Abstract
Porous poly(glycidyl methacrylate-co-ethyleneglycol dimethacrylate) samples with total porosity up to 91% were prepared by the polymerisation of high internal phase emulsions (HIPEs). Morphological features like cavity and interconnecting pore diameter were investigated and successfully controlled by changing the initiator system, surfactant ratio, pore volume ratio, stirring rate and reaction mixture temperature. All resulting porous polyHIPE monoliths had open cellular morphology with cavity diameters between sub-μm and 30 μm.
Introduction
Highly porous and permeable polymeric materials with a microcellular structure can be prepared conveniently via the polymerisation of high internal phase emulsions (HIPEs), where the internal phase exceeds 74% of total emulsion volume and continuous phase contains monomers.[Citation1] By the removal of internal phase, polymers with high porosity and interconnected porous morphology termed polyHIPEs are obtained.[Citation2–7] Porous polymers raised a lot of attention due to their exceptional properties such as low density and high permeability and are therefore used for various applications. The most studied polyHIPEs are based on styrene and its derivatives and divinylbenzene (DVB) as a cross-linker; however, various acrylates are recently used as monomers. Glycidyl methacrylate (GMA) is a functional monomer used in the preparation of polyHIPE materials and is usually cross-linked by ethyleneglycol dimethacrylate (EGDMA) or DVB.[Citation8,9] Epoxy groups, which can be further functionalised to, for example, ion exchange groups,[Citation10] make GMA suitable monomer for chromatographic applications.[Citation11] So far GMA-based polyHIPEs have been successfully prepared and used as catalyst supports,[Citation12] as filtration media[Citation13] for protein separation [Citation11,14–16] and as columns for liquid chromatography.[Citation17–19] For such applications, a significant pressure of mobile phase is usually used, and therefore, appropriate mechanical properties are also needed. We have previously shown that methyl methacrylate (MMA) can be used as an effective comonomer in combination with other acrylates for polyHIPE production via photopolymerisation and that monoliths with open porosity up to 85% and improved mechanical properties can be obtained.[Citation20] In order to gain more data on the influence of emulsion composition and polymerisation conditions on the morphology of GMA-based polyHIPEs, study of variations of initiator system, surfactant and pore volume, mixing speed and temperature was performed.
Experimental
Materials
GMA and EGDMA were passed through the layer of Al2O3 to remove the inhibitors. The surfactant Synperonic PEL 121, calcium chloride hexahydrate (CaCl2 × 6H2O), N,N,N′,N′-tetramethylethylenediamine (TEMED), ammonium persulfate (APS), azobisisobutyronitrile (AIBN) and ethanol (Merck) were used as received.
PolyHIPE preparation
GMA, EGDMA, AIBN and Synperonic PEL 121 were placed in a 250-ml round-bottomed three-necked reactor. The aqueous phase was prepared separately by dissolving initiator (APS) and calcium chloride hexahydrate. The organic phase was stirred at various stirring rates with an overhead stirrer. An appropriate amount of aqueous phase was added dropwise under continuous stirring. After addition of aqueous phase, stirring was continued for 1 h and TEMED was added just before the end of stirring followed by emulsion transfer into a polypropylene tube and was allowed to polymerise for 24 h at room temperature. PolyHIPEs were extracted in a Soxhlet apparatus with water for 24 h and absolute ethanol for a further 24 h and dried in vacuo at 40 °C for 24 h. Compositions of emulsions are given in Table .
Table 1. Compositions of emulsions.Table Footnotea
Characterisations
Polymer samples were characterised by nitrogen adsorption/desorption on a TriStar II 3020 porosimeter (Micromeritics) using a BET model for surface area evaluation. Porous structure typical for polyHIPEs was determined using scanning electron microscopy (SEM) Quanta 200 3D (FEI Company). Samples were mounted on metal stubs using graphite tape and sputter coated with a layer of gold. The average cavity sizes, cavity size distributions and interconnecting pore sizes were determined by SEM images analysis, using a correction factor of 2/√3 for cavity size, in order to compensate for the statistical error due to random sectioning of cavities at sample preparation.[Citation21] A minimum of 50 cavities per image was considered for size distribution graph construction and calculating average values.
Results and discussion
Preparation of polyHIPEs based on GMA and EGDMA and their use as separation media have already been reported.[Citation11] Besides improvement of mechanical properties, the manipulation of the morphology over a wide range is of great interest. So in order to successfully control the morphological features, influence of different polymerisation factors was investigated.
One of the factors is the initiator system used for polymerisation and the variations in the locus of initiation have already proven to be very important regarding resulting polyHIPE morphology in several cases.[Citation22–25] Initiators can be put in either organic or water phase or also in both phases [Citation22,26] and can be induced by thermal decomposition, redox reactions, photolysis, etc. In the case of water-in-oil emulsions (O/W), where monomers and initiator are both soluble in organic phase, the initiation takes place inside the continuous phase, and when initiator is soluble in water phase, the polymerisation starts at the interface of both phases. Since the aqueous-phase initiated system forms free radicals more rapidly and reaches the gel point faster, the droplets of internal phase are usually locked-in before destabilisation occurs, which results in the formation of smaller polyhedral pores. In the organic-phase initiated system, usually more extensive destabilisation occurs and reflects in the formation of bigger, more spherical pores.[Citation7]
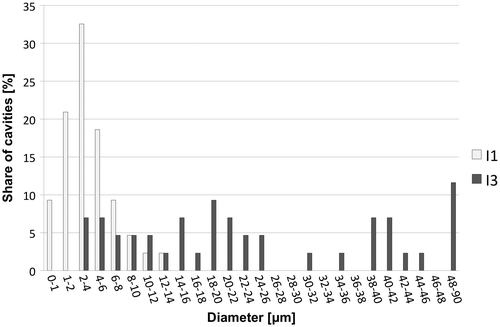
Within this study, two initiator systems were tested, namely oil phase included AIBN and redox initiator pair APS/TEMED. APS was included in the monomer non-containing aqueous phase, while AIBN on the other hand was dissolved in the monomer-containing oil phase. TEMED was added as an APS/TEMED redox component to the already formed APS containing emulsion just before the end of stirring. In both cases, obtained emulsions were stable (without TEMED) at room temperature for 24 h. Depending upon the initiator, the morphology resulted in either the formation of small and interconnected polyhedral pores or bigger spherical pores, with a lower degree of interconnectivity (Figure ). In the case where water soluble APS together with redox initiator TEMED was used, the polymerisation took place at the interface of both phases and locked-in the shape of the droplets, resulting in a polyHIPE sample I3 (Figure ) with the average diameter of cavities 3 μm and average diameter of interconnecting pores 400 nm. With the radical initiator AIBN, the polymerisation process was slower (24 h), and therefore, pores of obtained monoliths are significantly bigger. The average diameter of cavities is 30 μm, while the average diameter of interconnecting pores is 1.5 μm. Significant difference in average cavity and pore diameter is most probably the result of the difference in kinetics of radical formation.
Figure 1. SEM images; influence of initiator: (a) I1 – APS/TEMED and (b) I2 – AIBN; at 80% pore volume.
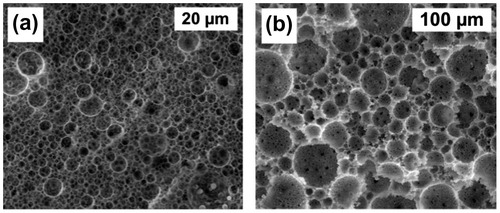
From Figure , it can be also clearly seen that in the case of aqueous-phase initiated sample I1, the cavities’ sizes are distributed more homogeneously compared to organic-phase initiated sample I2, which has a more hierarchical pore structure with significantly wider cavities’ size distribution.
An important role in polyHIPEs preparation play also the surfactants by lowering the surface tension between oil and water phase and preventing droplet coalescence. The amount of surfactant needed to produce open porous morphology depends on the amount of the oil phase of emulsion and usually ranges between 20 and 50 vol% with respect to the oil phase. With increasing the amount of surfactant, the thickness of the interfacial film is usually reduced and causes highly interconnected open porous morphology.[Citation27] In this work, two different block copolymers, namely polyethylene glycol-polypropylene glycol-polyethylene glycol block copolymer Synperonic PEL 121 were used, one with hydrophilic–lipophilic balance (HLB) of 0.5 and the second one with HLB of 1.5. Despite high polarity of GMA monomer, surfactant with low HLB did not produce a stable emulsion suitable for polymerisation. On the other hand, the surfactant Synperonic PEL 121 with HLB of 1.5 produced emulsions, which were stable for over 24 h. The surfactant amount was varied between 10 and 50% (based on monomer volume). The lowest amount of surfactant did not produce typical interconnected polyHIPE morphology but was more bi-continuous (Figure (a)). The use of 20% of surfactant resulted in the formation of rather closed polyHIPE morphology with cavities diameter of 2 μm and with few interconnecting pores with diameters approximately 200 nm (Figure (b)). On the other hand, the use of 30% of surfactant PEL 121 (HLB 0.5) produced highly interconnected morphology with smaller cavities (diameter approx. 1.5 μm) (Figure (c)). In the case of higher surfactant concentrations, the cavities were even smaller (less than 1 μm) and resulted in brittle monoliths (Figure (d)).
Figure 3. SEM images; influence of surfactant ratio: (a) 10%, (b) 20%, (c) 30% and (d) 40% and pore volume ratio: (e) 80% and (f) 90%.
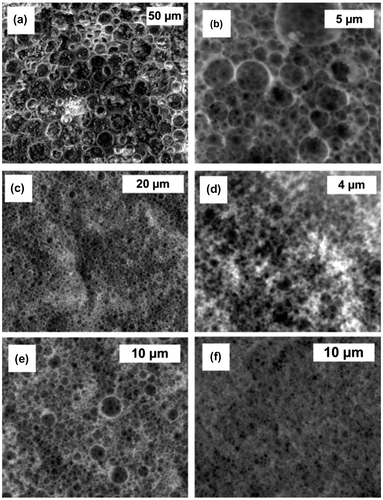
Pore volume was changed from 80 to 91%, which was also the maximum pore volume obtained for stable emulsions. Increasing the pore volume produced more viscous emulsions and after polymerisation more interconnected structure with smaller and more homogenous cavities. Such a trend has been observed previously for other GMA-based polyHIPEs.[Citation19]
Furthermore, stirring rate was varied from 100 to 300 rpm, and as it was expected, the cavity size decreased with increased stirring rate. Besides the viscosity,[Citation28] the increase in mixing speed influences an increase in shear stress, which causes the formation of smaller droplets of internal phase and also reduces the thickness of the interfacial film, causing more interconnected morphology after polymerisation.[Citation23,29,30] This can be nicely seen from the BET measurements listed in Table , where the surface area is increasing with increased stirring rate. Similar results have already been observed previously for MMA polyHIPE material.[Citation20]
Table 2. Influence of mixing speed and temperature on morphology.
The last set of experiments was done by increasing the reaction mixture temperature from 25 to 50 °C. The reaction mixture temperature influenced the stability of emulsions and consequently also the cavity size. With increased mixing temperature, we noticed an increase in the cavity size diameter since the stability of emulsions decreased. With the increased thermal energy of the system, also the rate of droplet coalescence increases [Citation31] and this was proven also in our case, at 40 °C, phase separation occurred. This is also clearly seen in Table , where the surface area is decreasing with increased mixing temperature on account of coalescence.
Conclusion
We can conclude that the biggest influence on morphological features was achieved by changing the initiator system from APS/TEMED to AIBN, where cavity diameter could be tailored in the range between sub-μm and 90 μm. The change of initiator also influenced the cavity size distribution, which was much more homogeneous in the case of APS compared to AIBN-initiated samples with more hierarchical pore structure with wider cavities’ size distribution. The biggest influence on interconnecting pore diameter was obtained by changing the surfactant and pore volume ratio. With increasing the surfactant and pore volume ratio, the resulting polymers had increased number of interconnecting pores.
Acknowledgements
This contribution was made within the framework of Centre for Open Innovation and Research of the University of Maribor (Core@UM) and within operation Creative Core VŠTP, both co-funded by the European Regional Development Fund (Operative Programme for Strengthening Regional Development Potentials for Period 2007–2013, priority aim 1.1.: improvement of the competitive capabilities of companies and research excellence).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lissant KJ. Emulsions and emulsion technology: part 1. New York: Marcel Dekker; 1974.
- Bartl VH, Von Bonin W. Über die Polymerisation in umgekehrter Emulsion [On the polymerisation in reversed emulsions]. Makromol. Chem. 1962;57:74–95.10.1002/macp.1962.020570105
- Bartl VH, Von Bonin W. Über die polymerisation in umgekehrter emulsion: II [On the polymerisation in reversed emulsions]. Makromol. Chem. 1963;66:151–156.10.1002/macp.1963.020660115
- Barby D, Haq Z, Unilever. Low density porous cross-linked polymeric materials and their preparation and use as carriers for included liquids. EU 60138. 1982.
- Cameron NR, Sherrington DC. High internal phase emulsions (HIPEs): structure, properties and use in polymer preparation. Adv. Polym. Sci. 1996;126:163–214.10.1007/3-540-60484-7
- Pulko I, Krajnc P. High internal phase emulsion templating – a path to hierarchically porous functional polymers. Macromol. Rapid Commun. 2012;33:1731–1746.10.1002/marc.v33.20
- Silverstein MS. PolyHIPEs: recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014;39:199–234.10.1016/j.progpolymsci.2013.07.003
- Barbetta A, Dentini M, Leandri L, Ferraris G, Coletta A, Bernabei M. Synthesis and characterization of porous glycidylmethacrylate – divinylbenzene monoliths using the high internal phase emulsion approach. React. Funct. Polym. 2009;69:724–736.10.1016/j.reactfunctpolym.2009.05.007
- Kimmins SD, Wyman P, Cameron NR. Photopolymerised methacrylate-based emulsion-templated porous polymers. React. Funct. Polym. 2012;72:947–954.10.1016/j.reactfunctpolym.2012.06.015
- Vlakh EG, Tennikova TB. Preparation of methacrylate monoliths. J. Sep. Sci. 2007;30:2801–2813.10.1002/(ISSN)1615-9314
- Krajnc P, Leber N, Štefanec D, Kontrec S, Podgornik A. Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media. J. Chromatogr. A. 2005;1065:69–73.10.1016/j.chroma.2004.10.051
- Majer J, Krajnc P. Amine functionalisations of glycidyl methacrylate based polyHIPE monoliths. Macromol. Symp. 2010;296:5–10.10.1002/masy.201051002
- Yang S, Zeng L, Wang Y, et al. Facile approach to glycidyl methacrylate-based polyHIPE monoliths with high epoxy-group content. Colloid Polym. Sci. 2014;292:2563–2570.10.1007/s00396-014-3295-8
- Pulko I, Smrekar V, Podgornik A, Krajnc P. Emulsion templated open porous membranes for protein purification. J. Chromatogr. A. 2011;1218:2396–2401.10.1016/j.chroma.2010.11.069
- Yao C, Qi L, Jia H, Xin P, Yang G, Chen Y. A novel glycidyl methacrylate-based monolith with sub-micron skeletons and well-defined macropores. J. Mater. Chem. 2009;19:767–772.10.1039/B816712E
- Yang J, Yang G, Liu H, Bai L, Zhang Q. Novel porous monolithic column using poly(high internal phase emulsion) methacrylate as materials for immunoglobulin separation performance on HPLC. Chin. J. Chem. 2010;28:229–234.10.1002/cjoc.v28:2
- Yao C, Qi L, Yang G, Wang F. Preparation of sub-micron skeletal monoliths with high capacity for liquid chromatography. J. Sep. Sci. 2010;33:475–483.10.1002/jssc.v33:4/5
- Junkar I, Koloini T, Krajnc P, Nemec D, Podgornik A, Štrancar A. Pressure drop characteristics of poly(high internal phase emulsion) monoliths. J. Chromatogr. A. 2007;1144:48–54.10.1016/j.chroma.2007.01.003
- Jerenec S, Šimič M, Savnik A, et al. Glycidyl methacrylate and ethylhexyl acrylate based polyHIPE monoliths: morphological, mechanical and chromatographic properties. React. Funct. Polym. 2014;78:32–37.10.1016/j.reactfunctpolym.2014.02.011
- Huš S, Krajnc P. PolyHIPEs from methyl methacrylate: hierarchically structured microcellular polymers with exceptional mechanical properties. Polymer. 2014;55:4420–4424.
- Carnachan RJ, Bokhari M, Przyborski SA, Cameron NR. Tailoring the morphology of emulsion-templated porous polymers. Soft Matter. 2006;2:608–616.10.1039/b603211g
- Gitli T, Silverstein MS. Bicontinuous hydrogel–hydrophobic polymer systems through emulsion templated simultaneous polymerisations. Soft Matter. 2008;4:2475–2485.10.1039/b809346f
- Gurevitch I, Silverstein MS. Polymerized pickering HIPEs: effects of synthesis parameters on porous structure. J. Polym. Sci. Polym. Chem. 2010;48:1516–1525.10.1002/pola.v48:7
- Livshin S, Silverstein MS. Crystallinity in cross-linked porous polymers from high internal phase emulsions. Macromolecules. 2007;40:6349–6354.10.1021/ma071055p
- Livshin S, Silverstein MS. Crystallinity and cross-linking in porous polymers synthesized from long side chain monomers through emulsion templating. Macromolecules. 2008;41:3930–3938.10.1021/ma800195w
- Kovačič S, Jerabek K, Krajnc P. Responsive poly(acrylic acid) and poly(N- isopropylacrylamide) monoliths by high internal phase emulsion (HIPE) templating. Macromol. Chem. Phys. 2011;212:2151–2158.
- Williams JM, Wrobleski DA. Spatial distribution of the phases in water-in-oil emulsions: open and closed microcellular foams from cross-linked polystyrene. Langmuir. 1988;4:656–662.10.1021/la00081a027
- Abbasian Z, Moghbeli MR. Open porous emulsion-templated monoliths: effect of the emulsion preparation conditions on the foam microstructure and properties. J. Appl. Polym. Sci. 2010;116:986–994.
- Lin TJ, Shen YF. Low-energy emulsification. Part Vl: applications in high-internal phase emulsions. J. Soc. Cosmet. Chem. 1984;35:357–368.
- Richez A, Deleuze H, Vedrenne P, Collier R. Preparation of ultra-low-density microcellular materials. J. Appl. Polym. Sci. 2005;96:2053–2063.10.1002/(ISSN)1097-4628
- Kunieda H, Solans C, Shida N, Parra JL. The formation of gel-emulsions in a water/nonionic surfactant/oil system. Colloid Surf. 1987;24:225–237.10.1016/0166-6622(87)80352-9