Abstract
Reactions of 3-aminophenol with dichlorodiphenylsilane in the presence of triethylamine resulted in the preparation of a diamine named as bis(m-aminophenoxy)diphenylsilane. Reaction of the diamine with two moles of trimellitic anhydride led to the preparation of a diacid with preformed imide structures. Silicon-containing poly(amide-imide)s were then prepared by polycondensation reactions of the diacid with different diamines in the presence of triphenyl phosphite. All the products and polymers were characterized and the physical properties of the polymers including solubility, solution viscosity, thermal stability, thermal behavior, flameretardency, crystallinity, and morphology were studied. The polymers showed high thermal stability and flameretardency, as well as enhanced solubility in polar solvents. The glass transition temperature of the polymers was about 187–196 °C, the temperature for 10% gravimetric loss was in the range of 375–400 °C, and also the weight of the polymer remaining at 700 °C was about 39–44%. They were flame-retardant, soluble in dipolar aprotic solvents, and showed some crystalline structures.
1. Introduction
High-performance polymers are an important category of polymers and their applications are growing progressively. Preparation of high-performance polymers has attracted much attention and focus on new materials for polymer syntheses has been achieved. Generally, they show high thermal stability, excellent mechanical properties, and good chemical resistance. Polyimides and polyamides are among the most important ones. The inclusion of rigid fragments into the polymer chain, especially in polyamide and polyimide is an effective technique to improve their thermal stability.[Citation1–4] While polyimides show higher thermal stability than polyamides, they are usually insoluble and infusible in the imide form. On the other hand, thermal stability of polyamides is lower than that of polyimides, but polyamides have superior solubility and processability than polyimides. So, poly(amide-imide)s were developed from high-performance polymers, which merge and inherit desirable properties from both polyamides and polyimides. They own superior thermal properties compared with polyamides, and improved processability than polyimides.[Citation5,6] To further improve their solubility in organic solvents, several methods to obtain polymers with chemically modified chain structures have been introduced for balancing the thermal stability and the processability. The most important methods include the inclusion of hinge atoms, kink units, or flexible spacer units into the backbone. The incorporation of bulky pendant (cardo) groups into the backbone of polymers is another approach to enhance solubility and thus processability of the poly(amide-imide)s.[Citation7–12]
It has been cleared that the problems in processing of these conventional aromatic polymers are resulted from the inherent molecular characteristics, such as the molecular chain stiffness, high polarity, and high intermolecular association interaction. Thus, the major structural alterations to get processable polymers have been carried out by introducing flexible spacers and also bulky substitutes such as pendent aryl rings into their chains.[Citation13–17]
Poly(amide-imide)s can be synthesized by polycondensation from different monomers containing anhydride, carboxylic acid, or aromatic amino groups. To prepare poly(amide-imide), it is common to use a dicarboxylic acid, dianhydride, diamine, amino acid, or anhydride acid, among which trimellitic anhydride (TMA) is a commercially accessible aromatic anhydride.[Citation16–18]
There are some reports on silicon-containing poly(amide-imide)s and some investigation of their properties. A series of aromatic silicon-containing poly(amide-imide)s were prepared by solution polycondensation reaction of a silicon-containing diacid chloride with diamines containing phenoxy units. The polymers showed good solubility in polar solvents and their initial decomposition temperature was about 410 °C.[Citation19] In another related work, Sava reported silicon-containing poly(amide-imide)s by direct polycondensation of different aromatic diamines with a dicarboxylic acid containing the dimethylsilylene group and preformed imide rings. These polymers were soluble in polar amidic solvents and they showed high thermal stability, of temperature for 5% weight loss about 400 °C and glass transition temperature in the range of 220–270 °C.[Citation20]
A silicone-diamine including ether and bulky aromatic groups was synthesized in this research. Afterward, the related diimide-diacid compound was prepared through reaction of the diamine with TMA. Silicon-containing poly(amide-imide)s were prepared from a polycondensation reaction of the diacid with different diamines. The polymers showed a nice balance of high thermal stability and improved solubility due to their unique feature of backbones.
2. Experimental
2.1. Reagents and solvents
All chemicals were purchased either from Merck or Aldrich Chemical Co. TMA, CaCl2, and LiCl were dried in a vacuum oven overnight. N-Methyl-2-pyrrolidone (NMP) and tetrahydrofuran (THF) were distilled under reduced pressure over CaH2 and sodium wire, respectively and stored over molecular sieves. 3-Amino phenol was sublimed under reduced pressure.
2.2. Instruments
Infrared measurements were performed on a Bruker-IFS 48 FTIR spectrometer (Ettlingen, Germany). 1H-NMR spectra were recorded in DMSO-d6 solution using a Bruker Avance DPX 400 MHz (GmbH, Germany). Elemental analyses were performed by a CHN-O-Rapid Heraeus elemental analyzer (Wellesley, MA). The molecular weights (Mn and Mw) of the polymers were determined in DMF at 30 °C at a flow rate of 1 mL/min using a Waters gel permeation chromatography (GPC) equipped with a Styragel column (Milford, MA). Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were recorded on a Stanton Redcraft STA-780 (London, UK) in air atmosphere at a heating rate of 10 °C/min. Wide-angle X-ray diffraction (WAXD) patterns were performed at room temperature on an X-ray diffractometer (Siemens model D 5000) using Ni-filtered Cu Ka radiation (40 kV, 25 mA) with a scanning rate of 3 °C/min. The fractured surfaces of the films, prepared after cooling in liquid nitrogen, were observed by SEM. The apparatus used was an S-360 (Cambridge, UK); and the surfaces of samples were prepared by sputter coating with a gold layer.
2.3. Monomer and polymer synthesis
2.3.1. Synthesis of bis(m-aminophenoxy)diphenylsilane [Citation21]
The typical procedure for the synthesis was as follows: a mixture of 0.10 mol (10.92 g) of 3-aminophenol, 0.11 mol (11.11 g) of triethylamine (TEA), 175 mL of THF, and 25 mL of toluene was introduced into a three-necked flask. Then, over a period of 2 h at 75 °C, 0.05 mol (12.67 g) of dichlorodiphenylsilane was added into the mixture drop-wise. The mixture was refluxed for 3 days. Filtration was used to remove the formed salt from the mixture and the filtrate was concentrated. Then it was dissolved in ether and the precipitated salt was separated by filtration. The basic product was crystallized from ether at 0 °C to yield brown-yellow crystals and dried in a vacuum oven at 60 °C for 3 days. The yield of reaction was 85%.
2.3.2. Synthesis of diimide-diacid
19.23 g (0.10 mol) of TMA was added to a solution of diamine (19.9 g, 0.05 mol) dissolved in 120 mL of NMP in a 500 mL flask. The reaction mixture was stirred and heated to 80 °C for 2 h under N2. Then, 50 mL of dry toluene was added to the mixture, and it was heated to reflux for about 12 h to distill off water azeotropically via a Dean–Stark trap. After cooling the mixture to room temperature, the precipitate was gathered by filtration, washed several times with methanol, and dried in a vacuum oven (Yield was about 90%).
2.3.3. Poly(amide-imide)s synthesis
A typical procedure was as follows: a mixture of 1.5 mmol of a commercial diamine, 1.5 mmol of diimide-diacid, 0.70 g of calcium chloride, 1.5 mL of triphenyl phosphite, 1.5 mL of pyridine, and 7 mL of NMP was stirred and refluxed for 3 h. After cooling, the reaction mixture was poured into a large amount of methanol with stirring. The precipitate was collected by filtration and washed with methanol and hot water. Then it was dried at 120 °C under vacuum overnight.
3. Results and discussion
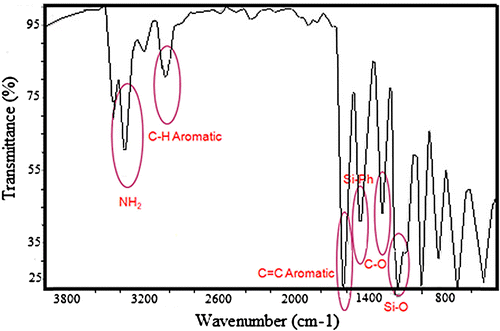
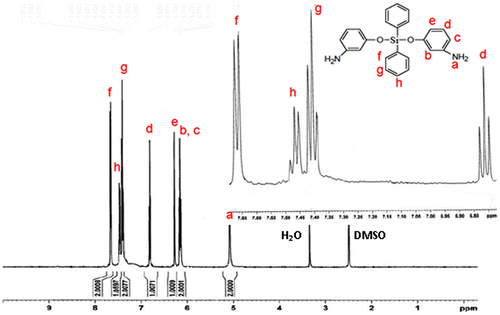
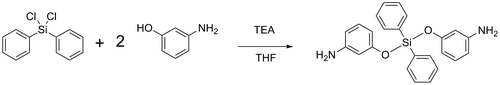
The main aim of this research was focused on the preparation of a new type of thermally stable polymer with improved solubility, and for this purpose silicon-containing poly(amide-imide)s were considered. The basic strategy for preparation of poly(amide-imide)s was around the preparation of a diamine and subsequently a diimide-diacid compound using TMA, and finally its reaction with diamines. In view of this fact, a silicon-based diamine named as bis(m-aminophenoxy)diphenylsilane was prepared (Scheme ) via reaction of 3-aminophenol with dichlorodiphenylsilane in THF in the presence of TEA.[Citation21] Common spectroscopic methods including FT-IR (Figure ) and 1H-NMR spectroscopy (Figure ) were used to confirm the structures of diamine. Also elemental analysis revealed good agreement between calculated and found amounts of carbon, hydrogen, and nitrogen contents.
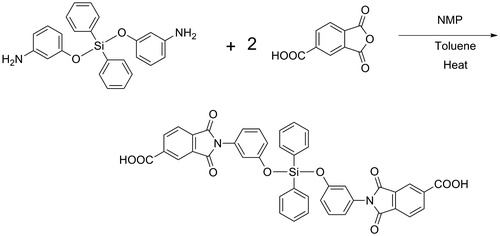
Bis(m-aminophenoxy)diphenylsilane was reacted with two moles of TMA resulting in the preparation of a diacid with preformed silicon, ether, and imide structures (Scheme ). Structure of the diimide-diacid was characterized and confirmed by FT-IR and 1H-NMR spectroscopic methods and also elemental analysis (Table ).
Table 1. Characteristic data of diimide-diacid compound.
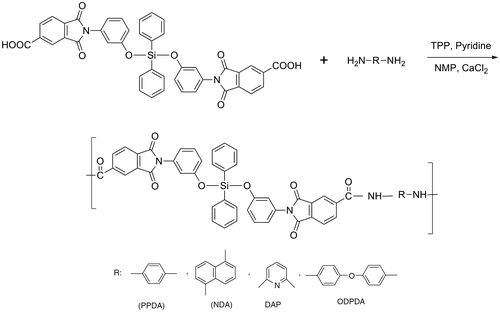
A series of silicon-containing poly(amide-imide)s were prepared via polycondensation reaction of the prepared diacid with various aromatic diamines in NMP by the Yamazaki reaction method using triphenyl phosphite and pyridine as condensing agents (Scheme ). Progress of these reactions was achieved via an acyloxy N-phosphonium salt of pyridine formed by dephenoxylation of triphenyl phosphite that was followed by aminolysis.[Citation22,23]
FT-IR and elemental analysis techniques were used to determine the chemical structures of all the polymers and related results are collected in Table .
Table 2. Characteristic data of polymers.
As a criterion for assessment of molecular weights, the inherent viscosity of the polymers was measured in NMP at a concentration of 0.5 g/dL at 30 °C, additionally, the molecular weights of polymers (Mw and Mn) were evaluated by GPC (Table ). The results disclosed reasonable molecular weights of polymers in comparison to common poly(amide-imide)s.
The polymers revealed good solubility (by heating and stirring) in dipolar aprotic solvents, including NMP, DMAc, DMF, DMSO, and also m-cresol (Table ). The improved solubility of the polymers could be attributed to the presence of flexible silicon and ether units. Also, meta-catenation (3-amino phenol group) and the presence of bulky phenyl groups on silicon led to improved solubility of the polymers because of increase of the free volume, and accordingly penetration of polar solvents into the polymer chains. In fact, decrease of symmetry led to lower chain–chain interaction and therefore penetration of the solvent into the polymer chain, and bulky groups decreased interactions of polymer chains and offered good solubility of the prepared polymers as well.
Table 3. Solubility behavior of polymers.
ODPDA-polymer revealed highest solubility among the prepared polymers that was related to the flexibility of this diamine (existence of ether group in ODPDA) in comparison to others.
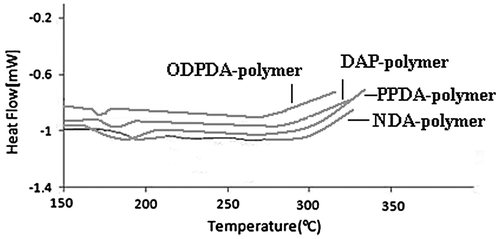
Thermal behavior and stability of polymers were studied by DSC and TGA techniques. The glass transition temperature (Tg) of the polymers was in the range of 187–196 °C attained from the midpoint temperature of the base line shift on the first heating of the DSC traces (Figure ).
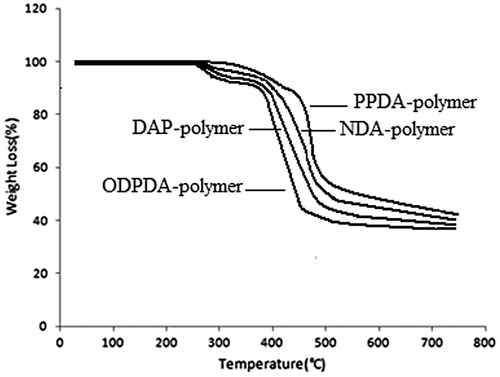
Thermal stability of the polymers in air at a heating rate of 10 °C was evaluated by TGA. The initial decomposition temperature (TIDT) of the polymers was in the range of 275–287 °C, and the temperature for 10% gravimetric loss (T10) that is a decisive factor for estimation of thermal stability was about 375–400 °C, and also the weight of the polymer remaining (char yield) at 700 °C was in the range of 39–44%. The residual weight of polymers at higher temperatures showed an increase in comparison to ordinary aromatic silicon-free polymers. This was obviously related to the inorganic nature of silicon atom and char formation due to its presence in the polymers. The results are gathered in Table and TGA curves of polymers are shown in Figure .
Table 4. Thermal properties and flame retardant data of polymers.
High thermal stability of the polymers might be attributed to the fully aromatic nature of the structures, as well as the presence of imide and silicone units. On the other hand, since the diacid was the same, PPDA-polymer showed the highest thermal stability with respect to the other polymers, which was related to the more rigid and symmetric structure of PPDA in comparison to other diamines.
According to Van Krevelen equation,[Citation24,25] an estimation of limiting oxygen index (LOI) was obtained via the following equation: LOI = 17.5 + 0.4 CR, in which CR is char yield. LOI of polymers are in the range of 33.1–35.1 (Table ), and as is declared in the literature, polymers with LOI values higher than 26 could be categorized as flame-retardant polymers,[Citation26] therefore such silicon-containing poly(amide-imide)s were flame-resistant polymers.
In general, it should be emphasized that the role of diphenylsilane unit in the polymer main chains for solubility and flame retardancy was important. Presence of flexible silicon unit and also bulky phenyl groups on silicon caused improved solubility of the polymers due to increased free volume of the backbones. Inflammable nature of the inorganic silicon unit gave a suitable flame-retardant character to the final polymers. On the other hand, because of the flexibility of the diphenylsilane unit, perhaps some thermal instability occurred in the polymers that might be compensated by the presence of two aromatic thermally stable phenyl groups.[Citation19,20]
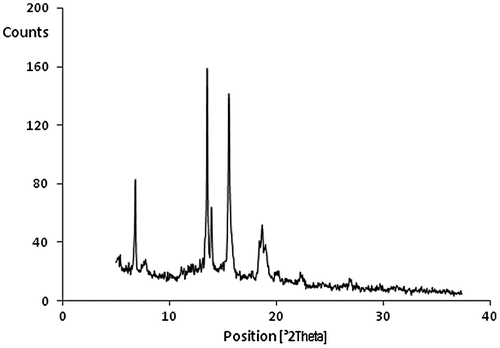
Crystallinity of the polymers was investigated using a wide-angle X-ray diffraction pattern with powder samples in the region of 2θ = 5–40° at room temperature. Some crystallinity was observed in the polymers that might be related to the overall rigid structure of polymers and phenylated backbones. In Figure a representative pattern was shown.
SEM on the fractured surfaces of the films that was prepared after cooling in liquid nitrogen was investigated. SEM results also confirmed crystallinity of the polymers that could be seen clearly in a sample image in Figure .
4. Conclusion
Bis(m-aminophenoxy)diphenylsilane as a diamine was prepared via the reaction of 3-aminophenol with dichlorodiphenylsilane. Subsequently, a diimide-diacid was obtained through the reaction of the diamine with TMA. Finally, silicon-containing poly(amide-imide)s were prepared by polycondensation reactions of the diacid with different commercially available diamines in the presence of triphenyl phosphite.
Totally, the main problem of thermally stable polymers, i.e. improving solubility while maintaining thermal stability, was agreeably overcome by the introduction of ether, silicon, meta-catenation, and bulky phenyl groups in favor of enhancing solubility whereas, avoid of weak linkages and incorporation of aromatic structures for maintaining high thermal stability of the poly(amide-imide)s.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cassidy PE. Thermally stable polymers. New York (NY): Marcel Dekker, Inc.; 1980.
- Wilson D, Stenzenberger HD, Hergenrother PM. Polyimides. London: Blackie; 1990.10.1007/978-94-010-9661-4
- Ghosh MK, Mittal KL. Polyimides: fundamentals and applications. New York (NY): Marcel Dekker, Inc.; 1996.
- Seymour RB. Engineering polymer sourcebook. New York (NY): McGraw-Hill Publishing; 1990.
- Sroog CE. Polyimides. J. Polym. Sci. Macromol. Rev. 1976;11:161–208.10.1002/pol.1976.230110105
- Jeong HJ, Kakimoto M-A, Imai Y, Imai Y. Synthesis and characterization of novel aromatic polyamides from 3,4-bis(4-aminophenyl)-2,5-diphenylpyrrole and aromatic diacid chlorides. J. Polym. Sci. Polym. Chem. 1991;29:767–772.10.1002/pola.1991.080290519
- Akbarian-Feizi L, Mehdipour-Ataei S, Yeganeh H. Investigation on the preparation of new sulfonated polyimide fuel cell membranes in organic and ionic liquid media. Int. J. Polym. Mater. Polym. Biomater. 2014;63:149–160.10.1080/00914037.2013.812086
- Abbasi A, Mehdipour-Ataei S. Novel sepiolite-based poly(amide-imide) nanocomposites: preparation and properties. Polym. Plast. Technol. Eng. 2014;53:596–603.10.1080/03602559.2013.854389
- Zeng K, Zou Y, Yang G. Synthesis and properties of polyimides derived from a new phthalonitrile-containing diamine with high polyaddition reactivity. Des. Monomers Polym. 2014;17:186–193.10.1080/15685551.2013.840500
- Tabatabaei-Yazdi Z, Mehdipour-Ataei S. Preparation, Characterization, and properties of novel category of processable thermally stable poly(ether ether imide)s. Colloid Polym. Sci. 2014;292:2145–2156.10.1007/s00396-014-3248-2
- Parhami A, Shojai Nasab S, Abbasi M, Jafarazad R, Bahmani R. Synthesis and characterization of novel nanostructured, organosoluble, thermally stable and wholly aromatic poly(amide-imide)s from a new diimide-diacid monomer by direct polycondensation. Des. Monomers Polym. 2014;17:736–745.10.1080/15685551.2014.918012
- Sabbaghian E, Mehdipour-Ataei S, Jalilian S, et al. Novel species of soluble thermally stable poly(keto ether ether amide)s: preparation, characterization, and properties. Polym. Adv. Technol. 2015;26:1–9.10.1002/pat.v26.1
- Rostami E. Synthesis and characterization of new polyamides containing pyridine thioether units in the main chain under microwave irradiation (MW) and their nanostructures. Int. J. Polym. Mater. Polym. Biomater. 2013;62:175–180.10.1080/00914037.2011.641632
- Mehdipour-Ataei, S, Babanzadeh, S, Abouzari-Lotf, E. Nicotinic-based poly(amide-ether-imide)s: a new category of soluble, heat-resistant, and flame-retardant polyimides. Des. Monomers Polym. 2015;18:451–459.
- Mehdipour-Ataei S, Aram E. New polyimide and nanoporous structures with low dielectric constant. Adv. Polym. Technol. 2014;33:21407–21417.
- Mehdipour-Ataei S, Amirshaghaghi A. Novel poly(amide-imide)s from 2,6-bis(5-amino-1-naphthoxy) pyridine. Eur. Polym. J. 2004;40:503–507.10.1016/j.eurpolymj.2003.10.024
- Mehdipour-Ataei S, Hatami M. Synthesis and characterization of novel heat resistant poly(amide-imide)s. Eur. Polym. J. 2005;41:2010–2015.10.1016/j.eurpolymj.2005.03.012
- Chol KH, Lee KH, Jung JC. Synthesis of new poly(amide-imide)s with (n-alkyloxy)phenyloxy side branches. J. Polym. Sci., Part A: Polym. Chem. 2001;39:3818–3825.
- Hamciuc E, Bruma M, Schulz B, Kopnick T. New silicon-containing poly(amide-imide)s. High Perform. Polym. 2003;15:347–359.10.1177/0954008303015003010
- Sava I, Bruma M, Schulz B, Kopnick T. Comparison of properties of silicon-containing poly(amide-imide)s. High Perform. Polym. 2005;17:483–495.10.1177/0954008305044162
- Babanzadeh S, Mahjoub AR, Mehdipour-Ataei S. Novel soluble thermally stable silane-containing aromatic polyimides with reduced dielectric constants. Polym. Degrad. Stab. 2010;95:2492–2498.10.1016/j.polymdegradstab.2010.08.001
- Yamazaki N, Matsumoto M, Higashi F. Studies on reactions of the N-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salt. J. Polym. Sci., Polym. Chem. 1975;13:1373–1380.
- Yamazaki N, Iguchi T, Higashi F. Preparation and reaction of diphenyl phosphite. Chem. Lett. 1977;19:185–188.10.1246/cl.1977.185
- Van Krevelen DW, Hoftyzer PJ. Properties of polymers. New York (NY): Elsevier; 1976.
- van Krevelen DW. Some basic aspects of flame resistance of polymeric materials. Polymer. 1975;16:615–620.10.1016/0032-3861(75)90157-3
- Lin CH, Wang CS. Novel phosphorus-containing epoxy resins Part I. Synthesis and properties. Polymer. 2001;42:1869–1878.10.1016/S0032-3861(00)00447-X