Abstract
A sulfone ether diamine was synthesized by three-step reactions. Firstly, 4-(4-nitrophenoxy) phenol was synthesized via nucleophilic substitution reaction of 1-fluoro-4-nitrobenzene with hydroquinone in the presence of K2CO3 in NMP. In the second step, 4-(4-nitrophenoxy) phenol was reduced to 4-(4-aminophenoxy) phenol by Pd/activated carbon catalyst in ethanolic hydrazine hydrate. Finally, 4-(4-aminophenoxy) phenol was reacted with 4,4′-dichlorodiphenyl sulfone (with 2:1 M ratio) to produce the sulfone ether diamine. Then three different kinds of poly(sulfone ether imide)s were prepared by solution polycondensation of synthesized diamine with commercially available aromatic dianhydrides including pyromellitic dianhydride, benzophenonetetracarboxylic dianhydride, and hexafluoroisopropylidene diphthalic anhydride. The structure of synthesized compounds, monomers, and prepared polymers was characterized using 1H NMR and FT-IR spectroscopy and also elemental analysis method. Thermal behavior and stability, solubility, solution viscosity, mechanical properties, crystallinity, water uptake, and optical properties of samples were studied.
1. Introduction
Polyimides are the most prominent group of all high-performance polymers.[Citation1,2] Aromatic polyimides have been extensively considered as engineering plastics because of their unique heat resistance and special physical and mechanical properties.[Citation3–6] Additionally aromatic ring in the polymer chain structure affords packing, crystallinity, melt viscosity, high thermo-oxidative stability, and high glass transition temperature. Therefore, due to their superior thermal, physical, mechanical, and electrical properties together with flame retardancy and oxidative stability they are widely used in complex, high-tech industries such as electronic and microelectronic, aerospace, fire resistant, and insulating materials, and so on.[Citation3,7–10]
In spite of mentioned advantages, using aromatic polyimides is limited because of some drawbacks such as insolubility in common solvents and infusibility in typical processing conditions.[Citation11–13] These disadvantages arise from their dipole–dipole intermolecular interactions and stiffness of aromatic rings. Therefore, serious efforts have been focused on finding ways for solving this problem. Chemical modification on the structure of polyimide backbone is the best strategy for synthesis of processable aromatic polyimides. Among various designs, the most important approach is incorporation of flexible linkages into the backbone to reduce molecular stiffness of chains.[Citation13–15] Therefore, polyimide copolymers have been synthesized to establish a balance between processing features and mechanical properties.[Citation16,17] Incorporation of flexible units including ether and sulfone is one of the most effective approaches to improve polyimide processability.[Citation18,19] The polymers containing both sulfone and ether groups have low glass transition, but show excellent mechanical properties and substantially improved solubility.[Citation18,20] So, presence of ether and sulfone groups lead to decreased rigidity and improved flow ability, allowing poly(sulfone ether imide)s to become one of the most suitable high-temperature resistant materials in such a way that processable and soluble aromatic polyimides having ether and sulfone linkage in their chemical structure have been developed for many applications including gas separation membrane.[Citation18,21,22]
The purpose of the present research is synthesis of a novel diamine with incorporation of ether and sulfone linkages into the structure in order to increase solubility and improve processability without decreasing thermal stability of final polymers. In this way, three novel polyimides were prepared from polycondensation reaction of the diamine with three different dianhydrides including pyromellitic dianhydride (PMDA), benzophenonetetracarboxylic dianhydride (BTDA), and hexafluoroisopropylidene diphthalic anhydride (6FDA).
2. Experimental
2.1. Materials
All chemicals were purchased either from Aldrich or Merck chemical Co. K2CO3 was vacuum-dried at 120 °C for 5 h. Hydroquinone and bis-(4-chlorophenyl) sulfone were recrystallized from ethanol. N-Methyl-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMAc) were dried by distillation under reduced pressure over calcium hydride. Toluene was distilled over sodium. Pyromellitic dianhydride (PMDA), benzophenonetetracarboxylic dianhydride (BTDA), and hexafluoroisopropylidene diphthalic anhydride (6FDA) were recrystallized from acetic anhydride and were dried in a vacuum oven at 120 °C overnight.
2.2. Instrumental
2.2.1. Spectroscopy and elemental analysis
1H NMR spectra were recorded on a Bruker Avance DPX 500-MHz instrument using dimethyl sulfoxide-d6 as solvent, and tetramethylsilane as the internal reference. Infrared measurements were performed on a Bruker-IFS 48 FT-IR spectrometer using potassium bromide pellet. Elemental analysis was run on a CHN-O rapid Heraeous analyzer.
2.2.2. Thermogravimetric analysis (TGA)
TGA was performed on a PL-1500 in air atmosphere at the temperature range of 25–750 °C and the data were collected at a heating rate of 10 °C/min.
2.2.3. Dynamic mechanical thermal analysis (DMTA)
DMTA was carried out on a polymer laboratories PL-TMA model using a thin film (20 mm length, 10 mm width, and 0.04–0.06 mm thickness) over a temperature range of 25–300 °C at a heating rate of 5 °C/min and a load frequency of 1 Hz in air.
2.2.4. Inherent viscosity
Inherent viscosities were measured using an Ubbelohde viscometer. It was measured at a concentration of 0.5 g/dL in NMP at 30 °C.
2.2.5. Mechanical analysis
Tensile test was conducted at room temperature for thin rectangular films with dimensions of 30 mm length, 10 mm width, and 0.05 mm thick, with a clamp distance of 20 mm using a mechanical (Universal) testing machine, SANTAM, STM-20, at a cross-head speed of 5 mm/min.
2.2.6. Water absorption
Water absorption values of poly(sulfone ether imide) films were recorded by weighting immersed sample in distilled water for 24 h at room temperature. The percentage gain after a measurement was calculated by the following equation:
Water absorption = [(W − W0)/W0] × 100%; where W0 and W denote weights of dry and immersed samples, respectively.
2.2.7. Wide-angle X-ray diffraction (WAXD)
WAXD spectra were collected in the range of 2θ = 5–70° with a scanning rate of 1.2°/min on a Siemens D-5000 diffractometer operated by a radiation source of Fe Kα at 25 mA and 35 kV.
2.2.8. Solubility
By immersing 0.30 g of each polymer specimen in 10 mL solution of different polar solvents in a test tube at room temperature, the solubility behavior of tree types of polymer films was studied. The temperature was raised to the range of 60–100 °C, where needed to achieve complete potential solubility.
2.2.9. UV–visible spectroscopy
The optical transparency of polymer films with average thickness of about 30 µm was evaluated on a SHIMADZU (UV-160) ultraviolet-visible spectrophotometer in the wavelength range of 200–800 nm at room temperature. To prevent interference of adsorbed moisture on final transparency results of films, samples were dried at 100 °C for 1 h before measurement. Light transmittance (%) values at the wavelength of 600 nm (T600) were considered as a criterion of coloration intensity. Cut-off wavelength (the wavelength at which light transmittance decreases to 0%) was also determined.
2.3. Preparation of monomers
2.3.1. Synthesis of 4-(4-nitrophenoxy) phenol (NPP) [Citation23]
Into a two-necked round-bottomed flask equipped with a dean-stark trap, a condenser and a nitrogen inlet tube and a magnetic stirrer, 1.439 g (0.01 mol) of 4-fluoronitrobenzene, 1.11 g (0.01 mol) of hydroquinone,1.52 g (0.01 mol) of K2Co3, 20 mL of dry NMP and 15 mL of dry toluene was placed. The reaction mixture was heated to 140 °C for 6 h and to 165 °C for 20 h with continuous stirring. After cooling, the resulting reaction mixture was cooled and poured into 200 mL of distillated water at room temperature under continuous stirring. Then the mixture was filtered and the obtained product was dried at 70–80 °C for 15 h.
2.3.2. Synthesis of 4-(4-aminophenoxy) phenol (APP) [Citation23]
2.3 g of synthesized NPP was mixed with 0.15 g of 10% Pd/C as a catalyst and 80 mL of absolute ethanol in a 250 mL, two-necked flask equipped with a magnetic stirrer, condenser, and a dropping funnel. After the reaction temperature reached to the 80 °C, 20 mL of ethanolic hydrazine hydrate was gently dropped into the reaction mixture during 1 h. The reaction was then continued for 4 h under reflux condition and then 20 mL of THF was added to the mixture until the precipitate completely dissolved and reaction continued for another 17 h. Then the solution was filtered to remove all the Pd/C, and the concentrated filtrate was poured into 200 mL of distilled water to precipitate the product. The product was then filtered, washed repeatedly with hot water and dried.
2.3.3. Synthesis of diamine (DA)
About 1.64 g (0.008 mol) of synthesized APP was reacted with 1.15 g (0.0038 mol) of 4,4-dichlorophenyl sulfone in 12 mL of dry NMP and 10 mL of dry toluene into a two-necked flask equipped with a magnetic stirrer, condenser, dean-stark trap, and a nitrogen inlet tube. Then 1.7 g (0.32 mol) of K2CO3 was added to the mixture and the reaction mixture was heated to 140 °C with continues stirring. The generated water was removed from the reaction by azeotropic distillation. The reaction temperature was increased to 160 °C and continued for 20 h in order to remove the remained water and toluene. The resulting reaction was cooled and poured into 200 mL of water, then 20 mL of 5% NaOH was added to the mixture and filtered. The mixture was washed repeatedly with 5% NaOH solution and water. The obtained diamine was dried in a vacuum oven at 70 °C for 24 h.
2.4. Polymer synthesis
2.4.1. Poly(amic acid) synthesis
The synthesized diamine (1.5556 g, 2.5 mmol) and DMAC (10 mL) were placed into a 100 mL, two-necked round-bottomed flask equipped with nitrogen inlet tube and a magnetic stirrer. The reaction mixture was stirred at room temperature until diamine was fully dissolved and then it was cooled in an ice bath for 30 min. After that, 2.5 mmol of dianhydride was added into the flask and the mixture was stirred at 0 °C for 1 h. Then the reaction mixture was continuously stirred at room temperature for 24 h. The viscous resulting polyamic acid solution was precipitated by pouring the flask content into 200 mL of distilled water. The precipitated poly(amic acid) was filtered, washed with water, and dried overnight in a vacuum oven at 50 °C (yields over 98%).
2.4.2. Preparation of poly(sulfone ether imide) film
Polyimide film was obtained as follows: 1.0 g of poly(amic acid) and 12.2 mL of DMAC were placed into a 100 mL, two-necked, round-bottomed flask equipped with a magnetic stirrer, nitrogen gas inlet tube and a reflux condenser. Then the mixture was heated at 50 °C and continuously stirred for 24 h. The PAA solution was cast into glass petri dish and thermal cyclo-dehydration imidization was carried out as follows: heating for 4 h at 60 °C, 1 h at 120 °C, 1 h at 160 °C, 1 h at 200 °C, and finally 1.5 h at 300 °C. Polyimide film was separated by immersing the glass petri dish into a water bath.
3. Results and discussion
The main purpose of this work was based on the synthesis of novel poly(sulfone ether imide)s with improved solubility and thermal stability. In this way, a novel sulfone ether diamine was prepared through a three-step synthetic route. At first, 4-(4-nitrophenoxy) phenol was produced from nucleophilic substitution reaction of 1-fluoro-4-nitrobenzene with hydroquinone in the presence of K2CO3 in NMP. In the second step, NO2 group was reduced to NH2 group using hydrazine hydrate and Pd/activated carbon catalyst and therefore 4-(4-aminophenoxy) phenol was synthesized. In the final step, 4-(4-aminophenoxy) phenol was reacted with 4,4′-dichlorodiphenyl sulfone with 2:1 M ratio under the similar reaction condition for the first step, to produce the diamine (Scheme ).
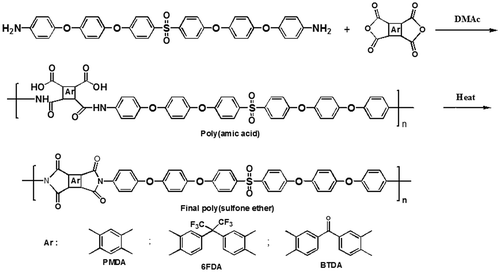
The prepared diamine was designed and prepared in such a way that combination of functional groups would induce certain properties to the final polymers. Therefore, the diamine was polycondensed with three different dianhydrides (PMDA, BTDA, and 6-FDA) in order to prepare related poly(sulfone ether imide)s via two-step imidization method (Scheme ). It was expected that presence of four ether groups in the diamine structure would induce excellent flexibility to final polyimides, existence of sulfone group not only would increase polarity and flexibility of the polymer, but also maintain thermal stability and also presence of six aromatic rings would afford suitable thermo-mechanical properties.[Citation13] In fact, incorporation of several phenylene rings and flexible ether and sulfone groups is an important approach to improve tractability of aromatic polyimides.[Citation19]
3.1. Characterization
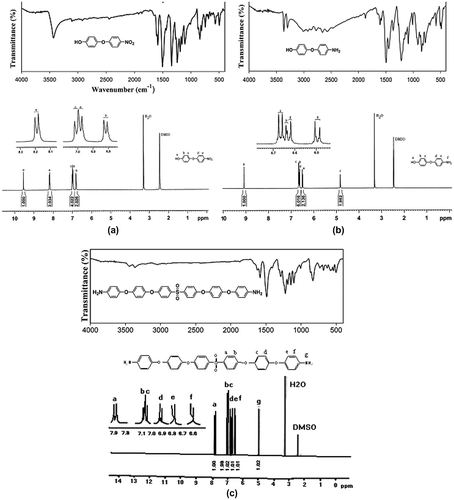
The structure of compounds was confirmed by FT-IR, 1H NMR (Figure ) and elemental analysis techniques and the data were tabulated in Table . All characteristics bands in FT-IR spectra and related peaks in 1H NMR of materials confirmed their structures. Also good agreement between calculated and found amounts of C, H, and N in elemental analysis approved the proposed structures.
Table 1. Name, structure, and characteristic data of synthesized materials.
Therefore, a new diamine containing six aromatic rings, four flexible ether linkages and also a sulfone group was synthesized. It was predicted that the diamine with such structural features could induce processability and thermal stability to final polyimides. Thus, three types of poly(sulfone ether imide)s were prepared via polycondensation reaction of the diamine with common aromatic dianhydrides including PMDA, BTDA, and 6FDA by conventional two-step polymerization method using chemical imidization. FT-IR spectrum of poly(amic acid) showed absorption bands of carboxylic O–H and amide N–H groups at 3300–3422 cm−1, and C=O of amide at 1660 cm−1. FT-IR spectra of synthesized polyimides showed characteristic bands at about 1776, and 1718 (imide C=O stretching), 716 (imide C=O bending), 1372 (C–N), 1153–1293 (ether and SO2), and 3052 cm−1 (C–H aromatic stretching). The OH, NH, and C=O of amic acid absorption bands were also disappeared. Also good agreement between calculated and found amounts of C, H, and N was observed in elemental analysis of polyimides approving the proposed structures (Table ).
Table 2. Polymer characterization data.
3.2. Solubility
The solubility behavior of polymers was evaluated in different solvents. All synthesized polymers were soluble in polar aprotic solvents including N-methyl-2-pyrrolidone (NMP), dimethylsulfoxide (DMSO), N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), and also in protic solvents such as m-cresol. Improved solubility of the polymers could be attributed to the incorporation of sulfone and ether groups in the main chains. Also, 6FDA- derived polyimide had higher degree of solubility due to the presence of bulky CF3 groups which reduced tractability (close packing) by increasing free volume between polymer chains. On the other hand, the poor solubility of PI-PMDA was related to its rigid and symmetric structure, and high interaction between chains. One of the main achievements of this research was excellent solubility of PI-6FDA in the CH2Cl2, i.e. solvent with medium polarity and low boiling point (39 °C).
3.3. Inherent viscosity
The inherent viscosity of prepared polyimides evaluated at a concentration of 0.5 g/dL in NMP at 30 °C using Ubbelohde viscometer was in the range of 0.68–0.78 dL/g (Table ). The obtained values of viscosity were reflecting almost high molecular weight of polymers.
3.4. Thermal properties
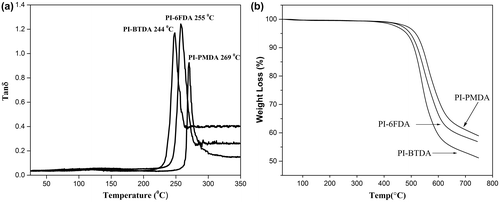
DMTA method was used to assess thermal behavior of polymers. DMTA profiles of samples were shown in Figure (a). The glass transition temperature of polymers was in the range of 244–269 °C. Comparing the results showed that the highest Tg value was related to the polyimide derived from PMDA, whereas polyimide obtained from BTDA showed the lowest Tg value. The higher Tg value of PI-6FDA in respect to PI-BTDA might be attributed to the high growth of molecular weight that was followed by the inherent viscosity results.
Thermal stability of poly(sulfone ether imide) films were measured by thermal gravimetric analysis (TGA techniques) in air atmosphere at a heating rate of 10 °C/min. PI-PMDA polymer showed higher thermal stability in comparison to the others, arising from its rigid, and symmetrical structure. High thermo-oxidative stability of polymers was realized from the fact that no remarkable weight loss was observed below 420 °C and all the polymers exhibited similar decomposition pattern. The 5% weight loss was in the range of at 483–516 °C and the temperature for 10 % weight loss was in range of 510- 540 °C . In general, the temperature for 10% weight loss has been considered as a main criterion for comparing the thermal stabilities of polymers. Also, maximum decomposition temperature of (Tmax) and char yields of polymers at 750 °C were evaluated and compared (Figure (b) and Table ).
Table 3. Thermal characteristic data.
3.5. Crystallinity
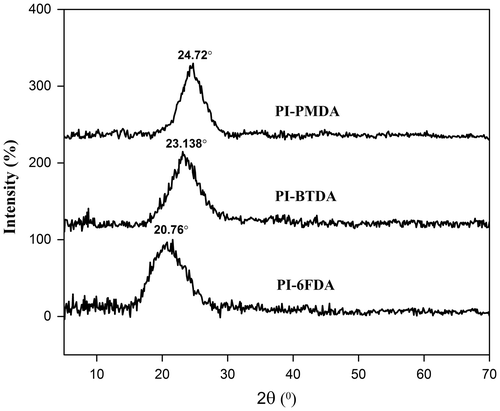
The crystallinity of prepared poly(sulfone ether imide)s was measured with X-ray diffractometer in the range of 2θ = 5–70° and related diffractograms were shown in Figure . The polymers exhibited almost amorphous patterns, however the PI-PMDA showed a more intense peak at higher 2θ in comparison to the others that indicated more crystallinity of this polymer due to more regularity and symmetry of its chemical structure. Also broader peak was observed for PI-6FDA due to the presence of bulky hexafluoroisopropylidene group leading to more amorphousness.
3.6. Tensile strength
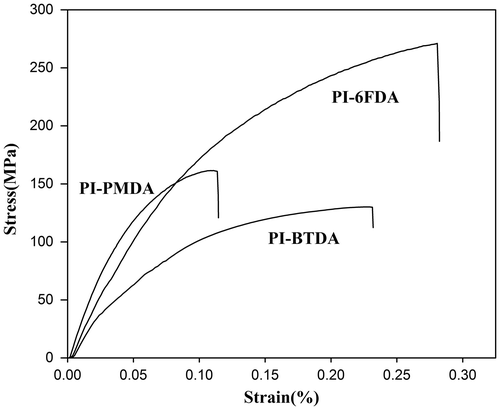
Mechanical properties of the samples were tabulated in Table and related curves were shown in Figure . The tensile strength of the prepared polyimides was about 130–271 MPa, elongation at break was 11.5–28.0%, and initial modulus was in the range of 1.89–3.20 GPa. 6FDA-based polyimide showed the best mechanical properties when compared with PI-BTDA and PI-PMDA which might be attributed to its higher molecular weight and the presence of CF3 flexible groups.
Table 4. Mechanical, physical, and optical properties of PSEI films.
3.7. Optical transparency
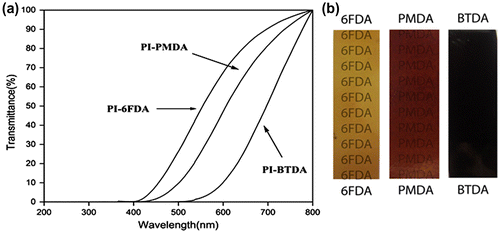
The optical transparency of samples was determined at the wavelength of 600 nm and the values reported in Table . Aromatic polyimides showed strong absorption in the ultraviolet visible region. Related UV–vis spectra were shown in Figure (a).
The results showed that the PI-6FDA film had a higher light transmission than others and therefore it was seen as a transparent light color film. It was with accordance to the fact that films derived from the polyimides prepared from asymmetric dianhydride had greater light transmission than those made from the symmetrical once. The PI-BTDA film had the lowest optical transparency due to the formation of charge transfer complexes and appeared as dark brown color (Figure (b)).
3.8. Water uptake
The water absorption of samples was measured by the immersion of dried films in distilled water at 23 °C for 24 h then immediate weighting after wiping of surface water .Also before measurement, the samples were dried under vacuum at 120 °C for 10 h. Due to the presence of heterocyclic imide ring, ether, and sulfone groups in the synthesized polyimides backbone, their moisture uptakes were in the range of 0.49–1.27%. Moisture absorption of polymer could be changed by chemical structure. In Table , water absorption of polyimides was tabulated. BTDA-polyimide showed highest moisture uptake in comparison to the others owing to the presence of carbonyl polar group and therefore hydrogen bonding with H2O. On the other hand, 6FDA-polyimide showed lowest value of water uptake because of hydrophobic nature of the fluorinated moiety that prevented water absorption on the surface.
4. Conclusion
In order to obtain processable polyimide with high thermal stability and remarkable solubility, a novel aromatic diamine with built-in one sulfone and four ether linkages was synthesized. Subsequently it was polymerized with different aromatic dianhydrides. The obtained results from TGA revealed high thermal stability of the polyimide films. Presence of four ether linkages and one sulfone groups in the diamine structure induced excellent solubility, flexibility, amorphousness, and toughness to the prepared films. 6FDA-based polyimide showed highest solubility, mechanical properties, optical transparency, and lowest water uptake and crystallinity among the prepared polyimides that make it as a potential insulator for many usages such as electronic applications.
References
- Hergenrother PM. The use, design, synthesis and properties of high performance/high temperature polymers: an overview. High Perform. Polym. 2003;15:375–394.
- Mittal KL. Polyimides and other high temperature polymers: synthesis, characterization and applications, volume 5. New York (NY): Taylor & Francis; 2009.10.1163/ej.9789004170803.i-424
- Mehdipour-Ataei S, Bahri-Laleh N. Synthesis and properties of polyimides and copolyimides containing pyridine units: a review. Iran Polym. J. 2008;17:95–124.
- Damaceanu MD, Constantin CP, Nicolescu A, et al. Highly transparent and hydrophobic fluorinated polyimide films with ortho-kink structure. Eur. Polym. J. 2014;50:200–213.10.1016/j.eurpolymj.2013.10.030
- Song G, Li X, Jiang Q, et al. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015;5:11980–11988.10.1039/C4RA14727H
- Kim YJ, Kim JH, Ha SW, et al. Polyimide nanocomposites with functionalized SiO 2 nanoparticles: enhanced processability, thermal and mechanical properties. RSC Adv. 2014;4:43371–43377.10.1039/C4RA04952G
- Ganeshkumar A, Bera D, Mistri EA, et al. Triphenyl amine containing sulfonated aromatic polyimide proton exchange membranes. Eur. Polym. J. 2014;60:235–246.10.1016/j.eurpolymj.2014.09.009
- Zeng K, Zou Y. Synthesis and properties of polyimides derived from a new phthalonitrile-containing diamine with high polyaddition reactivity. Des. Monomers Polym. 2014;17:186–193.10.1080/15685551.2013.840500
- Akbarian-Feizi L, Mehdipour-Ataei S, Yeganeh H. Investigation on the preparation of new sulfonated polyimide fuel cell membranes in organic and ionic liquid media. Inter. J. Polym. Mater. Polym. Biomater. 2014;63:149–160.10.1080/00914037.2013.812086
- Wilson D, Stenzenberger HD, Hergenrother PM. Polyimides. London: Blackie; 1990.10.1007/978-94-010-9661-4
- Liaw D, Liaw B, Li L-J, et al. Synthesis and characterization of new soluble polyimides from 3,3’,4,4’-benzhydrol tetracarboxylic dianhydride and various diamines. Chem. Mater. 1998;10:734–739.10.1021/cm970463c
- Mehdipour-Ataei S, Sarrafi Y, Hatami M. Novel thermally stable polyimides based on flexible diamine: synthesis, characterization, and properties. Eur. Polym. J. 2004;40:2009–2015.10.1016/j.eurpolymj.2004.05.027
- Bruma M, Mercer F, Fitch J, et al. Synthesis and characterization of fluorinated poly(imide–amide–sulfone)s. J. Appl. Polym. Sci. 1995;56:527–532.10.1002/app.1995.070560501
- Xie K, Zhang SY, Liu JG, et al. Synthesis and characterization of soluble fluorine-containing polyimides based on 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene. J. Polym. Sci. Part A Polym. Chem. 2001;39:2581–2590.10.1002/(ISSN)1099-0518
- Mehdipour-Ataei S, Babanzadeh S, Abouzari-Lotf E. Nicotinic-based poly(amide-ether-imide)s: a new category of soluble, heat-resistant, and flame-retardant polyimides. Des. Monomers Polym. 2013;16:417–424.
- Mehdipour-Ataei S, Hatami M. Synthesis and characterization of novel heat resistant poly(amide imide)s. Eur. Polym. J. 2005;41:2010–2015.10.1016/j.eurpolymj.2005.03.012
- Zhang T, Litt MH, Rogers CE. Synthesis and characterization of poly(ester-sulfone)s. J. Polym. Sci. Part A Polym. Chem. 1994;32:1351–1360.10.1002/pola.1994.080320716
- Mehdipour-Ataei S. Novel thermally stable poly(sulfone ether ester imide)s. Eur. Polym. J. 2005;41:91–96.10.1016/j.eurpolymj.2004.07.001
- De AJ, De CJG. Progress in polyimide chemistry I. Vol. 140. Berlin, Heidelberg: Springer; 1999.
- Adduci J, Chapoy LL, Jonsson G, et al. Synthesis, characterization, and study of thermal properties of methyl-substituted arylene sulfone ether polyimides, polyamides, and poly(amide–imides). J. Appl. Polym. Sci. 1983;28:2069–2081.10.1002/app.1983.070280619
- Mehdipour-ataei S. Novel thermally stable arylene sulphone ether. Iran Polym. J. 2002;11:251–256.
- Barikani M, Mehdipour-Ataei S. Synthesis, characterization, and thermal properties of novel arylene sulfone ether polyimides and polyamides. J. Polym. Sci. Part A Polym. Chem. 2000;38:1487–1492.10.1002/(ISSN)1099-0518
- Tabatabaei-Yazdi Z, Mehdipour-Ataei S. Preparation, characterization, and properties of novel category of processable thermally stable poly(ether ether imide)s. Colloid Polym. Sci. 2014;292:2145–2156.10.1007/s00396-014-3248-2