Abstract
Conventional crosslinked polymers and homopolymers both have their own limitations. As a result, core–shell polymer was synthesized to obtain cauliflower-shaped and highly hydroxyl functionalized polymer. For the core, acrylate-based copolymers were synthesized by varying crosslinkers and porogens at different crosslink density. Owing to high surface area (554 m2/g), poly(MMA-co-DVB) was used as a core and low-molecular weight (24,600 g/mol) poly(GMA) was used as a shell in core–shell approach. Average particle sizes of the core polymers were in the range of 15–75 μm. In order to evaluate reactivity efficiency of core–shell polymer, hydroxyl content was evaluated with a value of 3.97 mmol/g. Importantly, hydroxyl content demonstrated the successful increase in reactive sites of the core–shell polymer over conventional crosslinked hydroxyl polymer. Notably, synthesized core–shell polymer has more surface area and pore volume which substantially attributes for better polymer efficiency during application. Scanning electron microscopy images revealed the spherical, uniform, and slightly conglomerated properties of core–shell polymer. Due to higher reactivity, insolubility, and more surface area of hydroxyl functionalized core–shell polymer, its use become inevitably essential.
1. Introduction
Over the last decade, polymers have increasingly found potential use as a solid support in different fields.[Citation1–4] In solid phase synthesis, polymers are extensively used as a support [Citation5] for substrate, reagents, catalysts, enzymes, scavenger, protecting groups, photosensitizers, and so on. Different immobilization techniques including covalent, ionic, adsorption confinement, and entrapment methods [Citation6] are widely used to support the aforementioned compounds. Industrially, the use of polymer as a support in an asymmetric synthesis [Citation7] and chiral resolution [Citation8] became successful. However, smaller surface area [Citation9,10] and less reactivity is the major concern for solid support [Citation11] due to the fact that most of the functional groups are well buried into the polymer matrix and are not available for application purpose. Over the past few years, crosslinked polymer as well as homopolymer were used to support the desired compounds. Unfortunately, less reactivity is the major concern with conventional crosslinked polymer whereas insolubility and tunable surface area are some of the merits. On the other hand, high solubility in organic solvents, lack of strength, and low surface areas are the demerits of the homopolymer while high reactivity contributes to merit. This demonstrated that both types of polymers have their own merits and demerits. To overcome these difficulties of conventional crosslinked and homopolymer, core–shell polymer offers an attractive alternative. In the past, core–shell technology was used in semiconductor nanocrystal [Citation12,13] along with some other areas like polymer and catalysis
Ramli et al. [Citation14] demonstrated the possible composition of core–shell matrix. They concluded that core part may be solid, liquid, or gas whereas shell must be a solid part only. In the present study, we developed the core–shell polymer containing crosslinked polymer as a solid core and homopolymer also as a solid shell which increased the reactive functionality. In 2012, Todorovic et al. [Citation15] synthesized the methyl methacrylate copolymers bearing the surface area in the range of 9–17 m2/g. This low surface area is not effective for modification as a support or any other applications because polymer efficiency attenuates due to small surface area. In contrast, extremely porous polymer looses mechanical strength which creates problem during applications. In the present study, methyl methacrylate core polymer was successfully synthesized bearing high surface area (554 m2/g) using solvating (SOL) porogens by suspension polymerization. Lin et al. [Citation16] used the molecularly imprinted polymer for resolution wherein the effect of particle size on resolution was demonstrated. However, this paper showed the spherical and uniform core polymers having particle size is in the range of 15–75 μm.
In this work, cauliflower-shaped and highly hydroxyl functionalized core–shell polymer was successfully synthesized wherein we introduced the merits and eliminated the demerits of the conventional crosslinked polymers and homopolymers. Influences of physicochemical parameters on the polymer properties were also evaluated. Undoubtedly, core–shell polymer developed in the present work has more reactivity, insolubility, high surface area, porosity, and uniform particle size. To the best of our knowledge, core–shell polymer synthesized in the present work is not reported elsewhere. Undertaken study is not only promises to provide cauliflower-shaped and highly hydroxyl functionalized polymer but also provide high surface area, more porosity, and uniform particle size of polymer which have potential application in solid phase synthesis, biomedical, and pharmaceutical applications.
2. Experimental
2.1. Materials
Methyl methacrylate (99%), 1,1,2,2-tetrachloroethane (98%), 1,2-dichlorobenzene (99%), and 1,4-dioxane (99.9%) were procured from Loba chemie, Mumbai. Ethylene dimethacrylate (98%) and divinylbenzene (85%) were purchased from Sigma-Aldrich. 2,2′-Azobisisobutyronitrile (99%) was obtained from SAS chemicals, Mumbai, India. Glycidyl methacrylate (>97%) and poly(vinyl pyrrolidone) K-90 powder (PVP, mol. wt. 360,000 g/mol) were received from Fluka. Methyl ethyl ketone (>99%) was procured from Merck. All chemicals were used as received.
2.2. Synthesis of hypercrosslinked polymers by suspension polymerization: core polymer (A)
Suspension polymerization [Citation17] was carried out in a specially designed double-walled cylindrical glass reactor equipped with a condenser, nitrogen inlet, thermostat, and overhead stirrer for constant stirring. Oil (discontinuous) phase comprising monomer (methyl methacrylate), crosslinkers (ethylene dimethacrylate/divinylbenzene), initiator (2,2′-azobisisobutyronitrile), and porogens (1,2-dichlorobenzene/1,1,2,2,-tetrachloroethane) was added to the polymerization reactor containing 100 mL of aqueous (continuous) phase comprising 5 wt.% of poly(vinylpyrrolidone) as a protective colloid in distilled water with constant stirring speed of 500 rotations per minute. After complete addition of oil phase to the aqueous phase, reactor temperature was raised to 70 °C and maintained for 3 h to carry out the polymerization. On completion of reaction time, product obtained in the form of beads was cooled, filtered, and washed with water, methanol and dried in oven at 60 °C under reduced pressure for 8 h.
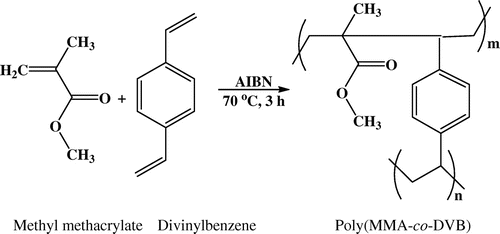
Copolymers obtained by suspension polymerization were further purified by methanol using soxhlet extractor.[Citation18] For this, the polymer beads were packed in a Whatmann paper and placed in the soxhlet apparatus. Approximately, 300 mL of methanol was added in a round bottom flask of the soxhlet apparatus and heated to reflux in order to maintain continuous methanol circulation. After five methanol cycles, copolymer beads were isolated from soxhlet extractor and washed with methanol. Copolymer beads were dried at 60 °C under reduced pressure for 8 h. The yield of the polymer was about 96–97%. Monomer–crosslinker feed composition in the suspension polymerization is depicted in Table . In this work, poly(MMA-co-EDMA) was synthesized using 1,1,2,2-tetrachloroethane/1,2-dichlorobenzene, are abbreviated as MET and MED, respectively. In another, poly(MMA-co-DVB) was synthesized using 1,1,2,2-tetrachloroethane/1,2-dichlorobenzene are abbreviated as MDT and MDD, respectively. Poly(MMA-co-EDMA) and poly(MMA-co-DVB) having differing crosslink density (CLDs) were synthesized as demonstrated in Schemes and , respectively.
Table 1. Monomer–crosslinker feed composition of poly(MMA-co-EDMA) and poly(MMA-co-DVB) at different CLD.
2.3. Synthesis of poly(GMA) by solution polymerization: shell polymer
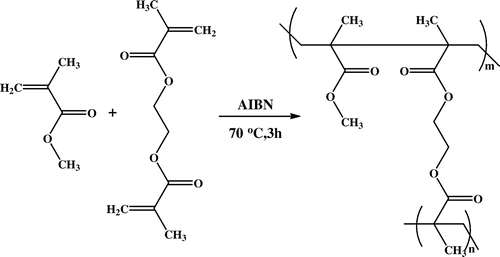
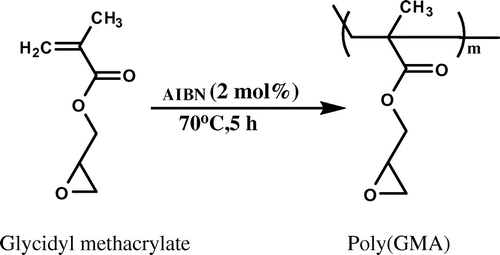
In most of the core–shell approach, shell part is always solid. In this work, poly(GMA) was synthesized by solution polymerization [Citation19] to obtain epoxy-based reactive polymer for application as a shell in core–shell approach. A 250 mL of double-walled cylindrical glass reactor was equipped with a mechanical stirrer and thermostat. To this, glycidyl methacrylate (20 g), AIBN initiator (2 mol%), and methyl ethyl ketone (20 mL) were added. Subsequently, reaction mixture was stirred at 70 °C for 5 h. On completion of reaction time, polymeric solution was precipitated in methanol, filtered, and dried in oven under reduced pressure at 60 °C for 8 h. Molecular weight of the synthesized poly(GMA) was 24,600 g/mol which was determined by gel permeation chromatography. This homopolymer was used as a shell polymer in core–shell approach. Synthesis of poly(GMA) is represented in Scheme .
2.4. Synthesis of core–shell polymer
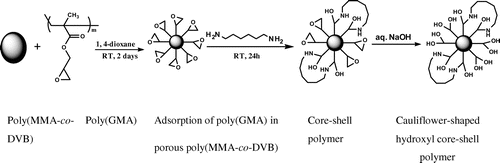
Nowadays, less reactivity is a major concern with crosslinked (insoluble) polymer support which tends to restrict polymer efficiency. On the contrary, homopolymer enhances the reactive sites but is soluble in organic solvents and consequently cannot be used as a solid support. To overcome these difficulties, core–shell is one of the ways which increases the reactive sites, insoluble property as well as strength of the polymer. Core–shell polymer [Citation20] was obtained using high surface area crosslinked polymer as a core which was obtained by suspension polymerization and homopolymer as a shell which was synthesized by solution polymerization. Following steps were used to obtain functional core–shell polymer.
2.4.1. Preparation of 10 wt.% poly(GMA) solution
Poly(GMA) obtained by solution polymerization was used as a shell in core–shell approach. This shell polymer was dissolved in 1,4-dioxane. To a 100 mL of stoppered conical flask, 5 g of poly(GMA) and 50 mL of 1,4-dioxane were added for dissolution. This solution was placed for 3 days to obtain clear polymer solution.
2.4.2. Coating (B) and crosslinking (C) of poly(GMA) onto the surface of hyper-crosslinked core polymer
In general, core–shell approach is useful to improve the properties of the conventional materials.[Citation21] Core–shell polymers have attracted a lot of attention due to their tunable properties to obtain desired polymer.[Citation22,23] The present study was devoted to the synthesis of hydroxyl functionalized core–shell polymer. For this, 20 g of high surface area and hypercrosslinked beaded polymer prepared by suspension polymerization (core polymer) was weighed in a stoppered conical flask to which 40 mL of poly(GMA) in 1,4-dioxane (10 wt.%) was added and placed for 2 days at ambient temperature. Polymer was dried in an oven under reduced pressure at 70 °C for 24 h to obtain crosslinked porous beads-coated poly(GMA). In a further step, this dried polymer (14 g) was treated with 7 mL of 1,6-hexamethylenediamine solution in methanol (1.47 g of 1,6-hexamethylenediamine was dissolved in 100 mL of methanol) and placed for a period of 24 h at ambient temperature to react amino groups with epoxy functionality (5 mol%) available on the surface of core polymer. Subsequently, this core–shell polymer was washed with methanol and dried in an oven under reduced pressure at 60 °C for 8 h. In this work, polymers synthesized by suspension polymerization and screened as a core polymer on the basis of high surface area are labeled as ‘A.’ This core polymer-coated poly(GMA) is labeled as ‘B.’ In further step, core-coated poly(GMA) was crosslinked (5 mol%) with 1,6-hexamethylenediamine (core-shell polymer) and labeled as ‘C.’
2.4.3. Cauliflower-shaped hydroxyl functionalized core–shell polymer
Conventional crosslinked polymers have less reactivity due to most of the functional groups are embedded into the polymer matrix which cannot be used for modification. A main objective of the present work is to synthesize the cauliflower-shaped and highly hydroxyl functionalized core–shell polymer. For this, epoxy-based core–shell polymer (2 g) was further treated with 20 mL (5%) of sodium hydroxide solution in deionized water and mixture was placed at ambient temperature for overnight to obtain highly hydroxyl functionalized core–shell polymer. Subsequently, core–shell polymer was filtered and washed with 20 mL of hydrochloric acid (5%) followed by deionized water till neutral pH. This hydroxyl functionalized core–shell polymer was dried at 80 °C under reduced pressure for 8 h. Synthesis of cauliflower-shaped hydroxyl functionalized core–shell polymer is represented in Scheme .
2.5. Characterization
Polymers purified by the soxhlet extraction and dried under reduced pressure at 60 °C were used for characterization. Fourier transform infrared (FT-IR) spectra were recorded on Perkin Elmer instrument (Model: Spectrum GX and serial number: 69229). Number of scans was 10 with resolution of 4 cm−1 and interval was 1. Surface area of polymers was determined by NOVA 2000e, Quantachrome instruments, Boynton, FL-33426. 13C NMR spectrum of core and core–shell polymer was recorded using adamantane (C10H16) as an internal standard (JEOL–400 MHz). The porous properties were evaluated using Poremaster (Quantachrome) and average particle size was determined by Accusizer 780 (Model: LE 2500-20) PSS.NICOMP Particle sizing system, Santa Barbara, California, USA. Gel permission chromatography was performed in chloroform using Viscotek 200 + RALLS, Model-Viscotek model 250, Column-3X mix B. Surface morphology and particle size visualization were analyzed using scanning electron microscope (Quanta 200 3D, dual beam SEM microscope) wherein the electron source was thermionic emission tungsten filament. Epoxy content was determined by HCl in dioxane whereas hydroxyl content was determined by acetic anhydride in pyridine method, titrimetrically.
3. Results and discussion
Monomer–crosslinker feed composition,[Citation24,25] crosslinker (hydrophilic/hydrophobic) [Citation26,27], and porogen (type/concentration) [Citation28,29] are important parameters in controlling the desired polymer properties such as reactivity, pore volume, pore size, morphology (conglomerated/non-conglomerated), particle size, strength, and stiffness of the beaded polymer. In the present work, methyl methacrylate copolymers were synthesized by suspension polymerization by varying the crosslinkers, porogens, and crosslink densities to obtain high surface area and porous polymer which was used as a core polymer in core–shell approach.
3.1. FT-IR spectroscopy
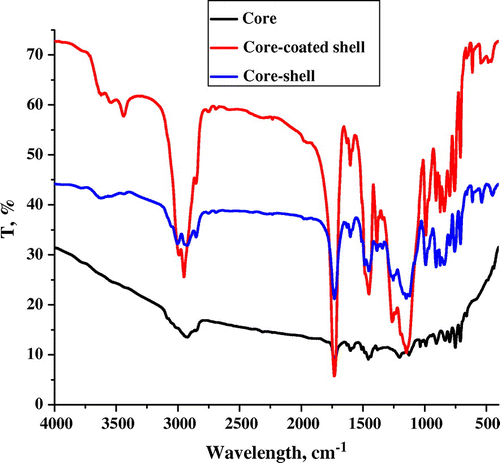
FT-IR spectroscopy is a well-established technique to confirm polymer synthesis (insoluble solid samples) and their modification. FT-IR analysis was carried out using Perkin Elmer (spectrum GX) instrument. FT-IR samples were prepared in the form of KBr pellet after drying the sample at 80 °C under reduced pressure for 8 h. Synthesis of the core polymer (A), core-coated poly(GMA) (B), and core–shell polymer (C) were confirmed by FT-IR spectroscopy (KBr pellet) and depicted in Figure . FT-IR spectrum (cm–1) demonstrated the peak of core polymer (A) at 1731 (–C(O)–C), 797 (aromatic C–H out-of-plane bending), 905 (disubstituted benzene) and 835 (para disubstituted benzene ring). In addition to the core polymer peaks, core-coated poly(GMA) (B) and core–shell (C) polymer revealed the appearance of confirmative peaks. FT-IR spectrum of core-coated poly(GMA) illustrated the appearance of peaks at 907 and 840 (epoxy C–O bonds) while 2990–2854 (C–H sym. and asymm. str. vib.). Moreover, core–shell polymer showed the peak at 3628 and 795 (N–H), 1149 (C–N str.) whereas 907 and 840 (epoxy str.).[Citation30] Presence of these confirmative peaks strongly support to successful synthesis of core–shell polymer.
3.2. 13C solid state NMR spectroscopy
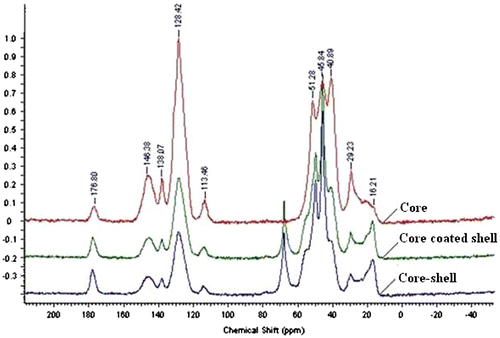
The synthesis of core and core–shell polymer was confirmed by FT-IR spectroscopy. In addition to FT-IR, 13C solid state NMR is also one of the most important tools to confirm polymer (solid/insoluble) synthesis. 13C NMR is widely used for solid (insoluble) polymer sample instead of 1H NMR due to easy interpretation of 13C solid state NMR of insoluble polymer samples, whereas 1H solid state NMR creates complication in data interpretation.[Citation31,32] As a result, 13C NMR was used to confirm core–shell polymer synthesis. In this work, 13C solid state NMR (400 MHz) of core polymer (A), core-coated poly(GMA) (B), and core–shell (C) polymer are represented in Figure which were performed using adamantane (C10H16) as an internal standard [Citation33] containing two carbon peaks (29.23, 40.89 ppm). 13C NMR (C10H16, 400 MHz) of core polymer (A): methyl methacrylate unit has δ 17.9 (–CH3), 51.28 (ester–CH3), 145.83 (tertiary carbon after polymerization), 177.78 (carboxyl), and divinylbenzene unit has 113.46 (ethylene–CH2–), 128.42 (ethylene–CH–), 138.07 (benzene–CH–). In addition to core polymer peak, core–shell polymer demonstrated some confirmative peaks. Core-coated poly(GMA) (B) revealed δ 17.01 (–CH3), 67.77 (–CH2– of epoxy) and core–shell polymer (C) demonstrated δ 16.95 (–CH3), 67.89 (–CH2– of epoxy), whereas peak of –CH2– in hexamethylenediamine is in the range of 26–42 ppm which was overlapped with core polymer peak in the same range. These confirmative peaks in 13C NMR also support to successful core–shell polymer synthesis.
3.3. Surface area determination
Surface area is the most important parameter which attributes to polymer efficiency. Four different series of methyl methacrylate monomer were synthesized using two different crosslinkers (EDMA/DVB) and two different porogens (1,1,2,2-tetrachloroethane/1,2-dichlorobenzene) to obtain high surface area core polymer.
3.3.1. Effect of crosslinkers
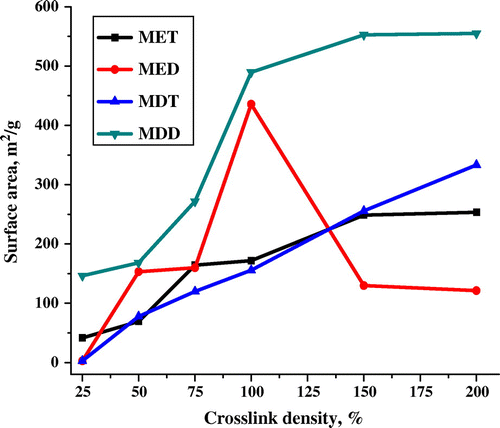
Indeed, number of physicochemical parameters attributes for the variation in surface area.[Citation34] Physical parameters such as stirring speed, reaction time, temperature, and reactor dimension whereas chemical parameter such as crosslinker (hydrophilic/hydrophobic), porogen (solvating/non-solvating), and their concentration significantly controls the surface area. However, crosslinker is the major parameter which attributes to variation in the surface area of polymer. In four polymer series, MET, MED, MDT, and MDD revealed the surface area of 41.5, 2.84, 2.82, and 146.2 m2/g for 25% CLD which were increased to 253.5, 121.2, 333.2, and 554.9 m2/g for 200% CLD, respectively. In the present work, surface area was increased with increase in CLD.[Citation35] Effect of crosslinkers on surface area is illustrated in Figure . Copolymer series of MET, MDT, and MDD revealed the increased surface area with CLD. However, MED series demonstrated the attenuated surface area after 100% CLD. This is possibly due to the polar crosslinker (EDMA) and less polar porogen (1,2-dichlorebenzene) pass a critical ratio which allowed to attenuate the surface area. Interestingly, polymer containing DVB crosslinker displayed the higher surface area than polymer containing EDMA crosslinker. Both, MDT and MDD series have better surface area than MET and MED series due to comparatively hydrophilic property of EDMA and has more affinity toward aqueous phase while DVB has more affinity toward organic phase. This is mainly due to the solubility of ethylene dimethacrylate in water (1.086 g/L) is much higher than divinylbenzene (0.005 g/L) at 20 °C. This causes the compatibility of DVB with organic phase resulting high surface area polymer whereas EDMA causes compatibility with aqueous phase resulting low surface area polymer. Nevertheless, this phenomenon creates the interfacial tension between aqueous and organic phase in a reaction mixture.[Citation36,37] Notably, poly(MMA-co-DVB) demonstrated the highest surface area of 554 m2/g at 200% CLD. As a result, this polymer was used as a core polymer in core–shell approach.
3.3.2. Effect of porogens
Porogen is one of the most crucial factors which substantially influence the surface area of beaded polymer synthesized by suspension polymerization. In the past, three types of porogens were used to generate the surface area into the beaded polymer i.e. solvating, non-solvating, and polymeric (POLY) porogen.[Citation38] Each porogen has own limitations. In this study, SOL porogens were used which are well known for generating the high surface area into the polymer beads. Recently, Clarisse et al. [Citation39] reported the surface area of poly(MMA-co-DVB) using a mixture of porogens. However, present work reported the much higher surface area using a single porogen instead of porogen mixture. Effect of porogens on surface area is illustrated in Figure wherein poly(MMA-co-DVB) revealed the high surface area with 1,2-dichlorobenzene than 1,1,2,2-tetrachloroethane. In contrast, poly(MMA-co-EDMA) revealed the maximum surface area with 1,1,2,2–tetrachloroethane than 1,2–dichlorobenzene. This is mainly due to the compatibility of monomer system with porogen as aforementioned in crosslinker effect. In case of poly(MMA-co-DVB), maximum surface area was observed in MDD series (554 m2/g), while MDT series demonstrated the surface area of 333 m2/g at 200% CLD. In case of poly(MMA-co-EDMA), MET series displayed the higher surface area of 253 m2/g whereas MED series revealed 121 m2/g at 200% CLD.
3.3.3. Effect of core polymer modification
In 2004, Jal et al. [Citation40] demonstrated that surface area of solid adsorbent was decreased after modification. They showed that surface area of unmodified silica (420.8 m2/g) was decreased after covalent modification (154.8 m2/g) with 1,3-propyldiamine. In this work, four series of core polymer were synthesized and characterized to evaluate surface area. Poly(MMA-co-DVB) having highest surface area of 554 m2/g at 200% CLD was screened as a core polymer in core–shell approach. Polymer surface area after poly(GMA) coating onto the surface of core polymer was 410 m2/g. Surface area was decreased from 554 to 410 m2/g due to poly(GMA) coating. In further step, surface area was again attenuated [Citation41] from 410 to 332 m2/g mainly due to 5 mol% crosslinking of the epoxy functionality using 1,6-hexamethylenediamine. Owing to high surface area (332 m2/g), core–shell polymer recognized as a versatile polymer.
3.4. Porosity determination
Pore volume is a measure of fraction of the volume of voids over the total volume of polymer matrix. Generally, interfacial tension formed between aqueous and organic phase attributes to polymer properties.[Citation42] Porogen is an important factor in controlling the polymer properties such as pore volume, pore size, and porosity.[Citation43] Porosity is one of the most crucial property of core polymer which significantly influences to shell immobilization in core–shell approach. Pore volume and porosity of core polymer synthesized by suspension polymerization at 25 and 200% CLD are illustrated in Table . Result illustrated that the high CLD polymers have more pore volume as well as porosity. Interestingly, pore volume revealed the similar observation like surface area result. In four polymer series, MET, MED, MDT, and MDD revealed the pore volume of 0.75, 0.75, 0.84, and 1.44 cc/g for 200% CLD, respectively. Notably, highest pore volume was observed for MDD series followed by MDT, MED, and MET series. This is because of crosslinker (DVB) and porogen (1,2-dichlorobenzene) is comparatively less hydrophilic and more affinity toward organic phase which allowed increasing pore volume resulting higher porosity in MDD series. However, more hydrophilicity of crosslinker (EDMA) and porogen (1,1,2,2-tetrachloroethane) attenuated the pore volume as well as porosity due to more affinity toward the aqueous phase. Owing to much higher surface area (554 m2/g), pore volume (1.44 cc/g) and porosity (64.85%) of MDD polymer at 200% CLD was selected as a core polymer. This is due to the fact that high surface area and porosity of the core polymer substantially attribute to shell polymer coating on core during core–shell polymer synthesis. In addition, core–shell polymer revealed the higher pore volume (1.06 cc/g) and porosity (41.99%) which illustrated the transcendent porous properties of core–shell polymer matrix.
Table 2. Pore volume (cc/g) and porosity (%) of poly(MMA-co-EDMA) and poly (MMA-co-DVB) at different CLD.
3.5. Particle size distribution
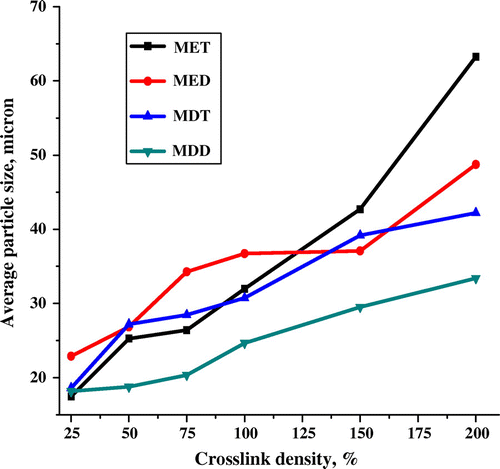
Average particle size is an important from application point of view. Recently, nano and micron sized particles have increased attention due to their application in solid phase synthesis,[Citation44] solid phase extraction, and biomedical applications.[Citation45] However, nano and micron sized polymer has their own merits and demerits. Nanoparticles are associated with large surface area and conglomerated property.[Citation46] On the other hand, micron particle are associated with comparatively small surface area and nonconglomerated property.[Citation47] Present study was interested in the synthesis of microbeads using suspension polymerization. Generally, suspension polymerization is well known to synthesize the beaded polymers having particle size is in the range of 5–200 μm. Various parameters including crosslinker, CLD, and porogen significantly affect the particle size. Indeed, SOL porogen is able to impart small-sized particles compared to NONSOL porogen. In the present study, 1,1,2,2-tetrachloroethane and 1,2-dichlorobenzene was used as a SOL porogens. Average particle size of poly(MMA-co-EDMA) and poly(MMA-co-DVB) synthesized at 70 °C for 3 h was studied as a function of CLD, crosslinker, and porogen. Effect of these parameters on the particle size is represented in Figure which demonstrated that particle sizes were slightly increased with increase in CLD [Citation48,49]. In four polymer series, MET, MED, MDT, and MDD revealed the average particle size of 17.45, 22.8, 18.60, and 18.16 μm at 25% CLD which were increased to 63.27, 48.73, 42.21, and 33.39 μm at 200% CLD, respectively. Remarkably, average particle sizes of the polymers synthesized by suspension polymerization were in the range of 15–70 μm. However, there is small difference in average particle size with respect to crosslinkers and porogens. Both, poly(MMA-co-EDMA) and poly(MMA-co-DVB) series displayed the larger particle size with 1,2-dichlorobenzene than 1,1,2,2-tetrachloroethane porogen. It must pointed out that divinylbenzene (DVB) containing polymer revealed the larger average particle size with same monomer (methyl methacrylate) and porogen (1,1,2,2-tetrachloroethane/1,2-dichlorobenzene) than polymer containing ethylene dimethacrylate (EDMA) crosslinker. This difference in average particle size distribution is due to more compatibility of EDMA as a crosslinker with 1,1,2,2-tetrachloroethane than 1,2-dichlorobenzene resulting smaller particle size in MET series and larger particle size in MED series. On the other hand, DVB is more compatible with 1,2-dichlorobenzene than 1,1,2,2-tetrachloroethane resulting smaller particles size in MDD series and larger particle size in MDT series. Overall, poly(MMA-co-EDMA) displayed the larger average particle size than poly(MMA-co-DVB) due to compatibility of monomer system with aqueous and organic phase, respectively.
3.6. Titrimetric determination of epoxy and hydroxyl content
In case of reactive polymer, quantitative determination of functional group content is essential to evaluate the polymer efficiency. The quantitative determination of epoxy functionality was performed by titrimetric method. Titrimetric method is well known and a reliable method for epoxy and hydroxyl content determination of the beaded polymer. Hydroxyl content of core–shell polymer determines the reactivity, eventually polymer efficiency. Core polymer-coated poly(GMA) was dried at 80 °C for 8 h and this dried polymer was used for quantitative determination of epoxy content. In this work, epoxy content [Citation50] of core-coated poly(GMA) before crosslinking was analyzed titrimetrically using HCl-dioxane method. The evaluated epoxy content was 2.48 mmol/g. Subsequently, this poly(GMA) was crosslinked (5 mol%) using 1,6-hexamethylenediamine to obtain core–shell polymer. Epoxy content of this core–shell polymer was evaluated with a value of 2.35 mmol/g. Thereafter, epoxy functionalized core–shell polymer was treated with sodium hydroxide to obtain cauliflower-shaped and highly hydroxyl functionalized core–shell polymer. This hydroxyl functionalized core–shell polymer was washed, dried at 80 °C for 8 h, and used for quantitative determination of hydroxyl content by acetic anhydride in pyridine method,[Citation50] titrimetrically. It is noteworthy that evaluated hydroxyl content was 3.97 mmol/g. Undoubtedly, result demonstrated the high hydroxyl content than conventional crosslinked hydroxyl polymer [Citation47] wherein the large number of functionality is well buried into the polymer matrix and is not available for application purpose. Thus, cauliflower-shaped highly hydroxyl functionalized core–shell polymer was obtained.
3.7. External morphology
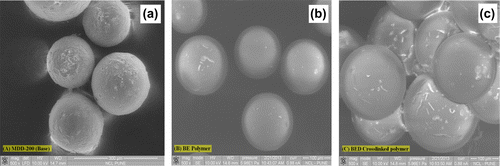
Scanning electron microscopy (SEM) images are a visual observation tool which confirms the size, shape, and surface morphology of the beaded polymer. In general, polymer synthesized by SOL porogens imparts high surface area, spherical, and nonconglomerated beads inversely polymer synthesized by NONSOL porogens have the property to impart nonspherical and conglomerated properties to the polymer beads.[Citation15,51] However, SOL porogens were used in the present work which demonstrated the similar observation. SEM images were scanned under 500× magnifications. SEM images of core polymer, core-coated poly(GMA), and core–shell polymer are represented in Figure (a)–(c), respectively. Figure (a) showed the SEM image of poly(MMA-co-DVB) core polymer prepared by suspension polymerization. SEM clearly illustrated that spherical, uniform, and quite smooth properties of the beaded polymers.[Citation52] Moreover, non-agglomeration and unique identity of beaded polymer was observed. Figure (b) revealed the SEM image of core polymer-coated poly(GMA) which demonstrated that the surface of core is coated with thin layer of poly(GMA) in the form of ring. Figure (c) illustrated, the SEM image of core–shell polymer. Owing to chemical crosslinking, polymer beads were agglomerated and coupled together. Interestingly, this morphology is obviously different than Figure (a) and (b) due to crosslinking with 1,6-hexamethylenediamine.
4. Conclusions
In conclusion, cauliflower-shaped hydroxyl functionalized core–shell polymer was successfully synthesized bearing high surface area and porous properties. Cauliflower-shaped polymer was increased the reactive sites resulting in more efficient polymer and consequently can be recognized as a versatile polymer support. Notably, core polymer was obtained bearing high surface area (554 m2/g) which substantially attributes to more coating of shell polymer onto the surface of core polymer. In spite of insoluble, more reactive, uniform, spherical and high surface area, the evaluated epoxy content of core–shell polymer was 2.35 mmol/g. In addition, evaluated hydroxyl content of core–shell polymer was 3.97 mmol/g. Indeed, hydroxyl content was strongly support to increased reactive sites of the core–shell polymer over conventional crosslinked polymer. Core–shell polymer revealed the high surface area (332 m2/g), pore volume (1.06 cc/g), and porosity (41.99%) which attributes to higher polymer efficiency. Overall, synthesized core–shell polymer has more reactivity, high surface area, and porosity. SEM images demonstrated the spherical nature of core–shell polymer with slight conglomeration. In still another aspect, reactive core–shell polymer attributes to more loading of catalyst, reagent substrate, photosensitizer, resolving agent, and chelating agent for solid phase synthesis and extraction. Thus, more reactive, high surface area, and excellent porosity of core–shell polymer was obtained. Owing to recover, recycle and reuse properties of core–shell polymer, its use offer economical and environmentally benign feature.
| Abbreviations | ||
| A (Core) | = | methyl methacrylate – divinylbenzene – 1,2-dichlorobenzene at 200% CLD |
| B | = | coating of poly(GMA) on the surface of core |
| C (core-shell polymer) | = | 5% epoxy functionality crosslinking of poly(GMA) coated on core using 1,6-hexamethylenediamine |
| CLD | = | crosslink density |
Funding
This work was financially supported by University Grant Commission (UGC) [20/06-2010(i) EU-IV].
Acknowledgement
This research was financially supported by University Grant Commission (UGC), New Delhi, India. We express our gratitude towards UGC for the award of research fellowship (Fellowship Award No. 20/06-2010(i) EU-IV).
Disclosure statement
The authors declare no conflict of interest.
References
- Guillier F, Orain D, Bradley M. Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem. Rev. 2000;100:2091–2158.10.1021/cr980040+
- Ley SV, Baxendale IR, Brusotti G, Caldarelli M, Massi A, Nesi M. Solid-supported reagents for multi-step organic synthesis: preparation and application. Il Farmaco. 2002;57:321–330.10.1016/S0014-827X(02)01210-7
- Malachowski L, Stair JL, Holcombe JA. Immobilized peptides/amino acids on solid supports for metal remediation. Pure Appl. Chem. 2004;76:777–787.
- Clapham B, Reger TS, Janda KM. Polymer-supported catalysis in synthetic organic chemistry. Tetrahedron. 2001;57:4637–4662.10.1016/S0040-4020(01)00298-8
- Kim BM, Sharpless KB. Heterogeneous catalytic asymmetric dihydroxylation: use of a polymer-bound alkaloid. Tetrahedron Lett. 1990;31:3003–3006.
- Oehme I, Prattes S, Wolfbeis OS, Mohr GJ. The effect of polymeric supports and methods of immobilization on the performance of an optical copper(II)-sensitive membrane based on the colourimetric reagent Zincon. Talanta. 1998;47:595–604.10.1016/S0039-9140(98)00084-8
- Baker GL, Fritschel SC, Stille JR, Stille JK. Transition-metal-catalyzed asymmetric organic synthesis via polymer-attached optically active phosphine ligands. 5. Preparation of amino acids in high optical yield via catalytic hydrogenation. J. Org. Chem. 1981;46:2954–2960.10.1021/jo00327a023
- Gübitz G, Schmid MG. Recent advances in chiral separation principles in capillary electrophoresis and capillary electrochromatography. Electrophoresis. 2004;25:3981–3996.10.1002/(ISSN)1522-2683
- Xu J, Geng H, Sun J, Ding L. Preparation and characterization of nanoporous nickel using PS bead as physical template. Rev. Adv. Mater. Sci. 2013;33:61–64.
- Demircelik AH, Andac M, Andac CA, Say R, Denizli A. Molecular recognition-based detoxification of aluminum in human plasma. J. Biomater. Sci. 2009;20:1235–1258.10.1163/156856209X452971
- Zhang Y, Xiang S, Wang G, Jiang H, Xiong C. Preparation and application of coconut shell activated carbon immobilized palladium complexes. Catal. Sci. Technol. 2014;4:1055–1063.10.1039/c3cy00460k
- Reiss P, Protière M, Li L. Core/shell semiconductor nanocrystals. Small. 2009;5:154–168.10.1002/smll.200800841
- Ivanov SA, Piryatinski A, Nanda J, et al. Type-II core/shell CdS/ZnSe nanocrystals: synthesis, electronic structures, and spectroscopic properties. J. Am. Chem. Soc. 2007;129:11708–11719.10.1021/ja068351m
- Ramli RA, Laftah WA, Hashim S. Core–shell polymers: a review. RSC Adv. 2013;3:15543–15565.10.1039/c3ra41296b
- Todorovic ZS, Nikolic LB, Nikolic VD, Vukovic ZM, Mladenovic-Ranisavljevic II, Takic LM. Cross-linked macroporous polymers and copolymers, their synthesis. Adv. Technol. 2012;1:11–19.
- Lin JM, Nakagama T, Uchiyama K, Hobo T. Molecularly imprinted polymer as chiral selector for enantioseparation of amino acids by capillary gel electrophoresis. Chromatographia. 1996;43:585–591.10.1007/BF02292972
- Lu L, Jiang C, Xiufang W, Pihui P, Zhuoru Y. Synthesis and characterization of suspension polymerized styrene-divinylbenzene porous microsphere using as slow-release-active carrier. Chin. J. Chem. Eng. 2006;14:471–477.
- Paukkeri R, Lehtinen A. Fractionation of polypropylenes using soxhlet extraction methods. Polymer. 1994;35:673–679.
- Ravve A, Khamis JT. Solution polymerization of styrene. J. Macromol. Sci. Chem. 1967;Al:1423–1431.
- Jin L, Deng Y, Hu J, Wang C. Preparation and characterization of core-shell polymer particles with protonizable shells prepared by oxyanionic polymerization. J. Polym. Sci., Part A: Polym. Chem. 2004;42:6081–6088.10.1002/(ISSN)1099-0518
- Aguiar A, González-Villegas S, Rabelero M, Mendizábal E, Puig JE. Core−shell polymers with improved mechanical properties prepared by microemulsion polymerization. Macromolecules. 1999;32:6767–6771.10.1021/ma981703s
- Sun J, Zhang L, Wang J, et al. Tunable rigidity of (polymeric core)–(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2014;27:1402–1407.
- Gan D, Lyon LA. Tunable swelling kinetics in core − shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001;123:7511–7517.10.1021/ja010609f
- Horák D, Pelzbauer Z, Bleha M, Ilavský M, Švec F, Kálal J. Reactive polymers: XXXII effect of composition of polymerization feed on morphology and some physical properties of macroporous suspension copolymers glycidyl methacrylate–ethylene dimethacrylate. J. Appl. Polym. Sci. 1981;26:411–421.10.1002/app.1981.070260203
- Hsieh TT, Hsieh KH, Simon GP, Tiu C, Hsu HP. Effect of crosslinking density on the physical properties of interpenetrating polymer networks of polyurethane and 2-hydroxyethyl methacrylate-teminated polyurethane. J. Polym. Res. 1998;5:153–162.10.1007/s10965-006-0051-x
- Lin S, Gu L. Influence of crosslink density and stiffness on mechanical properties of Type I collagen gel. Materials. 2015;8:551–560.10.3390/ma8020551
- Clayton AB, Chirila TV, Dalton PD. Hydrophilic sponges based on 2-hydroxyethyl methacrylate. III. Effect of incorporating a hydrophilic crosslinking agent on the equilibrium water content and pore structure. Polym. Int. 1997;42:45–56.10.1002/(ISSN)1097-0126
- Lu L, Jiang C, Xiufang W, Pihui P, Zhuoru Y. Synthesis and characterization of suspension polymerized styrene-divinylbenzene porous microsphere using as slow-release-active carrier. Chin. J. Chem. Eng. 2006;14:471–477.
- Hao DX, Gong FL, Wei W, Hu GH, Ma GH, Su ZS. Porogen effects in synthesis of uniform micrometer-sized poly(divinylbenzene) microspheres with high surface areas. J. Colloid Interface Sci. 2008;323:52–59.10.1016/j.jcis.2008.03.039
- Safa KD, Bahadori A, Tofangdarzadeh S, Nasirtabrizi MH. Trisyl modification of epoxy- and chloromethyl-polysiloxanes. J. Iran. Chem. Soc. 2008;5:37–47.10.1007/BF03245813
- Lubach JW, Padden BE, Winslow SL, et al. Solid-state NMR studies of pharmaceutical solids in polymer matrices. Anal. Bioanal. Chem. 2004;378:1504–1510.10.1007/s00216-003-2381-4
- Ali M, Apperley DC, Eley CD, Emsley AM, Harris RK. A solid-state NMR study of cellulose degradation. Cellulose. 1996;3:77–90.10.1007/BF02228792
- Morcombe CR, Zilm KW. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003;162:479–486.10.1016/S1090-7807(03)00082-X
- Barral S, Guerreiro A, Villa-García MA, Rendueles M, Díaz M, Piletsky S. Synthesis of 2-(diethylamino)ethyl methacrylate-based polymers. React. Funct. Polym. 2010;70:890–899.10.1016/j.reactfunctpolym.2010.08.003
- Kotha A, Raman RC., Ponrathnam S, Kumar K, Shewale JG. Beaded reactive polymers 3 effect of triacrylates as crosslinkers on the physical properties of glycidyl methacrylate copolymers and immobilization of penicillin G acylase. Appl. Biochem. Biotechnol. 1998;74:191–203.10.1007/BF02825965
- Okay O. Macroporous copolymer networks. Prog. Polym. Sci. 2000;25:711–779.10.1016/S0079-6700(00)00015-0
- Donahue DJ, Bartell FE. The boundary tension at water-organic liquid interfaces. J. Phys. Chem. 1952;56:480–484.10.1021/j150496a016
- Seidl J, Malinský J, Dušek K, Heitz W. Macroporous styrene-divinylbenzene copolymers and their application in chromatography and for the preparation of ion exchange resins. Adv. Polym. Sci. 1967;5/2:113–213.10.1007/BFb0051280
- Clarisse M, Queiros Y, Barbosa C, Barbosa L, Lucas E. Synthesis and characterization of polymeric resins based on methyl methacrylate and divinylbenzene. Chem. Chem. Technol. 2012;6:145–152.
- Jal PK, Patel S, Mishra BK. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta. 2004;62:1005–1028.10.1016/j.talanta.2003.10.028
- Norzilah AH, Fakhru'l-Razi A, Choong TSY, Chuah AL. Surface modification effects on CNTs adsorption of methylene blue and phenol. J. Nanomater. 2011;2011:1–18.10.1155/2011/495676
- Mayoral E, Achar EN. Study of interfacial tension between organic solvent and aqueous electrolyte solutions using electrostatic dissipative particle dynamics simulations. J. Chem. Phys. 2012;137:1–11.
- Mohamed MH, Wilson LD. Porous copolymer resins: tuning pore structure and surface area with non reactive porogens. Nanomaterials. 2012;2:163–186.10.3390/nano2020163
- Nahakpam L, Chingakham BS, Laitonjam WS. Polymer-supported tribromide as a new solid phase and recyclable catalyst for the synthesis of 2-( N -Arylamino)benzothiazoles under solvent-free microwave irradiation conditions. J. Heterocycl. Chem. 2015;52:267–272.10.1002/jhet.v52.1
- Mane S, Ponrathnam S, Chavan N. Hyperhydrophilic three-dimensional crosslinked beads as an effective drug carrier in acidic medium: adsorption isotherm and kinetics appraisal. New J. Chem. 2015;39:3835–3844.10.1039/C4NJ01999G
- Yao W, Guangsheng G, Fei W, Jun W. Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol. 2002;124:152–159.10.1016/S0032-5910(01)00491-0
- Mane S, Ponrathnam S, Chavan N. Synthesis and characterization of hypercrosslinked hydroxyl functionalized co-polymer beads. Eur. Polym. J. 2014;59:46–58.10.1016/j.eurpolymj.2014.07.001
- Gooch JW. Emulsification and polymerization of alkyd resins. Springer: New York (NY). 2002;XXII:28.
- Gong T, Wang CC. Preparation of highly cross-linked monodispersed functional polystyrene particles by utilizing the delayed addition method. J. Mater. Sci. 2008;43:1926–1932.10.1007/s10853-007-2441-9
- Vogel AI. Elementary practical organic chemistry part III quantitative organic analysis. CRC press: London 1958;XXXVIII.2:825.
- Yu S, Ng FL, Ma KCC, Mon AA, Ng FL, Ng YY. Effect of porogenic solvent on the porous properties of polymer monoliths. J. Appl. Polym. Sci. 2013;127:2641–2647.10.1002/app.37514
- Hwang ML, Lee YS, Kim TG, Yang GG, Park TS, Lee YS. Synthesis of hydroxylated macroporous polymer beads for microwave-assisted desorption of nonpolar volatile organic compounds. Bull. Korean Chem. Soc. 2010;31:2395–2398.10.5012/bkcs.2010.31.8.2395