ABSTRACT
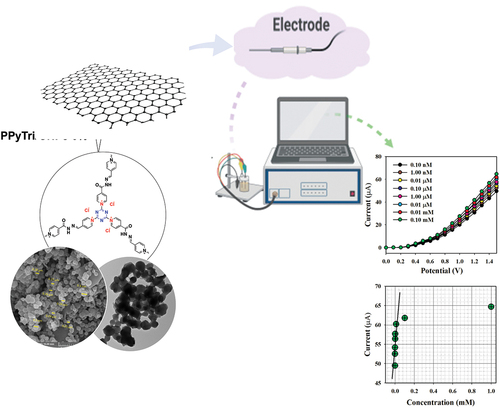
The C3-symmetry ionic polymer PPyTri has been designed with multi-walled carbon nanotubes (MWCNTs) or graphene nanoplatelets (GNPs) and studied as an ultrasensitive electrochemical sensor for trace Hg(II) detection. The synthesis approach incorporated attaching three pyridinium cationic components with chloride anions to the triazine core. The precursors, BPy, were synthesized using a condensation process involving 4-pyridine carboxaldehyde and focused nicotinic hydrazide. The polymer PPyTri was further modified with either MWCNTs or GNPs. The resulting ionic polymer PPyTri and its fabricated nanocomposites were characterized using infrared (IR), nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and powder X-ray diffraction (XRD). The analysis revealed that both the polymer and its nanocomposites have semi-crystalline structures. The electroactivity of the designed nanocomposites toward Hg + 2 ions revealed that among the nanocomposites and bare copolymer, the glassy carbon electrode (GCE) adapted with the PPyTri GNPs-5% exhibited the greatest current response over a wide range of Hg + 2 concentrations. The nanocomposite-modified electrode presented an excellent sensitivity of 83.33 µAµM − 1 cm − 2, a low detection limit of 0.033 nM, and a linear dynamic range of 0.1 nM to 0.01 mM (R2 = 0.9945).
1. Introduction
Heavy metal toxicity is critically important due to its poisonous effects on human health and ecological systems. Among all heavy metals, mercury (Hg) is one of the most universal and harmful pollutants found in natural environments and results from both natural and anthropogenic sources [Citation1]. Given its persistence in the environment, Hg is stored in aquatic ecosystems, water sources such as rivers, lakes, and oceans, and soil with high absorption in biological tissues and a slow elimination rate. The accumulation of Hg+2 ions is hazardous to humans and the environment, even at low concentrations. Scientific studies have revealed that the toxic impacts of Hg+2 ions can lead to various health issues in humans, such as damage to nervous and endocrine systems, adverse effects to the brain and kidneys, and conditions like Hunter – Russell, Alzheimer’s, and Minamata [Citation2–6].
A reliable and efficient method is needed to better detect Hg+2 ions. Several methods, such as inductively coupled plasma mass spectrometry, chemiluminescence, fluorescence, and atomic absorption spectrometry, have been extensively used to confirm the presence of Hg+2 ions [Citation7–10]. However, these methods have several drawbacks, such as high costs, complexity, bulky instrumentation, time-intensive processes, and impracticality for on-site measurements [Citation11–14].
Nanoporous ionic organic networks (NIONs) are a versatile class of materials that have recently emerged as promising candidates for electrochemical applications. These have covalently tethered cations and/or anions within a nanoporous structure, enhancing adsorbate – absorbent interactions through polarization effects and Coulombic forces. NIONs are readily modified through ion exchange reactions and can influence charge transport mechanisms, enhance ion conductivity, and introduce exotic photoelectrochemical properties. High ion densities within a conjugated skeleton indicate NIONs are a valuable structure for engineering ‘smart’ materials with tailored electrochemical responses [Citation15–34].
In parallel, materials with C3-symmetry have a unique structural platform that makes them suitable for various applications. These materials possess a three-fold rotational symmetry that impacts their electronic and optical properties. In electrochemical applications, materials with C3-symmetry provide a specific environment for incorporating functional moieties, such as triazine and pyridinium groups [Citation35–37]. Triazine and pyridinium moieties are known for their electrochemical activity and stability, making them ideal candidates for use in nanoporous materials. The triazine ring structure is stable and promising in facilitating electron transfer processes. Similarly, pyridinium moieties exhibit redox activity, enabling reversible electrochemical reactions. Combining these moieties in nanoporous materials with C3-symmetry can create tailored electrochemical interfaces with various applications from energy storage to sensing [Citation38–42].
The development of nanoporous materials is crucial for enhancing electrochemical performance. Multi-walled carbon nanotubes (MWCNTs) and graphene nanoplatelets (GNPs) are popular additives due to their unique properties. Integrating these nanomaterials improves composite conductivity, mechanical strength, and electrochemical stability. Combining organic networks with carbon materials enhances electron transfer, meeting electrochemical sensing requirements. Carbon nanoplatelets are widely used for their conductivity. Studies have shown that combining organic networks with carbon materials significantly enhances electrochemical performance [Citation43–45]. For example, Zhixiang Xu et al. [Citation46] modified a glassy carbon electrode with a graphene organic polymer composite, resulting in increased peak current and electroactive surface area. Similarly, Dawei Pan et al. [Citation47] synthesized organic networks with carbon materials, leading to excellent electrochemical performance. These findings validate the effectiveness of using organic networks/carbon materials for superior electrochemical sensors.
Thus, this study explores the synergies between nanoporous ionic organic networks, C3-symmetry materials, and electrochemical functionalities conferred by triazine and pyridinium moieties. Additionally, we investigate the impact of nanomaterial fabrication techniques, particularly the use of MWCNTs and GNPs, on enhancing the electrochemical performance of these materials. The PPyTri/GNPs or/CNTs were prepared and modified over a glassy carbon electrode (GCE) and studied for their electroactivity toward Hg+2 ions. The electrochemical performance of the sensor was thoroughly assessed in a phosphate buffer medium and found to be extremely sensitive and selective for Hg2+ ions.
2. Experiments
2.1. Materials and equipment
All solvents and chemicals were analytic grade and purchased from Sigma Aldrich. The MWCNTs and GNP were purchased from Aldrich Nano Tech Co. LTD. Egypt. Analytic grade inorganic salts Cu+2, Ni+2, Cd+2, Sn+2, Pb+2, Co+2, Hg+2, and Zn+2 were obtained from the supplier as required. All chemicals were used as received without further purification. Fourier transform infrared (FT-IR) spectra were recorded using a PerkinElmer Spectrum 100 FT-IR device in the range of 4000–500 cm−1. The morphologies and elemental distributions of the polymers were examined using scanning electron microscopy (SEM, TESCAN VEGA 3, Czech Republic). The samples were mounted on aluminum microscopy stubs using carbon tape and coated with gold (Au) for 120 s using a Quorum Techniques Ltd sputter coater (Q150t, UK). A JEOL JEM 1400 plus transmission electron microscope (TEM) investigated interactions between the polymers and nanomaterials. X-ray diffraction (XRD) data were collected using a Bruker D8 Advance X-ray diffractometer operating at 40 kV and 25 mA with a Cu Kα irradiation source. An electrochemical cell was constructed using a Keithley Electrometer to perform electrochemical (I-V) analysis.
2.2. Synthesis of Schiff base
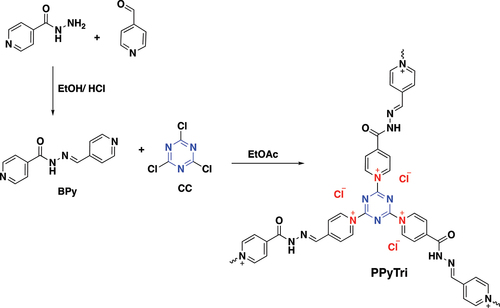
The Schiff base BPy was synthesized from the condensation reaction of 4-Pyridine carboxaldehyde (1 mmol) with nicotinic hydrazide (1 mmol). The mixture was dissolved in ethanol as a solvent with drops of concentrated HCl (37%) as a catalyst. The reaction mixture was equipped under a reflux system and stirred for 3 h at 70 °C. The obtained Schiff base was filtered, washed thoroughly with ethanol, and dried under vacuum. The recrystallization process used acetone to provide the pure Schiff base as white crystals (90%). FTIR: ν [cm‒1]: 3199 (Ar C-H stretch), 1684 (C=O, amide), 1599 (C=N, imine), 1567 (C=N, pyridine), 1412 (Ar C=C bending), 1287 (Ar C=N bending), 1149 (Ar C-N bending), and 827 (Ar C-C bending). 1H NMR (850 MHz, DMSO-d6): δH = 7.00 (d, 4 H, Ar-H), 7.09 (d, 4 H, Ar-H), 7.75 (s, 1 H, HC=N), 7.93 (d, 4 H, Ar-H), 8.03 (d, 4 H, Ar-H), and 11.78 (s, NH). 13C NMR (200 MHz, DMSO-d6): δC = 124, 126, 142, 145, 149, 152, 153, and 165.
2.3. Synthesis of PPyTri polymer
Cyanuric chloride (6.0 mmol, 1 eq) was dissolved in 30 mL ethyl acetate and added dropwise into a stirred solution of BPy (9.0 mmol, 1.5 eq) in 50 mL of ethyl acetate. Then, the reaction mixture was refluxed and stirred for 48 h. The obtained precipitate was then filtered and washed with ethyl acetate three times before drying in a vacuum, giving the product PPyTri as a light green powder (yield: > 90%). FTIR: ν [cm‒1]: 3199 (Ar C-H stretch), 1684 (C=O, amide), 1599 (C=N, imine), 1567 (C=N, pyridine), 1412 (Ar C=C bending), 1287 (Ar C=N bending), 1149 (Ar C-N bending), and 827 (Ar C-C bending). 1H NMR (850 MHz, DMSO-d6): δH = 7.43 (m, 4 H, Ar-H), 7.86 (m, 4 H, Ar-H), 7.05 (s, 1 H, HC=N), 8.12 (d, 4 H, Ar-H), 8.52 (m, 4 H, Ar-H), and 12.66 (s, NH). 13C NMR (200 MHz, DMSO-d6): δC = 129, 131, 133, 136, 137, 145, 150, 153, 154, 156, 157, 166, and 170.
2.4. Preparation of nanocomposite PPyTri polymer with GNPs or MWCNTs
A series of polymers with GNPs or MWCNTs were prepared via in-situ polymerization using different loadings (2 and 5%) of MWCNTs or (2 and 5%) of GNPs with respect to the unmodified copolymer. For each formulation, a mixture of 2 and 5% of MWCNTs or GNPs in H2O was added to equimolar amounts of the two monomers CC and BPy, and sonication was continued for 30 min followed by stirring for 16 h in ethyl acetate at 100 ºC. The process of analyzing the newly formed nanocomposites was similar to that of the bare copolymer nanocomposites as PPyTri/CNTs-5%, PPyTri/CNTs-2%, PPyTri/GNPs-5%, and PPyTri/GNPs-2%.
2.5. Fabrication of GCE with PPyTri/CNTs and PPyTri/GNPs nanocomposites
The I-V electroanalytical method modified the desired electrochemical sensor on the surface of a GCE with PPyTri/CNT or GNP nanocomposites. A thin homogenous layer of PPyTri/CNTs or GNPs was prepared in ethanol and deposited onto a GCE with a surface area of 0.0316 cm2. A drop of Nafion (5% Nafion suspended in ethanol) was added to the modified electrode after the drying step to obtain the desired stability. The GCE was subsequently subjected to thorough drying in an oven at 35 °C for an appropriate duration. An electrochemical cell was constructed using a Keithley electrometer, wherein either PPyTri/CNTs/binder/GCE or PPyTri/GNPs/binder/GCE was utilized as the working electrode, and a plain Pt wire was used as the counter electrode.
Then, a mercury (II) ion solution was prepared and used as the objective analyte. Linearity was established from the relationship between the current and concentration of Hg2+ ions, resulting in a calibration curve. Its slope assessed the fabricated sensor’s sensitivity and detection limit (DL). Calculating the linear dynamic range (LDR) involved determining the calibration curve’s maximum linearity (R2). The electrochemical investigation comprised maintaining a constant volume at 10.0 mL of a phosphate buffer solution in the detection beaker throughout the experiment. The electrochemical sensor utilized in this study employed a Keithley electrometer and featured a straightforward two-electrode system (working and counter electrodes).
3. Results and Discussions
3.1. Chemistry
The synthesis of C3-symmetry polymers requires considering the core structure and linkers. New ionic polymers with a C3-symmetry structure were created and evaluated for their electrochemical performance toward Hg+2 detection. The polymers incorporated a triazine core, viologen linkers, and MWCNTs or GNPs, improving their electrostatic and aromatic properties. This process enhanced their overall behavior using the electrochemical method.
3.2. Synthesis and characterization of Schiff bases and pure ionic copolymer (BPy, PPyTri)
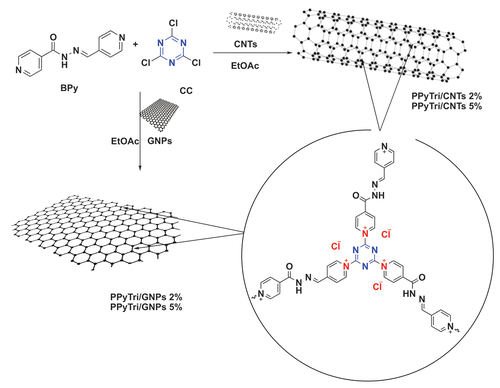
The synthesis of a pyridinium ionic polymer PPyTri (possessed C3-symmetry) and its nanocomposites was achieved through the synthetic method shown in and . In the first step, the precursor Schiff bases BPy were synthesized by thermally condensing 4-pyridine carboxaldehyde with nicotinic hydrazide in refluxing ethanol with a catalytic amount of acid (). The following step involved the Menshutkin reaction, widely used for producing polymers with quaternary pyridinium and ammonium salts. As illustrated in , one pot polymerization process occurred between cyanuric chloride as the core of the C3-symmetry construction with the synthesized Schiff bases BPy as a linker to form the desired cationic polymer PPyTri, where chloride plays the counter anion role.
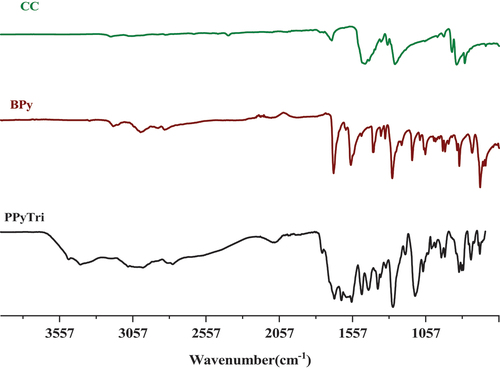
The success of synthesizing the targeted C3-symmetry ionic polymers and their corresponding monomers was supported by investigating their spectral data from the FT-IR and NMR results. The FT-IR spectra of the polymer PPyTri () showed the disappearance of the stretching vibration near 850 cm−1, attributed to the C-Cl band of their cyanuric chloride and supporting the quaternization of the pyridine ring in the monomer BPy. A new intense band belonging to pyridinium cations was observed near 1645 cm−1, further evidence of the successful quaternization reaction and the targeted ionic polymer PPyTri formation. The spectra also showed a shift in the C=N absorption bands of the triazine ring (1508 cm−1) compared to their precursor CC. The C=O amide linkage and C=N absorption of the imine and pyridine rings were observed at 1684, 1599, and 1567 cm−1, respectively.
3.3. Preparation and characterization of nanocomposites (PPyTri/CNTs and PPyTri/GNPs)
The fabrication procedure of bare copolymer incorporated loadings at various MWCNT or GNP ratios. The series of nanocomposites, PPyTri/CNTs-5%, PPyTri/CNTs-2%, PPyTri/GNPs-5%, and PPyTri/GNPs-2%, were produced through in-situ polymerization with a similar execution as the bare copolymer, as in . The distribution of the loaded nanostructure was reinforced through an ultrasonic technique [Citation46]. Ultrasonic irradiation is a versatile, eco-friendly, and straightforward approach for synthesizing nanostructured materials that are typically difficult to produce using conventional methods. Pressure oscillations are generated by subjecting liquids to ultrasonic waves, resulting in cavity formation. These cavities are effectively employed in homogenization to achieve uniform nanoparticle distributions throughout the final product. The nanocomposites were analyzed utilizing FT-IR spectroscopy, and their surfaces were examined using SEM, XRD, and TEM.
Scheme 2. Systematic illustration of nanocomposite fabrication for PPyTri/CNTs-5%, PPyTri/CNTs-2%, PPyTri/gnps-5%, and PPyTri/gnps-2%.
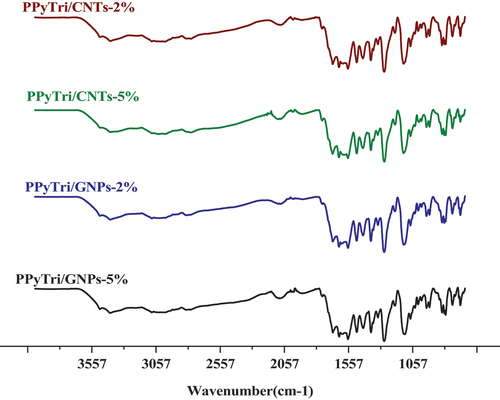
The FT-IR spectra of the created nanocomposites are presented in (). In the spectra, the band at 1612 cm−1 is assigned to the C=C stretching vibration of either MWCNTs or GNPs, while the broad peak at 1530 cm−1 is attributed to C-C plane vibrations of the graphitic walls of either MWCNTs or GNPs. The FT-IR characterization confirmed the successful formation of the copolymer and its nanocomposites. The influence of carbon-based nanoparticle coating is reflected in the vibrational mode with increased width and deformation compared to the bare copolymer [Citation46–50]. shows the1H NMR and13C for the monomer BPy and polymer PPyTri.
Figure 3. [Citation1]H NMR spectra for the monomer BPy and polymer PPyTri.
![Figure 3. [Citation1]H NMR spectra for the monomer BPy and polymer PPyTri.](/cms/asset/342e4350-d8cd-4287-acfa-c9a1123ba0cf/tdmp_a_2360746_f0003_oc.jpg)
Figure 4. [Citation13]C NMR1H NMR spectra for the monomer BPy and polymer PPyTri.
![Figure 4. [Citation13]C NMR1H NMR spectra for the monomer BPy and polymer PPyTri.](/cms/asset/be4d6a83-0d54-46c0-a019-931e66cb9aa5/tdmp_a_2360746_f0004_oc.jpg)
3.4. Morphology analysis
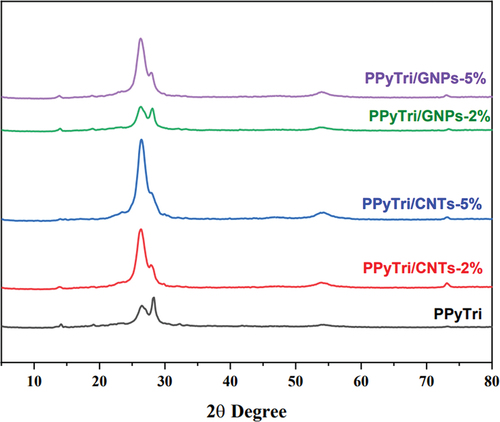
The surface morphologies of the synthesized copolymer and its nanocomposites were investigated by powder XRD (XRD), as shown in . The PXRD patterns of the pure copolymer, PPyTri, revealed a semi-crystalline structure with a small peak at 14º and a diffraction peak (110) at 2θ = 26º. These results indicate intermolecular π–π stacking of the parallel and perpendicular periodicities for the polymer chains [Citation51].
The spectra for the PPyTri/CNTs-5%, PPyTri/CNTs-2%, PPyTri/GNPs-5%, and PPyTri/GNPs-2% nanocomposites show bands at 26º, 28º, 54º, and 73º, attributed to the (002), (111), (220), and (400) planes of graphite. The peak intensity increased steadily as the MWCNT or GNP contents rose from 2 to 5%. Thus, the filler was effectively integrated into the copolymer molecules and dispersed throughout the polymer matrix [Citation52–58]. The diffraction spectra showed that the additional carbon-based nanostructure impacted the nanocomposite crystallinity. With an increased MWCNT or GNP loading, the characteristic diffraction peaks became more distinct, confirming the crystalline nature of the nanocomposites.
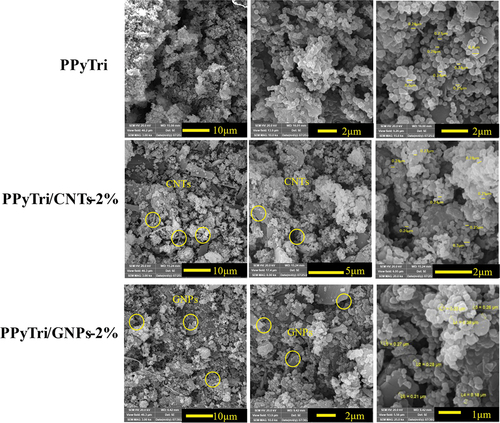
The SEM was utilized for three designed polymers (PPyTri, PPyTri/CNTs-2%, and PPyTri/GNPs-2%) with various magnifications from 3–25 Kx to better understand the impact of MWCNTs and GNPs on the modified copolymer surface as illustrated in . The SEM images revealed a porous network of interconnected nanofibers. The pure copolymer PPyTri showed a porous network of interconnected nanofibers with diameters ranging from 200–380 nm. While the surface morphology of the nanofibers was smooth, there were some visible striations along their length. The magnified nanofibers reveal their intricate and interconnected network structure.
The surface study of the nanocomposite PPyTri/CNTs-2% revealed a dense network of organic polymer fibers containing MWCNTs embedded throughout the micrographs. The polymer fibers exhibited a smooth surface morphology with visible striations along their length. The MWCNTs were dark, rod-like structures with diameters ranging from 20–50 nm and distributed within the polymer matrix. The PPyTri/GNPs-2% micrograph showed that GNPs were incorporated into the organic polymer matrix. The fact that the GNPs were partially embedded and had surface coverage indicates interactions between the two materials, potentially influencing the composite’s properties. The wrinkled and folded morphology of the GNPs increases the surface area available for interactions with the polymer or surrounding environment [Citation46,Citation47,Citation59].
Figure 6. SEM images of the pure copolymer and its nanocomposites PPyTri, PPyTri/CNTs-2%, and PPyTri/gnps-2%.
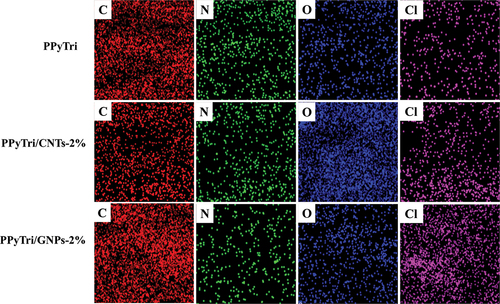
Further analysis of the elemental composition and homogeneity of the synthesized copolymer PPyTri and its nanocomposites with CNTs and GNPs was performed using energy-dispersive X-ray (EDX) mapping (). The mapping showed the presence of C, N, O, and Cl in the unmodified PPyTri and its nanocomposites. The incorporated desired monomers were uniformly distributed throughout the matrix. The results of the EDX mapping, in conjunction with previous SEM observations, demonstrate the effective synthesis of the copolymer and its nanocomposites via in situ polymerization.
Figure 7. EDX elemental maps of the pure copolymer and its nanocomposites PPyTri, PPyTri/CNTs-2%, and PPyTri/GNPs-2%.
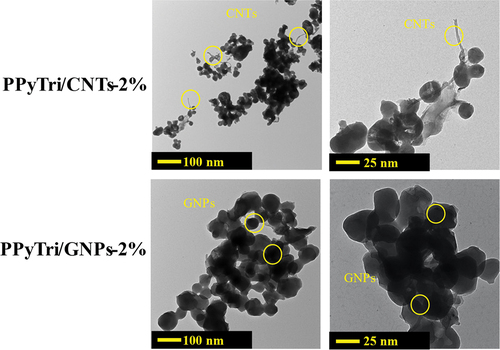
The TEM was used to better understand how MWCNTs and GNPs disperse within the synthesized copolymer matrix. The TEM images for the PPyTri/CNTs-2% and PPyTri/GNPs-2% nanocomposites are presented in . The images show a network of interconnected polymer fibers with a smooth surface and some discernible striations. For PPyTri/CNTs-2%, the MWCNTs appear as dark, tubular structures well-dispersed throughout the polymer matrix. For PPyTri/GNPs-2%, the GNPs appear as thin, plate-like structures with varying sizes and shapes. The TEM images agree with the SEM results of the polymer. The nanopolymer appears folded on the surface of the graphene nanosheet and forms a stacked cover. A closer look at the higher magnification inset image highlights the distributions of the polymer matrix with either MWCNTs or GNPs. The edges of some MWCNTs or GNPs appear seamlessly integrated, implying robust interfacial bonding and intermolecular interactions. This contact holds considerable promise for enhanced composite functionalities, paving the way for potential improvements in various properties. Specifically, the conductive nature of the nanomaterials coupled with the observed interfacial physical interactions suggests significant enhancements in electrical conductivity over the pure polymer[Citation60–65].
3.5. Thermal study
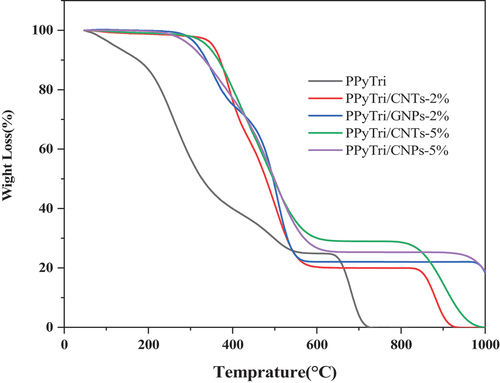
The thermal behaviors of the synthesized copolymers and nanocomposites were studied using thermogravimetric analysis (TGA) with temperatures from 25–1000 °C at a heating rate of 10 ºC/min (). The TGA curve of the polymer PPyTri showed it underwent three stages of weight loss. The first stage was observed between 25 and 190 °C due to water and moisture loss. The second and third stages occurred between 190 and 640 °C and 640 and 720 °C, respectively, and are attributed to the degraded polymer backbone [Citation62]. When a fixed loading of either GNPs or MWCNTs was introduced into the copolymer, the nanocomposites increased the thermal stability, which shifted both the first and second stages. The change in the first stage suggests the nanocomposites are not hygroscopic [Citation63], while the second stage change indicates the enhanced thermal stability from ether GNPs or CNTs [Citation66–68].
comprehensively compares the T10, T25, and T50, illustrating thermal decompositions at 10%, 25%, and 50%, respectively. The values of T10 show the high thermal stability for 2% MWCNTs, while the values of T25 and T50 indicate a pattern where the thermal stability increases with the loaded nanofiller. and demonstrate that a greater nanofiller content increases the thermal decomposition at both 25% and 50%. From a thermal perspective, the nanocomposites loaded with GNPs had a better effect on thermal stability than the corresponding CNTs. The nanocomposite PPyTri/CNTs-2% showed the lowest final temperature of polymer degradation (PDTfinal), while PPyTri/GNPs-5% exhibited the largest values at both degradation temperatures. The thermal analysis results agree with the electrochemical outcomes of the sensor performance, where the PPyTri/GNPs-5% registered the maximum response to the targeted analyte among all modified nanocomposites.
Table 1. Thermal behaviors of the polymer PPyTri and its nanocomposites.
3.6. Detection of mercury ions (Hg+2) employing PPyTri/CNTs/GCE and PPyTri/GNPs/GCE
The electroactivities of the modified sensors with PPyTri/CNTs or PPyTri/GNPs with both 2% and 5% nanostructure loadings were studied to detect Hg+2 ions on the GCE surface. Nafion supported the adhesion of the fabricated electrode as a conducting binding agent to create a thin, uniform layer on the GCE surface. It additionally worked as an adhesion between the nanocomposites and the electrode. Nafion has enhanced the electron transfer rate of the desired electrochemical sensors during I-V analysis [Citation69,Citation70]. The newly developed electrochemical sensor exhibited excellent performance in a phosphate buffer medium, displaying high sensitivity, a remarkably low DL, a wide LDR, and high stability with respectable reproducibility.
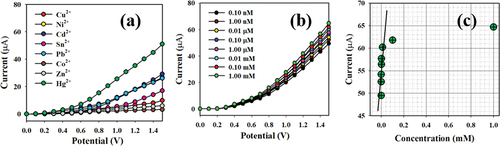
The initial stage of the I-V process includes investigating the selectivity of the modified senor toward Hg+2 ions. Various heavy metal ions at a concentration of 0.1 nM were examined under applied potentials of 0 to +1.5 V in a phosphate buffer with a pH of 7.0. As shown in , Hg+2 ions exhibit the most significant I-V response compared to the electrochemical responses of Cu+2, Ni+2, Cd+2, Sn+2, Pb+2, Co+2, and Zn+2 ions. The sensor performance against Hg+2 ions was demonstrated with extensive concentrations ranging from 0.1 nM to 1.0 mM. The electrochemical responses presented a high distinguishability of Hg+2 ions throughout low and high concentrations. The calibration curve for the projected Hg+2 ions relative to PPyTri/CNTs/GCE or PPyTri/GNPs/GCE assesses the analytical performance, as depicted in . The slope was measured to determine the sensitivity and detection limit of the Hg+2 sensor, recording values of 83.33 µAµM−1 cm−2 and 0.033 nM, respectively.
demonstrates the variable distribution of the current data across a linear plot spanning the concentration range of 0.1 nM to 0.01 mM at an applied potential of +1.5 V., which called as Linear Dynamic Range (LDR). There is an LDR over an extensive range of concentrations in the detection of target mercury ions using fabricated sensor probe with PPyTri/GNPs/GCE. Over the linear dynamic range, the sensor probe is started to be saturated upon addition of higher concentration until 0.1 mM. The linearity was calculated using the calibration plot drawn as current versus Hg+2 concentrations with a regression coefficient of r2 = 0.9945 under the linear dynamic range (0.10 nM to 0.01 mM).
Figure 10. Classification of the sensor behaviors using the electrochemical (I-V) approach. (a) Selectivity estimation, (b) I-V responses based on variations in the Hg+2 ion concentration from low to high, and (c) calibration curve.
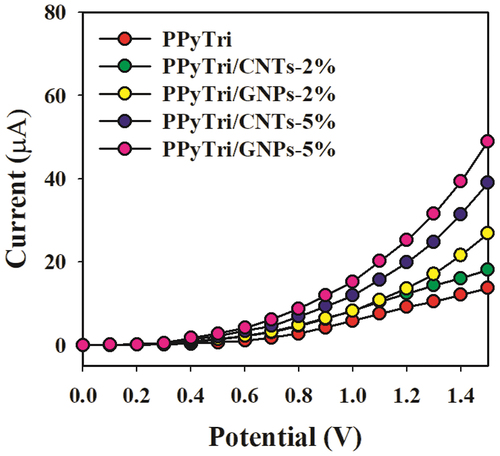
In the control study (), 0.1 nM Hg2+ solutions were utilized in a buffer medium with the adapted GCE comprising the bare copolymer PPyTri and the designed nanocomposites PPyTri/CNTs-2%, PPyTri/GNPs-2%, PPyTri/CNTs-5%, and PPyTri/GNPs-5%. Among the tested organic combinations, both hybrids PPyTri/GNPs-5% and PPyTri/CNTs-5%, with the highest carbon nanostructure loads, displayed the highest current response compared with the remaining investigated nanocomposites with the GCE. Between the two fabricated electrodes, PPyTri/GNPs-5% exhibited slightly more response to Hg+2 than PPyTri/CNTs-5% and was selected as the optimal composition for investigating Hg2+ ions based on the I-V approach. The expected behavior of the sensor performance is attributed to the impact of the carbon-based nanoparticles in the electron-transfer enhancement during sensing between the rich electron system and the cationic ions of Hg+2 [Citation71].
Figure 11. Control study executed at 0.1 µM Hg+2 solutions in a buffer medium with modified GCE containing PPyTri/GNPs or PPyTri/CNTs (2, 5%) nanostructure compositions.
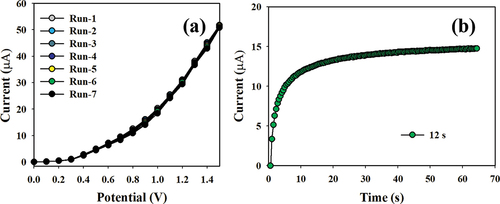
Ensuring the reproducibility of electrochemical sensors is critical for credibly testing the sensor performance. The procedure used a 0.1 nM concentration of Hg+2 ions and was subjected to potentials ranging from 0 to +1.5 V, as illustrated in . The reliability of the modified Hg+2 ion sensor was validated from the consistent I-V response, remaining unchanged even after washing the electrode following each of the seven replicated runs. Moreover, the response time can assess the efficiency of the formed electrochemical sensors, which is an important indicator. As shown in , the projected sensor demonstrates a short response time of 12.0 s using 0.1 nM of Hg+2 ions.
The performance of several recent investigations for detecting Hg+2 ions is compared with the fabricated electrochemical sensor from this work (PPyTri/GNPs-5%). shows the modified material on the electrode, the LDR, and the DL. The electrochemical sensor displays a low DL, a wide linear range, and high sensitivity. The hybrid sensor has a lower DL than our previous research using the same (I-V) method [Citation71].
Table 2. Performance comparison of different electrochemical sensors for Hg+2 ion detection.
3.7. Potential mechanism for high-sensitivity detection of Hg(II)
The electrochemical analysis is largely contingent on the effective analyte enrichment on the electrode surface [Citation72]. Increasing the number of enriched determinants provides a greater response signal. Hybrid materials based on carbon nanostructures (MWCNTs and GNPs) possess high conductivity with π-conjugated electron systems and charge transfer characteristics that are loaded on the fabricated electrode. The chloride counter anion and organic conjugated C3 symmetry structure enhance the system. The fabricated electrode with these properties demonstrates fast signal transduction. It further exhibits synergistic interactions between the graphene nanoparticles, PPyTri, and cationic Hg+2 ions, improving the selectivity and sensitivity of the sensing mechanism [Citation73–75].
3.8. Real sample analysis
Finally, the prepared PPyTri/GNPs-5% NCs as sensor probe was implemented to measure different real samples collected from various sources, which are included in the . The validation is performed by using the recovery technique, known as standard addition approach. The acquired results are presented in , indicating the successful detection of Hg2+ ion in various environmental real samples by using the PPyTri/GNPs-5% NCs sensor probe with electrochemical approach. The sensor probe was found to be reliable and satisfactory, indicating its potential for various environmental monitoring applications.
Table 3. Validation of PPyTri/GNPs-5% NCs fabricated sensor probe using real samples by recovery method.
4. Conclusion
The copolymer PPyTri was synthesized using a combination of triazine core hybrids and pyridinium cationic components, resulting in a C3-symmetry ionic polymer with promising structural features. The copolymer was modified with MWCNTs or GNPs with 2% and 5% loadings to enhance its electrochemical characteristics. Several characterizations were conducted to investigate the structure and morphology of the obtained nanocomposites, including IR, SEM, TEM, and PXRD. These studies provided valuable insights into the semi-crystalline nature of the polymer, the uniform distribution of nanoparticles within the polymer matrix, and the strong interactions between PPyTri and carbon nanomaterials. The results are improved mass transport and electron transfer efficiencies in electrochemical sensing applications. The electroactivity of the designed nanocomposites was studied towards different heavy metal ions with high sensitivity to Hg+2 ions. The GCE modified with the PPyTri/GNPs-5% NCs exhibited the greatest current response among the other combinations. The sensor performance showed excellent analytical performance in terms of sensitivity, LDR, and DL. The sensor presented consistent functioning with a short response time and ensured reproducibility.
Author contributions
M. A. Alshubramy: Methodology, Writing – Original Draft & Editing; K. A. Alamry: Conceptualization, Investigation; A. M. Asiri: Review & Editing – Final Draft; M. M. Alam: Methodology; M. A. Hussein: Conceptualization, Investigation, Writing – Review & Editing; M. M. Rahman: Methodology, Investigation, Writing – Review & Editing
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data has been illustrated in the manuscript text.
References
- Jeromiyas N, Elaiyappillai E, Kumar AS, et al. Bismuth nanoparticles decorated graphenated carbon nanotubes modified screen-printed electrode for mercury detection. J Taiwan Inst Chem Eng. 2019;95:466–474. doi: 10.1016/j.jtice.2018.08.030
- Kunthom R, Piyanuch P, Wanichacheva N, et al. Cage-like silsesequioxanes bearing rhodamines as fluorescence Hg2+ Sensors. J Photochem Photobiol Chem. 2018;356:248–255. doi: 10.1016/j.jphotochem.2017.12.033
- Bernard S, Enayati A, Redwood L, et al. Autism: a novel form of mercury poisoning. Med Hypotheses. 2001;56(4):462–471. doi: 10.1054/mehy.2000.1281
- Taki M, Akaoka K, Iyoshi S, et al. Rosamine-based fluorescent sensor with femtomolar affinity for the reversible detection of a mercury ion. Inorg Chem. 2012;51(24):13075–13077. doi: 10.1021/ic301822r
- Mutter J, Naumann J, Sadaghiani C, et al. Amalgam studies: disregarding basic principles of mercury toxicity. Int J Hyg Environ Health. 2004;207(4):391–397. doi: 10.1078/1438-4639-00305
- Boening DW. Ecological E€ects, Transport, and Fate of Mercury: A General Review. 2000. 2000;40(12):1335–1351. doi: 10.1016/S0045-6535(99)00283-0
- López-García I, Rivas RE, Hernández-Córdoba M. Hollow fiber based liquid-phase microextraction for the determination of mercury traces in water samples by electrothermal atomic absorption spectrometry. Anal Chim Acta. 2012;743:69–74. doi: 10.1016/j.aca.2012.07.015
- Li Y, Chen C, Li B, et al. Elimination Efficiency of Different reagents for the memory effect of mercury using ICP-MS. J Anal Spectrom. 2006;21(1):94–96. doi: 10.1039/B511367A
- Wang D, Guo L, Huang R, et al. Surface enhanced electrochemiluminescence for ultrasensitive detection of Hg2+. Electrochimica Acta. 2014;150:123–128. doi: 10.1016/j.electacta.2014.10.121
- Wang C-I, Huang C-C, Lin Y-W, et al. Catalytic gold nanoparticles for fluorescent detection of mercury(II) and Lead(II) Ions. Anal Chim Acta. 2012;745:124–130. doi: 10.1016/j.aca.2012.07.041
- Hussain MM, Asiri AM, Arshad MN, et al. Fabrication of a Ga3+ sensor probe based on methoxybenzylidenebenzenesulfonohydrazide (MBBSH) by an electrochemical approach. New J Chem. 2018;42(2):1169–1180. doi: 10.1039/C7NJ01891F
- Rahman MM, Alenazi NA, Hussein MA, et al. Hybride ZnCdCrO embedded aminated polyethersulfone nanocomposites for the development of Hg 2+ ionic sensor. Mater Res Express. 2018;5(6):065019. doi: 10.1088/2053-1591/aac681
- Khan AAP, Khan A, Alam MA, et al. Chemical Sensing Platform for the Zn+2 Ions Based on Poly(o-Anisidine-Co-Methyl Anthranilate) Copolymer Composites and Their Environmental Remediation in Real Samples. Environ Sci Pollut Res. 2018;25(28):27899–27911. doi: 10.1007/s11356-018-2819-z
- El-Shishtawy RM, Al-Ghamdi HA, Alam MM, et al. Development of Cd2+ Sensor Based on BZNA/Nafion/Glassy Carbon Electrode by electrochemical approach. Chem Eng J. 2018;352:225–231. doi: 10.1016/j.cej.2018.07.034
- Ghasimi S, Prescher S, Wang ZJ, et al. Heterophase photocatalysts from water‐soluble conjugated polyelectrolytes: an example of self‐initiation under visible Light. Angew Chem Int Ed. 2015;54(48):14549–14553. doi: 10.1002/anie.201505325
- Dawson R, Cooper AI, Adams DJ. Nanoporous organic polymer networks. Prog Polym Sci. 2012;37(4):530–563. doi: 10.1016/j.progpolymsci.2011.09.002
- McKeown NB, Budd PM. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem Soc Rev. 2006;35(8):675. doi: 10.1039/b600349d
- Thomas A. Functional materials: from hard to soft porous frameworks. Angew Chem Int Ed. 2010;49(45):8328–8344. doi: 10.1002/anie.201000167
- Wu D, Xu F, Sun B, et al. Design and preparation of porous polymers. Chem Rev. 2012;112(7):3959–4015. doi: 10.1021/cr200440z
- Das S, Heasman P, Ben T, et al. Porous organic materials: strategic design and structure–function correlation. Chem Rev. 2017;117(3):1515–1563. doi: 10.1021/acs.chemrev.6b00439
- Kandambeth S, Mallick A, Lukose B, et al. Construction of crystalline 2D Covalent organic frameworks with remarkable chemical (Acid/Base) stability via a combined reversible and irreversible route. J Am Chem Soc. 2012;134(48):19524–19527. doi: 10.1021/ja308278w
- Wang K, Yang L, Wang X, et al. Covalent triazine frameworks via a Low‐Temperature polycondensation approach. Angew Chem Int Ed. 2017;56(45):14149–14153. doi: 10.1002/anie.201708548
- Vilela F, Zhang K, Antonietti M. Conjugated porous polymers for energy applications. Energy Environ Sci. 2012;5(7):7819. doi: 10.1039/c2ee22002d
- Bildirir H, Gregoriou VG, Avgeropoulos A, et al. Porous organic polymers as emerging new materials for organic photovoltaic applications: Current status and future challenges. Mater Horiz. 2017;4(4):546–556. doi: 10.1039/C6MH00570E
- Baroncini M, d’Agostino S, Bergamini G, et al. Photoinduced reversible switching of porosity in molecular crystals based on star-shaped azobenzene tetramers. Nat Chem. 2015;7(8):634–640. doi: 10.1038/nchem.2304
- Sun J-K, Antonietti M, Yuan J. Nanoporous ionic organic networks: from synthesis to materials applications. Chem Soc Rev. 2016;45(23):6627–6656. doi: 10.1039/C6CS00597G
- Zhang P, Qiao Z-A, Jiang X, et al. Nanoporous ionic organic networks: stabilizing and supporting gold nanoparticles for catalysis. Nano Lett. 2015;15(2):823–828. doi: 10.1021/nl504780j
- Byun J, Patel HA, Thirion D, et al. Reversible water capture by a charged metal-free porous polymer. Polymer. 2017;126:308–313. doi: 10.1016/j.polymer.2017.05.071
- Li B, Zhang Y, Ma D, et al. Mercury nano-trap for effective and efficient removal of Mercury(II) from aqueous solution. Nat Commun. 2014;5(1):5537. doi: 10.1038/ncomms6537
- Lu W, Yuan D, Sculley J, et al. Sulfonate-grafted porous polymer networks for preferential CO 2 adsorption at low pressure. J Am Chem Soc. 2011;133(45):18126–18129. doi: 10.1021/ja2087773
- Yuan Y, Sun F, Li L, et al. Porous aromatic frameworks with anion-templated pore apertures serving as polymeric sieves. Nat Commun. 2014;5(1):4260. doi: 10.1038/ncomms5260
- Buyukcakir O, Je SH, Choi DS, et al. Porous cationic polymers: the impact of counteranions and charges on CO 2 capture and conversion. Chem Commun. 2016;52(5):934–937. doi: 10.1039/C5CC08132G
- Zhang P, Jiang X, Wan S, et al. Charged Porous Polymers using a solid C?O cross‐coupling reaction. Chemistry A European J. 2015;21(37):12866–12870. doi: 10.1002/chem.201501814
- Zhang Y, Thomas A, Antonietti M, et al. Activation of carbon nitride solids by protonation: morphology changes, enhanced ionic conductivity, and photoconduction experiments. J Am Chem Soc. 2009;131(1):50–51. doi: 10.1021/ja808329f
- Cherioux F, Guyard L. Synthesis and electrochemical properties of novel 1,3,5-Tris(Oligothienyl)Benzenes: a new generation of 3D reticulating agents. Adv Funct Mater. 2001;11(4):305–309. doi: 10.1002/1616-3028(200108)11:4<305:AID-ADFM305>3.0.CO;2-Y
- Xu G, Liu H, Zhou Z, et al. α ‐Cyano Triaryl[3]Radialene: unsymmetrical stereo‐configuration, clustering‐enhanced excimer emission, and radical‐involved multimodal information switching. Angew Chem. 2023;135(27):e202305011. doi: 10.1002/ange.202305011
- Idzik KR, Beckert R, Golba S, et al. Synthesis by Stille cross-coupling procedure and electrochemical properties of C3-symmetric oligoarylobenzenes. Tetrahedron Lett. 2010;51(18):2396–2399. doi: 10.1016/j.tetlet.2010.02.051
- Hryniewicka A, Breczko J, Siemiaszko G, et al. Three-dimensional organization of Pyrrolo[3,2-b]Pyrrole-based triazine framework using nanostructural spherical carbon: enhancing electrochemical performance of materials for supercapacitors. Sci Rep. 2023;13(1):10737. doi: 10.1038/s41598-023-37708-7
- Zhang P, Li M, Yang B, et al. Polymerized ionic networks with high charge density: Quasi‐solid electrolytes in lithium‐metal batteries. Adv Mater. 2015;27(48):8088–8094. doi: 10.1002/adma.201502855
- Troschke E, Leistenschneider D, Rensch T, et al. In situ generation of electrolyte inside pyridine‐based covalent triazine frameworks for direct supercapacitor Integration. ChemSuschem. 2020;13(12):3192–3198. doi: 10.1002/cssc.202000518
- Park JH, Lee CH, Ju J, et al. Bifunctional covalent organic framework‐derived electrocatalysts with modulated p ‐band centers for rechargeable Zn–air batteries. Adv Funct Mater. 2021;31(25):2101727. doi: 10.1002/adfm.202101727
- Imato K, Enoki T, Uenaka K, et al. Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–) 2 a fluorescent dyes. Beilstein J Org Chem. 2019;15:1712–1721. doi: 10.3762/bjoc.15.167
- Cheng Q, Tang J, Ma J, et al. Graphene and Carbon Nanotube Composite Electrodes for supercapacitors with ultra-high energy density. Phys Chem Chem Phys. 2011;13(39):17615. doi: 10.1039/c1cp21910c
- Kamedulski P, Lukaszewicz JP, Witczak L, et al. The importance of structural factors for the electrochemical performance of graphene/Carbon nanotube/melamine powders towards the catalytic activity of oxygen reduction reaction. Materials. 2021;14(9):2448. doi: 10.3390/ma14092448
- Dul S, Ecco LG, Pegoretti A, et al. Graphene/Carbon nanotube hybrid nanocomposites: effect of compression molding and fused filament fabrication on properties. Polymers. 2020;12(1):101. doi: 10.3390/polym12010101
- Sun Y, He J, Waterhouse GIN, et al. A Selective Molecularly Imprinted Electrochemical Sensor with GO@COF Signal Amplification for the Simultaneous Determination of Sulfadiazine and Acetaminophen. Sens Actuators B Chem. 2019;300:126993. doi: 10.1016/j.snb.2019.126993
- Pan F, Tong C, Wang Z, et al. Nanocomposite based on graphene and intercalated covalent organic frameworks with hydrosulphonyl groups for electrochemical determination of heavy metal Ions. Microchim Acta. 2021;188(9):295. doi: 10.1007/s00604-021-04956-1
- AL-Refai HH, Ganash AA, Hussein MA. Sensitive and Selective Voltammetric Sensor Based on Polythiophene Nanocomposite Mixed MWCNT-G for the Determination of Tartrazine. Synth Met. 2021;280:2021. doi: 10.1016/j.synthmet.2021.116875
- Masarra N-A, Batistella M, Quantin J-C, et al. Fabrication of PLA/PCL/Graphene nanoplatelet (GNP) electrically conductive circuit using the fused filament fabrication (FFF) 3D printing technique. Materials. 2022;15(3):762. doi: 10.3390/ma15030762
- Katowah DF, Hussein MA, Alam MM, et al. Poly(Pyrrole- Co-o -Toluidine) wrapped CoFe 2 O 4/R(GO–OXSWCNTs) ternary composite material for Ga 3+Sensing ability. RSC Adv. 2019;9(57):33052–33070. doi: 10.1039/C9RA03593A
- Asiri AM, Hussein MA, Abu‐Zied BM, et al. Enhanced Coating Properties of Ni‐La‐ferrites/Epoxy resin nanocomposites. Polym Compos. 2015;36(10):1875–1883. doi: 10.1002/pc.23095
- Asiri AM, Hussein MA, Abu-Zied BM, et al. Effect of NiLaxFe2−xO4 nanoparticles on the thermal and coating properties of epoxy resin composites. Compos Part B Eng. 2013;51:11–18. doi: 10.1016/j.compositesb.2013.02.023
- Sarwar A, Ali M, Khoja AH, et al. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J CO2 Util. 2021;46:101476. doi: 10.1016/j.jcou.2021.101476
- Ding KH, Wang GL, Zhang M. Characterization of mechanical properties of epoxy resin reinforced with submicron-sized ZnO prepared via in situ synthesis method. Mater Des. 2011;32(7):3986–3991. doi: 10.1016/j.matdes.2011.03.038
- Rahman MM, Hussein MA, Alamry KA, et al. Polyaniline/Graphene/Carbon nanotubes nanocomposites for sensing environmentally hazardous 4-aminophenol. Nano-Struct Nano-Objects. 2018;15:63–74. doi: 10.1016/j.nanoso.2017.08.006
- Moriche R, Prolongo SG, Sánchez M, et al. Morphological changes on graphene nanoplatelets induced during dispersion into an epoxy resin by different methods. Compos Part B Eng. 2015;72:199–205. doi: 10.1016/j.compositesb.2014.12.012
- Liu Y, Babu HV, Zhao J, et al. Effect of Cu-Doped Graphene on the Flammability and Thermal Properties of Epoxy Composites. Compos Part B Eng. 2016;89:108–116. doi: 10.1016/j.compositesb.2015.11.035
- Hussein MA, El-Shishtawy RM, Obaid AY. The impact of graphene nano-plates on the behavior of novel conducting polyazomethine nanocomposites. RSC Adv. 2017;7(17):9998–10008. doi: 10.1039/C6RA28756E
- Hussein MA, El-Shishtawy RM, Alamry KA, et al. Efficient water disinfection using hybrid Polyaniline/Graphene/Carbon nanotube nanocomposites. Environ Technol. 2019;40(21):2813–2824. doi: 10.1080/09593330.2018.1466921
- Savk A, Özdil B, Demirkan B, et al. Multiwalled Carbon Nanotube-Based Nanosensor for Ultrasensitive Detection of Uric Acid, Dopamine, and Ascorbic Acid. Mater Sci Eng C. 2019;99:248–254. doi: 10.1016/j.msec.2019.01.113
- Shao W, Mai J, Wei Z. Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor. Chemosensors. 2022;10(2):83. doi: 10.3390/chemosensors10020083
- Katowah DF, Hussein MA, Rahman MM, et al. Fabrication of Hybrid PVA-PVC/SnZnOx/SWCNTs Nanocomposites as Sn 2+ Ionic Probe for Environmental Safety. Polym-Plast Technol Mater. 2020;59(6):642–657. doi: 10.1080/25740881.2019.1673409
- Kang H, Xu L, Cai Y, et al. Using Boronic Acid Functionalization to Simultaneously Enhance Electrical Conductivity and Thermoelectric Performance of Free-Standing Polythiophene Film. Eur Polym J. 2021;144:110208. doi: 10.1016/j.eurpolymj.2020.110208
- Feng W, Kamide K, Zhou F, et al. Optical and field emission investigations for multiwalled carbon nanotubes with different functionalized groups. Jpn J Appl Phys. 2004;43(1A/B):L36–L39. doi: 10.1143/JJAP.43.L36
- Mohammed Z, Tcherbi-Narteh A, Jeelani S. Effect of graphene nanoplatelets and montmorillonite nanoclay on mechanical and thermal properties of polymer nanocomposites and carbon fiber reinforced composites. SN Appl Sci. 2020;2(12):1959. doi: 10.1007/s42452-020-03780-1
- Singh R, Bajpai AK, Shrivastava AK. CdSe QDs Reinforced Poly(1, 8 Diaminonaphthalene) (PDAN) offers improved thermal and AC conductivity properties. SN Appl Sci. 2019;1(8):815. doi: 10.1007/s42452-019-0835-3
- Biswas Y, Banerjee P, Mandal TK. From polymerizable ionic liquids to Poly(Ionic Liquid)s: structure-dependent thermal, crystalline, conductivity, and solution thermoresponsive behaviors. Macromolecules. 2019;52(3):945–958. doi: 10.1021/acs.macromol.8b02351
- Kumar D, Jindal P. Effect of multi-walled carbon nanotubes on thermal stability of polyurethane nanocomposites. Mater Res Express. 2019;6(10):105336. doi: 10.1088/2053-1591/ab3ad7
- Nikolaeva AL, Gofman IV, Yakimansky AV, et al. Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers. 2020;12(9):1952. doi: 10.3390/polym12091952
- Qazi RA, Khattak R, Ali Shah L, et al. Effect of MWCNTs functionalization on thermal, electrical, and ammonia-sensing properties of MWCNTs/PMMA and PHB/MWCNTs/PMMA thin films nanocomposites. Nanomaterials. 2021;11(10):2625. doi: 10.3390/nano11102625
- Ren S, Li C, Zhao X, et al. Surface modification of sulfonated Poly(Ether Ether Ketone) membranes using nafion solution for direct methanol fuel cells. J Membr Sci. 2005;247(1–2):59–63. doi: 10.1016/j.memsci.2004.09.006
- Wang Z, Liu G, Zhang L, et al. Electrochemical Detection of Trace Cadmium in soil using a Nafion/Stannum film-modified molecular wire carbon paste electrodes. Ionics. 2013;19(11):1687–1693. doi: 10.1007/s11581-013-0891-4
- Fu L, Xie K, Wang A, et al. High selective detection of mercury (ii) ions by thioether side groups on metal-organic frameworks. Anal Chim Acta. 2019;1081:51–58. doi: 10.1016/j.aca.2019.06.055
- Gao C, Yu X-Y, Xiong S-Q, et al. Electrochemical detection of Arsenic(III) completely free from noble metal: Fe 3 O 4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal Chem. 2013;85(5):2673–2680. doi: 10.1021/ac303143x
- Han L, Shen H, Zhu J-X, et al. Mini Review: Electrochemical Electrode Based on Graphene and Its Derivatives for Heavy Metal Ions Detection. Talanta Open. 2022;6:100153. doi: 10.1016/j.talo.2022.100153
- Liu Z, Xia X, Guan H-K, et al. Hypersensitized Electrochemical Detection of Hg(II) based on tunable sulfur-doped porous Co3O4 nanosheets: Promotion Co2+/Co3+ valence change cycle and adsorption via introducing S. Chem Eng J. 2022;435:134950. doi: 10.1016/j.cej.2022.134950
- Hussain MM, Rahman MM, Arshad MN, et al. Hg 2+ sensor development based on (E)- N ′-nitrobenzylidene-benzenesulfonohydrazide (NBBSH) derivatives fabricated on a glassy carbon electrode with a nafion matrix. ACS Omega. 2017;2(2):420–431. doi: 10.1021/acsomega.6b00359
- Wang X, Fang Z, Li Z, et al. R-Phycoerythrin proteins@ZIF-8 composite thin films for mercury ion detection. The Analyst. 2019;144(12):3892–3897. doi: 10.1039/C9AN00449A
- Janani B, Syed A, Raju LL, et al. Highly selective and effective environmental mercuric ion detection method based on starch modified Ag NPs in presence of glycine. Opt Commun. 2020;465:125564. doi: 10.1016/j.optcom.2020.125564
- Katowah DF, Alqarni S, Mohammed GI, et al. Selective Hg 2+ sensor performance based various carbon‐nanofillers into CuO‐PMMA nanocomposites. Polym Adv Technol. 2020;31(9):1946–1962. doi: 10.1002/pat.4919