found to be the most interfacially active. The data can be applied to the investigation of features of the monomers (co)polymerization in water and emulsion polymerization in aqueous–organic medium. The classification allows performing quantitative comparison of the distribution of the monomers between hydrophilic and hydrophobic regions of heterogeneous systems and the distribution of monomers between the interface and the bulk phase.
The monomers position in the two-dimensional diagram can be considered along with other factors when predicting the amphiphilic properties of the monomer units in homopolymers and block copolymers. However, further researches are needed in this direction.
Acknowledgment
This work was supported by the Russian Foundation for Basic Research under project No. 14-03-00762 A and 14-03-31549 mol_A.
References
- Heitner HI. Flocculating agents. In: Kroschwitz JI, Howe-Grant M, editors. Kirk-Othmer encyclopedia of chemical technology. Vol. 11. New York (NY): Wiley; 2004. p. 623–647.
- Hagiopol C, Johnston JW. Chemistry of modern papermaking. New York (NY): CRC Press; 2012.
- Sakai E, Ishida A, Ohta A. New trends in the development of chemical admixtures in Japan. J. Adv. Concr. Technol. 2006;4:211–223.10.3151/jact.4.211
- Papayianni I, Tsohos G, Oikonomou N, Mavria P. Influence of superplasticizer type and mix design parameters on the performance of them in concrete mixtures. Cement Concrete Comp. 2005;27:217–222. doi:10.1016/j.cemconcomp.2004.02.010
- Plamper FA, Ruppel M, Schmalz A, Borisov O, Ballauff M, Muller AHE. Tuning the thermoresponsive properties of weak polyelectrolytes: aqueous solutions of star-shaped and linear poly(N,N-dimethylaminoethyl methacrylate). Macromolecules. 2007;40:8361–8366.10.1021/ma071203b
- Schmalz A, Hanisch M, Schmalz H, Muller AHE. Double stimuli-responsive behavior of linear and star-shaped poly(N,N-diethylaminoethyl methacrylate) in aqueous solution. Polymer. 2010;51:1213–1217.10.1016/j.polymer.2009.11.023
- Cotanda P, Wright DB, Tyler M, O’Reilly RK. Comparative study of the stimuli-responsive properties of DMAEA and DMAEMA containing polymers. J. Polym. Sci. A. 2013;51:3333–3338.10.1002/pola.v51.16
- Cho SH, Jhon MS, Yuk SH. Temperature-sensitive swelling behavior of polymer gel composed of poly(N,N-dimethylaminoethyl methacrylate) and its copolymers. Eur. Polym. J. 1999;35:1841–1845.
- Savoji MT, Strandman S, Zhu XX. Switchable vesicles formed by diblock random copolymers with tunable pH- and thermo-responsiveness. Langmuir. 2013;29:6823–6832.10.1021/la4009625
- Becer CR, Hahn S, Fijten MWM. Libraries of methacrylic acid and oligo(ethylene glycol) methacrylate copolymers with LCST behavior. J. Polym. Sci. A. 2008;46:7138–7147. doi:10.1002/pola.23018
- París R, Quijada-Garrido I. Swelling behaviour of thermo-sensitive hydrogels based on oligo(ethylene glycol) methacrylates. Eur. Polym. J. 2009;45:3418–3425. doi:10.1016/j.eurpolymj.2009.09.012
- Fournier D, Hoogenboom R, Hanneke ML, Paulus TR, Schubert US. Tunable pH- and temperature-sensitive copolymer libraries by reversible addition fragmentation chain transfer copolymerizations of methacrylates. Macromolecules. 2007;40:915–920.10.1021/ma062199r
- Uzgun S, Akdemir O, Hasenpusch G, Maucksch C, Golas MM, Sander B, Stark H, Imker R, Lutz J-F, Rudolph C. Characterization of tailor-made copolymers of oligo(ethyleneglycol) methyl ether methacrylate and N,N-dimethylaminoethyl methacrylate as nonviral gene transfer agents: influence of macromolecular structure on gene vector particle properties and transfection efficiency. Biomacromolecules. 2010;11:39–50.10.1021/bm9008759
- Kuckling D, Doering A, Krahl F, Arndt K-F. Stimuli-responsive polymer systems. Polym. Sci.: A Comprehensive Reference. 2012;8:377–442. doi:10.1016/B978-0-444-53349-4.00214-4
- Liu R, Fraylich M, Saunders BR. Thermoresponsive copolymers: from fundamental studies to applications. Colloid Polym. Sci. 2009;287:627–643. doi:10.1007/s00396-009-2028-x
- Vasilevskaya VV, Ermilov VA. Computer simulation of macromolecular systems with amphiphilic monomer units: biomimetic models. Polym. Sci. Ser. A. 2011;53:846–866.10.1134/S0965545X11090148
- Okhapkin IM, Malhaeva EE, Khohlov AR. Water solutions of amphiphilic polymers: nanostructure formation and possibilities for catalysis. In: Khohlov AR, editor. Conformation-dependent design of sequences in copolymers I. Adv. Polym. Sci. 2006; 195:177–210.10.1007/11569572
- Fink JK. Handbook of engineering and speciality thermoplastics. Vol. 2, Water soluble polymers. Salem: Wiley; 2011.
- Lutz J-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: toward new generations of smart biocompatible materials. J. Polym. Sci. Part A: Polym. Chem. 2008;46:3459–3470.10.1002/(ISSN)1099-0518
- Gromov VF, Bune EV, Teleshov EN. Characteristic features of the radical polymerisation of water-soluble monomers. Russ. Chem. Rev. 1994;63:507–517. doi:10.1070/RC1994v063n06ABEH000101
- Sivokhin AP, Kazantsev OA, Shirshin KV, Samodurova SI. Effect of association processes on polymerization of N-[3-(dimethylamino) propyl] methacrylamide salts in aqueous solutions. Russ. J. Appl. Chem. 2010;83:2041–2046. doi:10.1134/S1070427210110285
- Kazantsev OA, Shirshin KV, Sivokhin AP, Igolkin AV, Goncharova OS, Kamorin DM. Copolymerization of sodium 2-acrylamido-2-methylpropane sulfonate with acrylamide and acrylonitrile in water: an effect of conditions on the compositional heterogeneity. J. Polym. Res. 2012; 19:1–10. doi:10.1007/s10965-012-9886-5
- Schott H. Hydrophilic–lipophilic balance and distribution coefficients of nonionic surfactants. J. Pharm. Sci. 1971;60:648–649.10.1002/(ISSN)1520-6017
- Sangster J. Octanol–water partition coefficients: fundamentals and physical chemistry. Chichester: Wiley; 1997.
- James AD, Wates JM, Wyn-Jones E. Determination of the hydrophilicities of nitrogen-based surfactants by measurement of partition coefficients between heptane and water. J. Colloid Interface Sci. 1993;160:158–165. doi:10.1006/jcis.1993.1379
- Okhapkin IM, Makhaeva EE, Khokhlov AR. Two-dimensional classification of amphiphilic monomers based on interfacial and partitioning properties. 1. Monomers of synthetic water-soluble polymers. Colloid Polym. Sci. 2005; 284:117–123. doi: 10.1007/s00396-005-1342-1
- Salehi R, Irani M, Rashidi M-R, Aroujalian A, Raisi A, Eskandani M, Haririan I, Davaran S. Stimuli-responsive nanofibers prepared from poly(N-isopropylacrylamide-acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Des. Monomers Polym. 2013;6:515–527. doi:10.1080/15685551.2013.771303
- Lozinsky VI. Approaches to chemical synthesis of protein-like copolymers. In: Khohlov AR, editor. Conformation-dependent design of sequences in copolymers II. Adv. Polym. Sci. 2006; 196:87–127.10.1007/11570325
- Lozinsky VI, Simenel IA, Semenova MG, Belyakova LE, Il’in MM, Grinberg VYa, Dubovik AS, Khokhlov AR. Behavior of protein-like N-vinylcaprolactam and N-vinylimidazole copolymers in aqueous solutions. Polym. Sci. Ser. A. 2006;48:435–443. doi:10.1134/S0965545X06040134
- Okhapkin IM, Askadskii AA, Markov VA, Makhaeva EE, Khokhlov AR. Two-dimensional classification of amphiphilic monomers based on interfacial and partitioning properties. 2. Amino acids and amino acid residues. Colloid Polym. Sci. 2006;284:575–585. doi: 10.1007/s00396-005-1447-6
- Bezzaoucha F, Lochon P, Jonquieres A, Fischer A, Brembilla A, Ainad-Tabet D. New amphiphilic polyacrylamides: synthesis and characterisation of pseudo-micellar organisation in aqueous media. Eur. Polym. J. 2007;43:4440–4452.10.1016/j.eurpolymj.2007.07.005
- Wilkinson MC. Extended use of, and comments on, the drop-weight (drop-volume) technique for the determination of surface and interfacial tensions. J. Colloid Interface Sci. 1972;40:14–26.10.1016/0021-9797(72)90169-5
