Abstract
A novel fully aromatic diamine containing highly pyridine units, amide, and ether groups was prepared via two-step reactions: nucleophilic substitution reaction of 2,6-diaminopyridine with 6-chloronicotinoyl chloride led to preparation of a dichloro-diamide compound. Subsequent reaction of this compound with 5-amino-1-naphthol resulted in synthesis of the particular diamine. After complete characterization, polycondensation reaction of the diamine with different dianhydrides led to preparation of related poly(amide-ether-imide)s. The polymers were characterized and their physical and thermal properties including viscosity, solubility, water absorption, thermal behavior and stability, flame retardancy, and crystallinity were studied. The nicotinic-based poly(amide-ether-imide)s showed high heat and flame resistant, low water absorption in conjunction with improved solubility in polar solvents.
1. Introduction
Today, with advances in science and technology applications of polymers, mainly high performance polymers have been increased extensively. Needs for polymers with superior properties including high heat resistant as well as easier solubility and processability are felt more. Among various high performance polymers, polyimides received more attentions due to their favorable balance of physical and chemical properties.[Citation1–4] Wholly aromatic polyimides are engineering plastics and are gaining broad acceptance by various industries due to their unique features. These comprise outstanding physical properties, retention at elevated temperature and in wet environments, almost constant electrical properties over a wide range of temperatures, superior chemical resistance, and nonflammability properties.[Citation5–10] However, one of the drawbacks to the employment of these high performance polymers is the difficulty in processing due to their high melting temperatures or high glass transition temperatures. Strong interaction between polyimide chains and their rigid structure are the major causes for these behaviors.[Citation11–17]
Different methods have been reported to overcome the problem by improving their solubility without sacrifice of heat resistance. Most efforts for enhancing solubility and processability of rigid-chain polymers have been made through synthetic modification by introduction of flexible or kinked linkages, asymmetric or non-coplanar units, and bulky pendant groups to the polymer backbone.[Citation18–25] Another significant modification for making the polyimides processable is copolymerization. In this way, copolyimides including poly(amide imide)s, poly(ester imide)s, poly(ether imide)s, poly(imide carbonate)s, poly(amide ester imide)s have been prepared.
In general, incorporation of flexible units such as –NHCO–, –O–, and –SO2– into rigid polyimide backbones is an important way to improve the solubility of the polymers. Poly(amide imide)s and poly(ether imide)s have the advantages, because solubility and processability can be improved without considerable sacrifice of thermal and mechanical properties. Therefore, poly(amide imide)s and poly(ether imide)s afford a favorable balance between processability and performance and are useful in several applications in electrical wire enamel, adhesives, and injection-molding and extrusion products.[Citation26–29] Although due to more difficultly, synthetic procedures inclusion of both amide and ether groups into the backbone of polyimides have been reported less, the synergist effect on the polyimide would be remarkable.
On the other hand, the selection of heterocyclic rings in the main backbone of the synthetic polymer is to impart certain properties to the polymer. Among the diverse heterocyclic rings, the benefit of using a pyridine nucleus is based on its high heat resistance resulting from its molecular symmetry and aromaticity. Also, presence of nitrogen atom in the structure creates a polarized bond which improves the solubility of the ultimate polymers due to increased dipole–dipole interaction of polymer-solvent system.[Citation30–32]
In this article, in order to obtain new category of soluble heat-resistant polyimides, design and synthesis of a novel diamine (as a key monomer in preparation of final polymers) containing amide, ether, pyridine, and bulky naphthyl units was considered. Nicotinic-based poly(amide-ether-imide)s were prepared thereof via polycondensation with three different dianhydrides.
2. Experimental
2.1. Materials
Pyromellitic dianhydride (PMDA), 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA), N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAc), dimethyl sulfoxide (DMSO), m-cresol, toluene, K2CO3, methanol, pyridine, 2,6-diaminopyridine, and acetic anhydride were obtained from Merck Chemical Co. 5-Amino-1-naphthol, 6-chloronicotinoyl chloride, and 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) were purchased from Aldrich Chemical Co. PMDA, BTDA, and 6FDA were dried in vacuum oven at 110 °C for 5 h. NMP, DMAc, DMF, and toluene were purified by vacuum distillation over calcium hydride.
2.2. Instruments
Infrared measurements were performed on a Bruker-IFS48 FT-IR spectrometer (Ettlingen, Germany). The 1H-NMR spectra were recorded in DMSO-d6 solution using a Bruker Avance 300 MHz (GmbH, Germany). Elemental analyses were performed by a CHN-O-Rapid Heraeus elemental analyzer (Wellesley‚ MA). Differential scanning calorimetric (DSC) and thermogravimetric analysis (TGA) were recorded in air at a heating rate of 10 °C/min on a Stanton Redcraft STA-780 (London, UK). Flame retardancy of polyimides was evaluated by calculating the limiting oxygen index (LOI) values according to Van Krevelen equation. Inherent viscosities were measured using an Ubbelohde viscometer at a concentration of 0.5 g dL−1 in DMAc at 30 °C. For estimation of the equilibrium water absorption values of poly(amide-ether-imide)s films firstly, the vacuum-dried polyimide film specimens at 100 °C were weighted and then were immersed in distilled water for 24 h at room temperature. Water absorption was estimated from the equation:
where W0 and W were the weights of vacuum-dried film specimens just after removal from oven and tissue-dried films after immersion in water, respectively. To reduce experimental errors, large film specimens of 0.1 g weight were used in this test.
Wide angle X-ray diffraction patterns were performed at room temperature on an X-ray diffractometer (Siemens model D 5000) using Ni-filtered Cu Kα radiation (40 kV, 25 mA) with scanning rate of 3°/min.
2.3. Monomer synthesis
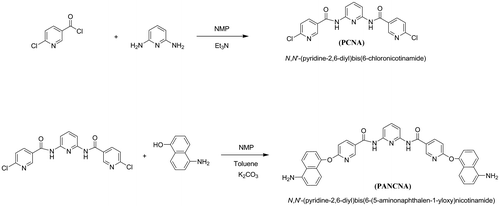
2.3.1. Synthesis of dichloro compound: N,N′-(pyridine-2,6-diyl)bis(6-chloronicotinamide) (PCNA)
Into a 100-ml, two-necked, round-bottomed flask equipped with drying tube (CaCl2), a nitrogen inlet tube, an ice bath, and a magnetic stirrer were placed with 1.68 g (15.0 mmol) of 2,6-diaminopyridine. Then, 60 ml of NMP was added to the flask and it was maintained at 0 °C for 30 min with continuous stirring under inert nitrogen gas. Then, 15 ml of triethyl amine was added to the mixture and stirred at the same temperature for a few minutes. A total of 6.05 g (33.3 mmol) of 6-chloronicotinoyl chloride was added to the mixture and the temperature was raised to ambient temperature and kept for 8 h. After that, the flask content was poured into 500 ml of distilled water with stirring. The precipitated product was washed with hot water and methanol, repeatedly. It was dried in an oven at 70 °C for about 8 h. The yield of the reaction was about 88%. m.p = 263–265 °C. [IR (KBr); υ 3469–3326 (N–H), 3061 (aromatic C–H), 1677 (C=O), 1584 (bending N–H), 801 cm−1 (C–Cl); 1H-NMR (DMSO-d6); δ 10.88 (s, 2H, NH), 8.91 (s, 2H, pyridine), 8.31 (d, 2H, pyridine), 7.90 (d, 2H, pyridine), 7.86 (t, 1H, pyridine), 7.67 (d, 2H, pyridine) ppm; elemental analysis calculated for C17H11N5O2Cl2: C, 52.58%; H, 2.84%; N, 18.04%; found: C, 52.65%; H, 2.80%; N, 18.11%].
2.3.2. Synthesis of diamine: N,N′-(pyridine-2,6-diyl)bis(6-(5-aminonaphthalen-1-yloxy) ((chloronicotinamide) (PANCNA)
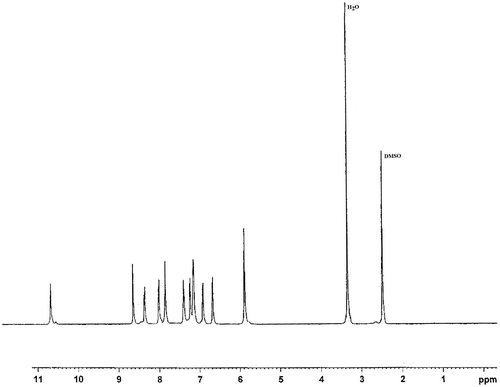
Into a 100-ml, three-necked, round-bottomed flask equipped with a Dean–Stark trap, a condenser, a nitrogen inlet tube, a thermometer, an oil bath, and a magnetic stirrer were placed with 2.33 g (6.0 mmol) of PCNA, 2.46 g (15.0 mmol) of 5-amino-1-naphthol, 30 ml of NMP, and 18 ml of dry toluene. Then, 11.19 g (26.1 mmol) of K2CO3 was added to the mixture and the reaction mixture was heated to 140 °C for 6 h with continuous stirring. By azeotropic distillation, the generated water was removed from the reaction mixture. The reaction temperature was raised to 165 °C and kept at the same temperature for 21 h. Then, the resulting reaction mixture was cooled and poured into water and the mixture was filtered and washed with hot water. The crude mixture was washed repeatedly with hot water and methanol. The obtained product was dried in an oven at 80 °C. Yield of the reaction was about 90%. m.p = 171–173 °C. [IR (KBr): υ 3400–3250 (N–H), 3065 (aromatic C–H), 1672 (C=O), 1593 (bending N–H), and 1240 cm−1 (C–O); 1H-NMR (DMSO-d6): δ 10.67 (s, 2H, amide), 8.64 (s, 2H, pyridine), 8.36 (d, 2H, pyridine), 8.00 (d, 2H, pyridine), 7.87 (d, 2H, pyridine), 7.85 (t, 1H, pyridine), 7.39 (d, 2H, naphthyl), 7.31 (d, 2H, naphthyl), 7.15 (d, 4H, naphthyl), 6.91 (d, 2H, naphthyl), 6.67 (d, 2H, naphthyl), 5.88 (s, 4H, amine) ppm; elemental analysis calculated for C37H27N7O4: C, 70.14%; H, 4.26%; N, 15.48%; found: C, 70.03%; H, 4.33%; N, 15.56%].
2.4. Polymer synthesis
2.4.1. Two-step method of imidization
2.4.1.1. Synthesis of poly(amic acid)
Into a round-bottomed flask equipped with a nitrogen inlet tube, a drying tube, a magnetic stirrer, and an ice bath, 3.00 mmol of diamine, 20 ml of NMP were stirred for 30 min at 0 °C. Then, 3.00 mmol of dianhydride was added and the mixture was stirred for 1 h. The reaction temperature was stirred at room temperature for 24 h. The mixture was poured into 300 ml of water/methanol 1:3 volume ratios. Then, it was filtered, washed with hot water, and dried under vacuum oven for 12 h at 40 °C.
2.4.1.2. Cyclization of poly(amic acid)
A total of 1.00 g of poly(amic acid) and 5 ml of dry NMP was placed into a 100-ml, two-necked, round-bottomed flask equipped with a magnetic stirrer, nitrogen gas inlet tube, and a reflux condenser. After stirring, 5 ml of acetic anhydride and 2.5 ml of pyridine were added to the mixture. The mixture was stirred for 30 min and then slowly heated to 140 °C and held for 6 h at the same temperature. Then, the mixture was cooled and poured into water, it was filtered, washed with hot water and methanol, and dried under vacuum at 120 °C overnight.
3. Results and discussion
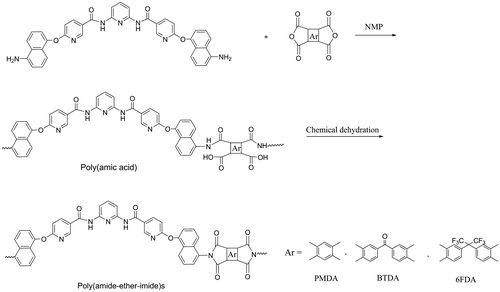
In order to obtain a new category of polyimides with the aim of overcoming their main technical difficulty, i.e. weak solubility and processability while maintaining heat resistance, a new diamine with unique structure was prepared in two steps: firstly, nucleophilic substitution reaction of 2,6-diaminopyridine with 6-chloronicotinoyl chloride in the presence of triethyl amine as an acid scavenger led to preparation of a dichloro-diamide compound named as N,N′-(pyridine-2,6-diyl)bis(6-chloronicotinamide) (PCNA). In the second step, reaction of this compound with 5-amino-1-naphthol resulted in preparation of a new and unique nicotinic-based diamine (PANCNA) containing amide, ether, pyridine, and bulky naphthyl groups (Scheme ).
The structures of PCNA and PANCNA were completely characterized using melting point, FT-IR, 1H-NMR, and elemental analysis methods. In preparation of PCNA, presence of amide linkage was confirmed by existence of a band at 1677 cm−1 in FT-IR attributed to the amide carbonyl bonds. Also, appearance of a peak at δ 10.88 in 1H-NMR was assigned to the amide N–H bonds. In the step of diamine synthesis, a band related to C–O ether group at 1240 cm−1 in FT-IR in addition to a peak related to amine bonds at δ 5.88 in 1H-NMR was observed (Figure ). Also, good agreement between calculated and found amounts of elemental analysis results was confirmed the proposed structures as well.
Polycondensation reactions of this nicotinic-based diamine with different dianhydrides including PMDA, BTDA, and 6-FDA via two-step chemical imidization led to preparation of poly(amide-ether-imide)s in high yields (Scheme ). In the first step, related poly(amic acid)s were prepared that were then cyclo-dehydrated in the presence of acetic anhydride and pyridine to form corresponding polyimides.
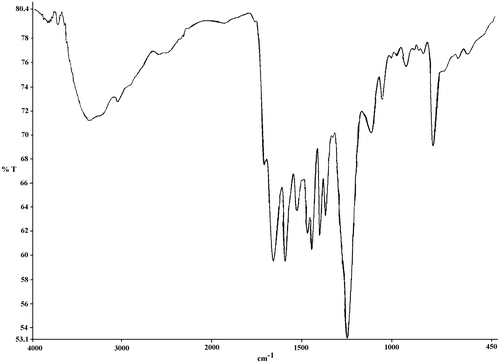
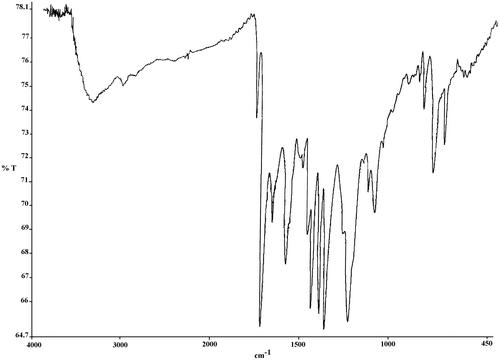
FT-IR spectroscopy was used for characterization of poly(amic acid)s (Figure ). The broad, continuous bands at about 3368–3200 cm−1 were related to O–H and N–H groups of amic acids, and two bands at about 1664–1660 cm−1 were attributed to the acid and amide carbonyl groups of amic acids. Also, poly(amide-ether-imide)s showed bands at about 1175–1780, 1724–1730, 1365–1380, and 713–724 cm−1 in the FT-IR spectra related to four specific imide structure (Figure ). Elemental analysis was applied for characterization of polymers as well. Suitable agreement between calculated and found amounts of elemental analysis was observed (Table ).
Table 1. Yield, viscosity, and elemental analysis of poly(amide-ether-imide)s.
As a criterion for estimation of molecular weight, the inherent viscosity of the polyimides was measured at a concentration of 0.5 g dL−1 in DMAc at 30 °C. Reasonable viscosity (in the range of 0.59–0.62 dL−1 g) was observed for the polymers indicating suitable growth of molecular weights.
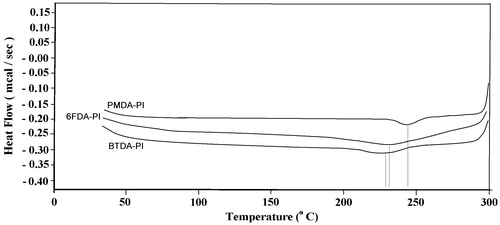
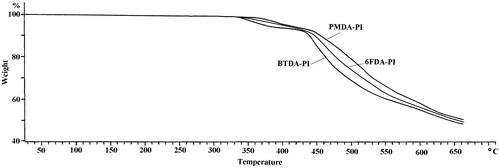
Thermal behavior and stability data of the poly(amide-ether-imide)s obtained by DSC and TGA were summarized in Table . The glass transition temperature (Tg) of the polymers was obtained from the midpoint temperature of baseline shift on the first heating traces in the DSC curve (Figure ). Polymers showed no clear melting endotherm in DSC thermograms, indicating the amorphous nature of the polymers. TGA was used for evaluation of thermal stability in air at a heating rate of 10 °C. The initial decomposition temperatures (Td) of the polyimides were about 340–365 °C, and the temperatures for 5% gravimetric loss (T5) – a decisive factor for evaluation of thermal stability – were in the range of 385–428 °C and also char yields of polymers at 600 °C were about 57–59%. The TGA curves are shown in Figure .
Table 2. Thermal characteristic data.Table Footnote1
According to the obtained results, the polymers revealed high heat resistant due to the incorporation of imide units, preformed amide groups, and phenylation of the backbone. Also, more rigid polymer (PMDA-based) showed higher thermal stability among the polymers. Less efficient chain close packing and thus decreasing the thermal stability were observed for BTDA- and 6FDA-derived polyimides due to the presence of keto and hexafluoroisopropylidene spacer groups, respectively.
According to the residual weight of each sample at 600 °C in air atmosphere using Van Krevelen equation, the values of LOI were determined with the intention of finding a perspective to flame-retardant character of synthesized polymers. The following equation was used:
where p is the residual weight at 600 °C in air atmosphere.[Citation33]
The obtained results that tabulated in Table (LOI = 40.3–41.1) showed that these polyimides had good flame-retardant properties (Table ). As it is reported in literature, polymers with LOI values higher than 26 could be regard as flame-retardant polymers.[Citation34]
The solubility behaviors of the polymers were studied in different solvents. The solubility of the polymers was about 1.3–1.7 g dL−1 in dipolar aprotic solvents including DMF, DMAc, NMP, DMSO and also in less efficient solvent m-cresol. This improved solubility (common solubility of polyimide is about 0.5 dL−1) was as a result of introducing flexible ether groups, bulky naphthyl groups into the polymer backbone, presence of amide groups in the polyimide chains. Also existence of three pyridine units in the backbone enhanced the solubility of polymers. This was attributed to the presence of nitrogen atom in the structure that created a polarized bond and improved the solubility of the prepared polymers due to increased dipole–dipole interactions in the polymer-solvent system. Therefore, the major benefit of using pyridine in the backbone of polyimides was to increase their solubility while maintaining their heat resistant (due to aromatic character). It should be mentioned that the presence of hexafluoroisopropylidene flexible unit in the 6FDA- based polyimide caused higher solubility of this polymer in respect to other polymers. Existence of hexafluoroisopropylidene group reduced the intensity of dipole–dipole inter-chain interactions and charge transfer complex, culminating to enhanced solubility by assisting the penetration of solvent molecules into the polymer chain.
Very low water absorption values were obtained for these polyimide films in comparison with common commercial polyimides. Water absorption (WA) values were all less than 1.2%, ranging from 0.65% for 6FDA-derived polyimide and 0.96 and 1.15% for PMDA- and BTDA-derived polyimides, respectively. Favorable low WA values might be related to a major decrease in the content of highly polarizing imide functional units caused by lengthy diamine moieties that could function as spacer units in the polymer backbones, and therefore decrease chain polarizability to some extent. Also, 6FDA-derived polyimide revealed the lowest amount of water absorption that could be obviously attributed to the hydrophobic nature of trifluoromethyl groups.[Citation35–37]
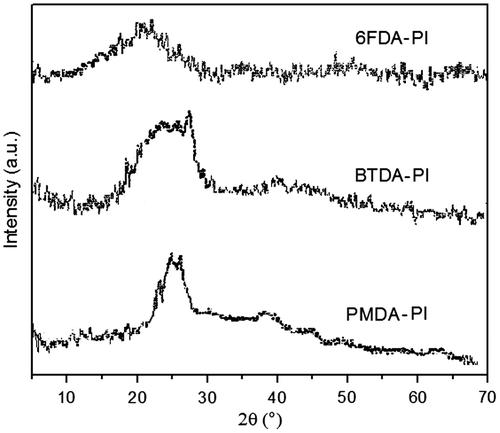
The morphology of the polymers was studied using wide angle X-ray diffraction on powder specimens (Figure ). The results indicated that they were almost amorphous. This could be attributed to the incorporation of flexible ether groups with free internal rotation about C–O bond and also packing-disruptive bulky naphthyl side groups that led to increased chain distances and decreased chain interactions. On the other hand, crystallinity of polyimides was also depended upon the structure of employed dianhydrides. 6FDA-derived polyimide was amorphous with no sharp peak. This was attributed to the presence of bulky –C(CF3)2– groups in dianhydride moieties that avoid dense packing of polymer chains. BTDA-derived polyimide showed only very small peaks along with a series of broad diffraction peaks. PMDA-derived polyimide had small degree of crystallinity confirmed by a moderately sharp peak. This might be related to the rigid structure of polymer backbone coming up from the dense packing of polymer chains. As it was seen, the good solubility behaviors of resulting polyimides could be explained by their amorphous nature.
4. Conclusion
A new special nicotinic-based diamine was prepared and characterized. Presence of amide, ether, bulky naphthyl, and pyridine units from one side and fully aromatic nature of the diamine from another side and the incorporation of these structures into the back bone of polymers led to the preparation of a new category of polyimides with favorable balance of properties including high heat resistant and improved solubility.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Liaw DJ, Hsu PN, Chen WH, Lin SL. High glass transitions of new polyamides, polyimides, and poly(amide−imide)s containing a triphenylamine group: synthesis and characterization. Macromolecules. 2002;35:4669–4676.
- Mehdipour-Ataei S, Amirshaghaghi A. Novel poly(amide-imide)s from 2,6-bis(5-amino-1-naphthoxy) pyridine. Eur. Polym. J. 2004;40:503–507.
- Mehdipour-Ataei S, Amirshaghaghi A. Preparation and properties of new thermally stable poly(ether-imide-amide)s. Polym. Int. 2004;53:1186–1190.
- Mehdipour-Ataei S, Sarrafi Y, Pirjel MA. Synthesis, characterization, and properties of novel poly(ether-urea)s. J. Appl. Polym. Sci. 2004;93:961–965.
- Maglio G, Palumba R, Vignola MC. Main chain modification of aromatic polyamides by incorporation of kinked, flexible monomers. Macromol. Chem. Phys. 1995;196:2775–2783.
- Hsiao SH, Yu CH. Aromatic polyamides bearing ether and isopropylidene or hexafluoroisopropylidene links in the main chain. J. Polym. Res. 1996;3:247–256.
- Babanzadeh S, Mehdipour-Ataei S. Effect of nano-silica on the dielectric properties and thermal stability of polyimide/SiO2 nano-hybrid. Mahjoub AR. Des. Monomers Polym. 2013;16:417–424.
- Hsiao SH, Yang CP, Chen SH. Synthesis and properties of ortho-linked polyamides based on a bis(ether-carboxylic acid) or a bis(ether amine) derived from 4-tert-butylcatechol. Polymer. 2000;41:6537–6551.
- Zenga K, Zoua GY. Synthesis and properties of polyimides derived from a new phthalonitrile-containing diamine with high polyaddition reactivity. Des. Monomers Polym. 2014;17:186–193.
- Espeso JF, De La Campa JG, Lozano AE, De Abajo J. Synthesis and characterization of new soluble aromatic polyamides based on 4-(1-adamantyl)-1, 3-bis(4-aminophenoxy)benzene. J. Polym. Sci., Part A: Polym. Chem. 2000;38:1014–1023.
- Liou GS, Hsiao SH. Polyterephthalamides with naphthoxy-pendent groups. J. Polym. Sci., Part A: Polym. Chem. 2002;40:1781–1789.
- Liaw DJ, Liaw BY, Yang CM. Synthesis and characterization of new soluble cardo aromatic polyamides bearing diphenylmethylene linkage and norbornyl group. Macromol. Chem. Phys. 2001;202:1866–1872.
- Espeso JF, Ferrero E, De La Campa JG, Lozano AE, De Abajo J. Synthesis and characterization of new soluble aromatic polyamides derived from 1,4-bis(4-carboxyphenoxy)-2,5-di-tert-butylbenzene. J. Polym. Sci., Part A: Polym. Chem. 2001;39:475–485.
- Liaw DJ, Liaw BY, Jeng MQ. Synthesis and properties of new polyamides and polyimides derived from 2,2′-dimethyl-4,4′-bis(4-aminophenoxy)biphenyl. Polymer. 1998;39:1597–1607.
- Imai Y. Synthesis of novel organic-soluble high-temperature aromatic polymers. High Perform. Polym. 1995;7:337–345.
- Wu SC, Shu CF. Synthesis and properties of soluble aromatic polyamides derived from 2,2′-bis(4-carboxyphenoxy)-9,9′-spirobifluorene. J. Polym. Sci., Part A: Polym. Chem. 2003;41:1160–1166.
- Imai Y. Recent advances in synthesis of high-temperature aromatic polymers. React. Funct. Polym. 1996;30:3–15.
- Siddique H, Bhole Y, Peeva LG, Livingston AG. Pore preserving crosslinkers for polyimide OSN membranes. J. Membr. Sci. 2014;465:138–150.
- Vora RH, Goh SH. Designed poly(ether-imide)s and fluoro-copoly(ether-imide)s: synthesis, characterization and their film properties. Mater. Sci. Eng., B. 2006;132:24–33.
- Zhou M, Sun Y, Yu Y, Cai M. Synthesis and properties of novel random copolymers of poly(ether ketone ether ketone ketone)–poly(ether ketone sulfone imide). High Perform. Polym. 2013;25:526–534.
- Bretas RES, Baird DG. Miscibility and mechanical properties of poly(ether imide)/poly(ether ether ketone)/liquid crystalline polymer ternary blends. Polymer. 1992;33:5233–5244.
- Ramani R, Alam S. Influence of poly(ether imide) on the free volume hole size and distributions in poly(ether ether ketone). J. Appl. Polym. Sci. 2012;125:3200–3210.
- Chinpa W. Fabrication and properties of poly(ether imide)/poly(vinyl alcohol) composite ultrafiltration membrane. Key Eng. Mater. 2012;531–532:18–21.
- Zhu M, Liu X, Liu B, Jiang Z, Matsumoto T. Poly(ether ketone azomethane)s and poly(ether ketone imide)s containing naphthylene moieties. Polym. Bull. 2011;67:1761–1771.
- Phomdum P, Chinpa W. Preparation and properties of poly (ether imide) ultrafiltration membrane modified with polyether diamine. Adv. Mater. Res. 2014;931–932:63–67.
- Abbasi A, Mehdipour-Ataei S. Novel sepiolite-based poly(amide-imide) nanocomposites: preparation and properties. Polym. Plast. Technol. Eng. 2014;53:596–603.
- Mehdipour-Ataei S. Novel thermally stable poly(sulfone ether ester imide)s. Eur. Polym. J. 2005;41:91–96.
- Beltramo M. The high performance thermoplastic. Eng. Plast. 1993;6:40–79.
- Billerbeck CJ, Henke SJ. Engineering thermoplastics. New York (NY): Dekker; 1985.
- Wilson D, Stenzenberger HS, Hergenrother PM. Polyimide. Glasgow: Blackie; 1991.
- Liaw DJ, Liaw BY, Li LJ, Sillion B, Mercier R, Thiria R, Sekiguchi H. Synthesis and characterization of new soluble polyimides from 3,3′,4,4′-benzhydrol tetracarboxylic dianhydride and various diamines. Chem. Mater. 1998;10:734–739.
- Sabbaghian E, Mehdipour-Ataei S, Jalilian S, Esfahanizadeh M, Salehi AM, Khodabakhshi F, Jalalian E. Novel species of soluble thermally stable poly(keto ether ether amide)s: preparation, characterization, and properties. Polym. Adv. Technol. 2015;26:1–9.
- Van Krevelen DW, Hoftyzer PJ. Properties of polymers. New York, (NY): Elsevier; 1976.
- Lin CH, Wang CS. Novel phosphorus-containing epoxy resins part I. Synthesis and properties. Polymer. 2001;42:1869–1878.
- Hasegawa M, Kasamatsu K, Koseki K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012;48:483–498.
- Hsiao SH, Guo WJ, Chung CL, Chen WT. Synthesis and characterization of novel fluorinated polyimides derived from 1,3-bis(4-amino-2-trifluoromethylphenoxy)naphthalene and aromatic dianhydrides. Eur. Polym. J. 2010;46:1878–1890.
- Shang Y, Fan L, Yang S, Xie X. Synthesis and characterization of novel fluorinated polyimides derived from 4-phenyl-2,6-bis[4-(4′-amino-2′-trifluoromethyl-phenoxy)phenyl]pyridine and dianhydrides. Eur. Polym. J. 2006;42:981–989.