?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives:
A study was undertaken to determine whether benefits gained by providing small-quantity lipid-based nutrient supplements (SQ-LNS) from age 6–12 months were maintained at age 18 months compared with a delayed intervention.
Design:
Children who completed a randomised controlled trial were enrolled at age 12 months (n = 392) and followed-up until age 18 months (n = 252; dropout rate 35.7%). Two previously exposed (PE and PE-plus) groups (received SQ-LNS from 6–12 months, but no supplement from 12–18 months) were compared with the delayed intervention (DI) group (received no supplement from 6–12 months, but received SQ-LNS from 12–18 months).
Methods and outcome measures:
At age 12 and 18 months, weight, length, haemoglobin (Hb) and psychomotor development were measured.
Setting:
The study was carried out in peri-urban Jouberton area, Klerksdorp, South Africa.
Subjects:
Children aged 12–18 months.
Results:
Compared with DI, negative effects (either a trend or statistically significant) were observed for PE and PE-plus for length-for-age Z-scores (LAZ) (p = 0.091 and p = 0.075, respectively), PE-plus for weight-for-age Z-scores (WAZ) (p = 0.027), and PE and PE-plus for Hb (p = 0.080 and p = 0.033, respectively); and a positive effect for PE-plus for eye–hand coordination (p = 0.086). The odds for anaemia were higher for PE-plus compared with DI (OR = 1.68; 95% CI 0.91, 3.09). Regardless of group, prevalence of anaemia and stunting increased from age 12 to age 18 months.
Conclusions
Benefits of providing SQ-LNS from age 6–12 months were not sustained at age 18 months, compared with providing SQ-LNS from age 12–18 months. Studies to determine the optimum supplementary period to achieve sustainable benefits of SQ-LNS on linear growth and iron status are warranted.
Introduction
Globally in 2019, 144 million children aged under 5 years were stunted and 47 million were wasted, of whom 14.3 million were severely wasted.Citation1 From 2000 to 2019 the global prevalence of stunting showed a steady decline, from 32.4% to 21.3%. Although the prevalence of stunting in the African region declined from 37.9% to 29.1%, it is the only region where the number of stunted children has increased over this time period, from 49.7 million in 2000 to 57.5 million in 2019,Citation1 due to population growth. In South Africa, however, an increase in the prevalence of stunting has been reported. In 2016, 27% of children under five years were stunted,Citation2 compared with 21.6% in 2012.Citation3 This increase in the prevalence of stunting is a concern.
Poor nutrition leads to growth retardation, which both lead to serious public health implications and contribute to early-age morbidity and mortality.Citation4 Stunting and underweight in young children are associated with poor cognitive development, which leads to poor school performance later in life.Citation5–7 Although cognitive gains are not assured as children grow older, there is a possibility for a child to catch up in height-for-age after the age of 24 months.Citation5,Citation8 In addition, Casale and Desmond indicated that early intervention (before two years of age) is the best way to improve a child’s cognitive development.Citation5
Prevention of stunting can bring multiple benefits that include cognitive development, school achievement and better wages earned in adulthood.Citation9 However, intervention strategies should target the first 1 000 days of life (from conception to age two years),Citation9,Citation10 during which a child has an increased metabolic demand, increased nutrient needs, major tissue deposition, and rapid growth and development.Citation10 If nutrition intervention is not undertaken during the first 1 000 days, a child can be at risk of impaired development and increased risk of mortality.Citation10 At present the focus is on the first 1 000 days, which is the crucial period for childhood development,Citation11 and therefore the appropriate window of opportunity for nutritional interventions for improved growth and development of children.
The need for a stronger focus on the nutritional quality of the complementary diet was emphasised by Faber and colleagues.Citation12 Point-of-use fortification using small-quantity lipid-based nutrient supplements (SQ-LNS) is a strategy that can be used to improve the nutritional quality of the complementary diet.Citation13,Citation14 SQ-LNS contain over 20 micronutrients including zinc, iron and vitamin A, essential fatty acids and a small amount of protein.Citation15 Providing SQ-LNS to young children from 9–18 months of age resulted in improved growth and reduced stunting, wasting and anaemia in Burkina Faso.Citation16 In contrast, in a study conducted in Malawi by Maleta and colleagues,Citation17 provision of a lipid-based nutrient supplement (LNS) to young children from 6 to 18 months did not improve linear growth or prevent growth faltering/stunting. Findings from studies on the effect of SQ-LNS in children aged 6–18 months are inconsistent.Citation16–19 To our knowledge, there is less information available on post-intervention studies that provided SQ-LNS to children.
A recent study in South Africa investigated the effect of two novel SQ-LNS products on linear growth and motor development in children in a randomised controlled trial (RCT; referred to as the Tswaka-RCT). Prior to the RCT, both products were shown to be acceptable to the caregivers, who indicated that their children liked the taste when mixed into maize porridge and/or other complementary foods.Citation20 The six-month intervention study showed an early transient intervention effect on linear growth and improved locomotor development for one of the products (SQ-LNS-plus), and both SQ-LNS products showed positive intervention effects for anaemia and iron status compared with a no-supplement control group.Citation21 All children in the no-supplement control group who completed the Tswaka-RCT were provided with SQ-LNS to be taken daily from age 12–18 months. The current study (Tswaka post-intervention study) is a follow-up study including children who completed the Tswaka-RCT and whose parents consented for the follow-up study. The aim of this study was to determine the nutritional status and psycho-motor development at age 18 months of children who received SQ-LNS daily from the age of 12–18 months compared with two previously exposed groups who received the same intervention daily from age 6–12 months but no intervention from age 12–18 months.
Methods
Study population and study design
This study included children who completed the Tswaka-RCT,Citation21 and were followed from age 12–18 months. The study participants resided in the peri-urban Jouberton and Alabama areas of the greater Matlosana (Klerksdorp) municipality, Dr Kenneth Kaunda District, North West province of South Africa. The study site is 200 km from the nearest metropolitan area (Johannesburg).
The Tswaka-RCT investigated the effects of SQ-LNS on growth, psychomotor development, iron status and morbidity among 6- to 12-month-old infants.Citation21 The study used a randomised controlled design. Block randomisation of sizes 3, 6 and 9 was used to randomly allocate infants (n = 750) into one of three groups, namely SQ-LNS (n = 250), SQ-LNS-plus (n = 250) and a no-supplement control group (n = 250). All children in the no-supplement control group who completed the Tswaka-RCT were provided with SQ-LNS to be taken daily from age 12–18 months; while all children in the two SQ-LNS groups who completed the Tswaka-RCT were monitored during the first month post-intervention but did not receive any supplements.
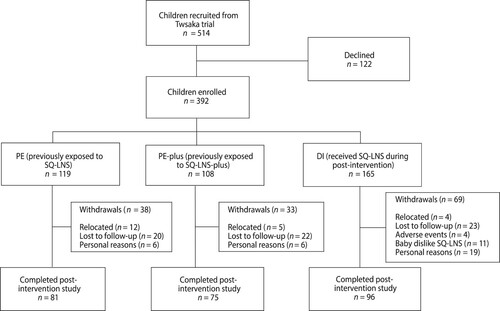
Recruitment for the Tswaka post-intervention study was done by a trained fieldworker when the mother came to the central study site when the baby was 10 months old during the Tswaka-RCT. The fieldworker explained the nature and purpose of the post-intervention study, and the mother was given an information sheet to take home. The recruitment process was repeated when the baby was 12 months old. At completion of the Tswaka-RCT, when the baby was 12 months old, eligible babies whose mothers consented were enrolled into the post-intervention study. Only babies whose mothers who planned to reside in the study area for the next 6 months and were willing to bring the child to the central study site at age 18 months were eligible for enrolment. Of the 514 children who completed the Tswaka-RCT, 392 were enrolled (see ).
Figure 1: Flow diagram of children in Tswaka post-intervention study from enrolment to end of study.
The post-intervention study included three groups, namely (i) PE group, previously exposed to SQ-LNS (received SQ-LNS from age 6–12 months, but no supplement from age 12–18 months), (ii) PE-plus group, previously exposed to SQ-LNS-plus (received SQ-LNS-plus from age 6–12 months, but no supplement from age 12–18 months), and (iii) the DI group, delayed intervention (received no supplement from age 6–12 months, but received SQ-LNS from age 12–18 months). Mothers/caregivers of children in the DI group were advised to feed the child one sachet (20 g) of SQ-LNS paste daily for 6 months, as part of the first meal, and to mix it with the child’s usual complementary foods. The nutrient content of the SQ-LNS is given in .
Table 1: Composition of SQ-LNS per 20 g portion
Fieldworkers visited each mother at home, bi-weekly, to deliver supplements, monitor morbidity and collect forms used by caregivers to record daily amount of supplement consumed by the infant using a four-point pictorial scale. The fieldworker asked about supplements used, not used and counted empty sachets at each visit.
Compliance was calculated based on mean weekly consumption expressed as a percentage of the recommended amount (infants had to consume 20 g of the SQ-LNS daily). Compliance was calculated based on the formula:
Data collection
End results (at age 12 months) of the Tswaka-RCT were used as baseline data for the post-intervention study. For outcome measurements at age 18 months, mothers and/or caregivers brought their children to the central study site for weight and length measurements, psychomotor milestones assessment and to measure haemoglobin. The psychomotor milestone questionnaires were administered by trained field workers who interviewed the mothers in their preferred language.
Measurements
Anthropometric measurements were taken by fieldworkers who were trained on the WHO Training Course on Child Growth Assessment for children. Anthropometric status was assessed using the WHO Child Growth Standards. Weight and recumbent length were taken according to WHO standardised techniques.Citation22 Children were undressed and weighed to the nearest 0.01 kg using a digital baby scale (Seca model 354, Seca GmbH & Co. KG, Hamburg, Germany, maximum weight 20 kg). Recumbent length was measured to the nearest 0.1 cm using an infantometer (Seca model 416, Seca GmbH & Co. KG, Hamburg, Germany). All measurements were done in duplicate and if the first two measurements differed by > 0.05 kg for weight or by > 0.3 cm for length a third measurement was done, and the two closest values were recorded. Anthropometric indices were generated using WHO Anthro 2005 software. Length-for-age Z-scores (LAZ) and weight-for-length (WLZ) were calculated using World Health Organization growth curves.Citation23 Stunting was classified as LAZ <−2SD, wasting as WLZ < −2SD, underweight as WAZ <−2SD and overweight as WLZ >+2SD.Citation23
Two trained fieldworkers administered the Kilifi Developmental Inventory (KDI) and South African Parent Rating Scale.Citation24,Citation25 The Kilifi Developmental Inventory (KDI) was developed in Africa by adapting a range of early childhood development instruments and is a continuous measure used for the assessment of psychomotor development in a resource-poor setting.Citation24 The KDI assesses both locomotor skills and eye–hand coordination. For the Parent Rating Scale, the mother or primary caregiver provides a ranking of the child’s gross motor developmental milestones. KDI scores and parent rating scores were calculated by adding up the scores recorded by the fieldworkers.
A blood sample was collected by finger prick and was done by a qualified nurse. Haemoglobin concentration was measured using the Hemocue method (Ames Mini-Pak haemoglobin test pack and Ames Minilab, Bio Rad Laboratories (Pty) Ltd, Gladesville, Australia). Anaemia was defined as haemoglobin (Hb < 110 g/l).Citation26
Data management and analysis
All team members were trained to conduct the study according to good clinical practice (GCP). The source data were identified by date, participant number and signature of the data collector and then stored in individual participant folders as per GCP requirements. All data were entered into an EpiInfo database (https://www.cdc.gov/epiinfo/index.html) and were captured by one trained research assistant. Quality control was conducted to correct obvious errors in the data set.
Data were analysed using IBM SPSS for Windows, version 23 (IBM Corp, Armonk, NY, USA) and STATA V14 (StataCorp, College Station, TX, USA). Characteristics of the children at age 12 and 18 months are presented as frequencies (categorical data) and means and standard deviations (continuous data). To test whether the three groups differed at age 12 months (baseline), categorical data were analysed using Pearson’s chi-square test and continuous data using ANOVA. To test for intervention effects at 18 months, PE and PE-plus respectively were compared with DI (similar to the analysis done in the Tswaka-RCT, except that DI was now the active intervention group). For LAZ, WAZ, parent rating, locomotor scores and eye–hand coordination scores median regression analysis was used. For Hb concentration, median regression analysis adjusted for baseline (12 months) was used. The intervention effect for anaemia was assessed using binary logistic regression analysis, and the odds ratio (OR) and 95% CI were calculated.
Ethical considerations
The study was approved by the ethics Committee of North-West University (NWU-00060-17-A1). The provincial, district and community’s approval to conduct the study was obtained through engagement with relevant stakeholders. Written informed consent was obtained from the mothers before all interviews and measurements were conducted and all interviews and measurements were conducted in a specified separate room to maintain confidentiality. Names of the children were not recorded on the questionnaires, but only the study identity number. All questionnaires were kept in a locked cupboard and the electronic database was secured, and only the principal investigator had access to the database.
Results
A total of 514 children completed the Tswaka-RCT and were recruited, among whose mothers 122 declined to participate, while 392 consented and were enrolled into the current study (). Of these 392, 252 children completed the post-intervention study. The dropout rate was therefore 35.7%. The reasons for withdrawal included loss to follow-up, mothers relocating out of the study area or changing address without notifying study staff, children refusing the SQ-LNS in the DI group, adverse event (AE) related concerns and personal reasons. In total, one serious adverse event (SAE) and 27 AEs were reported for the duration of the study. The child who was hospitalised due to an SAE did not drop out of the study and amongst those who had AEs, only four withdrew from the study. Mean weekly consumption (compliance) based on recorded daily consumption using a four-point pictorial scale was 92%.
summarises the main characteristics of the children, as well as the findings on anthropometric indices, psychomotor development, parent rating scores and anaemia at age 12 months. Of the 392 study children who enrolled in the post-intervention study, 51.9% were males and 48.1% were females; the sex distribution across the three study groups differed statistically significant (p = 0.012). At age 12 months, 39.9% of the children were stunted, 13.6% were underweight, 2.1% were wasted, 5.1% were overweight and 27.6% were anaemic. There was no statistically significant difference between the groups for any of the anthropometric measurements, the eye–hand coordination score, locomotor development score or parental rating. The mean Hb concentration differed significantly across the three groups (p = 0.003). The percentage of children with anaemia did not differ statistically significantly across the groups, although there was a trend towards significance (p = 0.051). The DI group had a higher percentage of children (33.9%) who were anaemic compared with the PE (24.3%) and PE-plus (21.5%) groups.
Table 2: Characteristics of children at age 12 months; baseline for post-intervention study (n = 392)
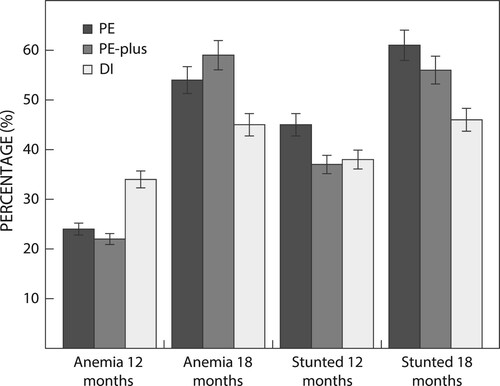
summarises the outcome of the study at age 18 months, as well as the estimated effects (PE and PE-plus respectively versus DI) for growth, psychomotor development scores and Hb concentrations. Because of the small sample size at age 18 months, we highlight results that are statistically significant (p < 0.05) as well as those that show a trend towards statistical significance (p < 0.1). shows that when comparing PE and PE-plus respectively with DI, with respect to LAZ there was a trend towards a negative effect for both PE (p = 0.091) and PE-plus (p = 0.075), indicating poorer linear growth in the two previously exposed groups compared with the DI group. As shown in , overall, at age 18 months 53.4% of the children were stunted, while 60.5%, 56.0% and 45.8% were stunted in the PE, PE-plus and DI groups respectively.
Table 3: Outcomes at follow-up at age 18 months and estimated effects for growth, psychomotor development scores, and anaemia and iron status indicator
Table 4: Anthropometric status of children at 18 months
There was a significant negative effect for WAZ when comparing PE-plus with DI (p = 0.027), indicating that WAZ deteriorated in children previously exposed to SQ-LNS-plus compared with the delayed intervention group. However, there was no statistically significant effect for WAZ when comparing PE versus DI.
There were no statistically significant effects for locomotor development and parental rating scores at age 18 months (). However, there was a trend towards a significant effect for PE-plus compared with DI (p = 0.086) for eye–hand coordination, indicating that children previously exposed to SQ-LNS-plus performed better than children in the DI group.
For Hb concentration, there was a trend towards a negative effect for children in the PE group compared with the DI group (p = 0.080), and a significant negative effect for children in the PE-plus group compared with the DI group (p = 0.033). For anaemia, there was no effect for the PE group compared with the DI group, while there was a trend towards an effect for children in PE–plus group compared with the DI group (p = 0.097). Children in PE-plus had a 68% higher chance of being anaemic compared with children in the DI group (OR = 1.68; 95% CI 0.91, 3.09). At age 18 months, 52.0% of children presented with anaemia: PE 54.0%, PE-plus 58.7% and DI 45.4%. As shown in , the percentage of children with anaemia at age 12 months was highest for the DI group. However, at age 18 months, the DI group had the lowest percentage of children with anaemia and stunting (). Regardless of the group, we observed that anaemia prevalence generally tended to increase from 12 to 18 months.
Discussion
In the recently completed Tswaka RCT, daily provision of two novel SQ-LNS products to infants from age 6–12 months showed positive intervention effects for anaemia and iron status, but only one of the products (SQ-LNS-plus) showed a transient intervention effect for linear growth at age 8 and 10 months, but not at age 12 months, as well as improved locomotor development score and parental rating scores.Citation21 The aim of the post-intervention study was to investigate whether the benefits gained by providing SQ-LNS from age 6–12 months would be maintained when no further supplements were provided for a period of 6 months, compared with control children who received SQ-LNS from age 12–18 months but not from age 6–12 months.
At age 18 months, the effect for LAZ showed a trend towards significance for the two previously exposed groups (p = 0.091 and p = 0.075, PE and PE-plus respectively) when compared with children who received SQ-LNS from age 12–18 months (DI). In addition, a significant effect for WAZ was observed when comparing PE-plus with DI. These effects were all negative, meaning that previously exposed children (PE and PE-plus) showed poorer growth compared with the delayed intervention group (DI). In other words, stating the reverse, children who received SQ-LNS from age 12–18 months showed better growth compared with previously exposed children. These findings are in line with the results of a study conducted in Ghana, which showed that SQ-LNS provided to mothers during pregnancy until 6 months postpartum and then to their children from 6 to 18 months had a positive impact on children’s weight and length by age 18 months compared with children in the two control groups.Citation27 The fact that we observed a trend towards significance only for LAZ is no surprise due to the small sample size, and a meta-analysis by Ramakrishnan and colleagues showed that, although multiple micronutrient interventions improved linear growth in children aged under five, the effect was small.Citation28 The negative effect observed for the two previously exposed groups suggests provision of the two SQ-LNS products probably should have been continued after the age of 12 months.
Although children in the DI group showed a trend towards better linear growth compared with the other two groups, linear growth deteriorated during the post-intervention period in all three groups, as reflected by the shift in the mean LAZ scores towards more negative values as well as the higher percentage of children who were stunted at age 18 months, compared with age 12 months. We postulate that providing 20 g SQ-LNS is insufficient and that higher amounts are needed to impact child growth and intakes should be adjusted/increased as the child gets older. However, a study in Haiti showed that positive effects on HAZ and WAZ of providing small amounts of LNS can be sustained six months post-intervention.Citation29
Our study results showed no statistically significant effects for developmental outcomes at 18 months. These results are consistent with previous studies done in GhanaCitation30 and Malawi,Citation31,Citation32 which also showed that provision of small amounts of LNS had no significant effect on developmental outcomes at age 18 months. Contrary to our study, a significant effect of supplementing children after 6 months of age with SQ-LNS on motor development at 18 months of age was seen in Bangladesh,Citation33 and in Burkina Faso, where higher mean scores were seen for language development in children who received SQ-LNS supplementation compared with those who received no intervention.Citation34 In the Tswaka-RCT, provision of SQ-LNS-plus resulted in improved locomotor development scores and parental rating scores at age 12 months,Citation21 but no significant effect was observed at age 18 months in the post-intervention study. Therefore, the lack of effects on developmental outcomes at age 18 months observed in our study and in other studies suggests that this early intervention effect might not be sustained.Citation31,Citation32,Citation35
It should be noted though that the Tswaka-RCT showed that provision of SQ-LNS-plus had a positive effect on locomotor development and parental rating scores, but no effect on eye–hand coordination.Citation21 At age 12 months, the mean eye–hand coordination scores did not differ between the three groups. At age 18 months, there was a trend (p = 0.086) towards a positive effect for eye–hand coordination for children in the PE-plus group compared with the DI group. The better performance in eye–hand coordination by previously exposed children compared with children in the delayed intervention group may indicate a delayed intervention effect for earlier provision of SQ-LNS-plus.
In the Tswaka-RCT, provision of both SQ-LNS products showed positive intervention effects for anaemia and iron status,Citation21 while the post-intervention study showed a deterioration in Hb concentration in the two previously exposed groups compared with the DI group. The significantly better Hb concentrations in the DI group compared with the previously exposed groups is supported by Hess and colleagues, who reported that children in the intervention groups (given SQ-LNS without zinc and SQ-LNS containing zinc) had a greater increase in Hb at 18 months compared with a control group.Citation16 In addition, the provision of a fat-based spread to undernourished children 6–17 months old in Malawi also showed a positive effect on Hb concentration.Citation36 In our study, we compared PE and PE-plus respectively with DI, which is similar to the approach used in the Tswaka-RCT.Citation21 In the Tswaka-RCT, the intervention effects for Hb when comparing SQ-LNS and SQ-LNS-plus with the no-supplement control group were 3.94 and 4.81 respectively; in the post-intervention study the effects when comparing the same groups were −3.64 and −4.63 respectively, indicating similar effect sizes. Therefore, provision of SQ-LNS improved Hb concentration, regardless of age when the SQ-LNS was provided. Although the percentage of children with anaemia increased in all three groups from age 12 to age 18 months, DI had the lowest percentage of children with anaemia at age 18 months, although the effect on anaemia was not statistically significant, probably because of the small sample size. Our results for Hb concentrations at both age 12 months and age 18 months, together with the percentage of children with anaemia, suggest that providing SQ-LNS may have protective effects on anaemia, regardless of the age of the children (whether provided at 6–12 months or 12–18 months). The results further demonstrate that children residing in this low-economic setting did not consume complementary food sufficient to prevent anaemia. Osei and colleagues showed that supplementation with micronutrient powders may reduce anaemia and iron deficiency in children,Citation37 while Bauserman and colleagues showed that provision of micronutrient-rich caterpillar cereal resulted in reduction of the risk of anaemia and improved Hb concentrations at age 18 months when compared with the control group.Citation38
Both anaemia and stunting deteriorated from age 12 to age 18 months, but the DI group (received SQ-LNS from age 12–18 months) deteriorated less than the two previously exposed groups (). At age 18 months, the two previously exposed groups presented with higher percentages of stunting (56% and 60.4%, respectively) compared with 45.8% of children in the DI group. Although the study sample was not representative of the study population, the stunting prevalence at age 18 months (53.6%) is very high based on the WHO thresholds, indicating a serious public health concern.Citation39 The current stunting prevalence is substantially higher than the findings from the 2016 South African Demographic and Health Survey, which reported 31.4% children of age 12–17 months being stunted.Citation2 Available evidence shows that stunting is associated with poor cognitive and physical development, reduced productivity and may cause an increased risk of chronic diseases in adulthood,Citation40 which further indicates the need for preventing stunting. In this setting SQ-LNS supplementation will need to be coupled with other proven stunting reduction interventions.Citation41
Limitations of the study include the large number of mothers of children who completed the Tswaka-RCT who did not consent to the post-intervention study, and the high number of dropouts (35.7%) between age 12 and 18 months. As a result, the final sample size was smaller than anticipated, and firm conclusions can therefore not be drawn from our results. Also, the study groups were not representative of the original randomised groups and the actual percentages for stunting and anaemia should be interpreted with caution.
Conclusions and recommendations
The Tswaka-RCT showed that provision of two SQ-LNS products from age 6–12 months had a positive effect on anaemia and iron status, while SQ-LNS-plus showed an early transient effect on linear growth and improved locomotor development, compared with a no-supplement control group.Citation21 However, when they no longer received SQ-LNS from age 12–18 months, and compared with the DI group, the intervention effects were reversed. While anthropometric status and anaemia deteriorated from age 12–18 months in all three groups, the DI group, who received SQ-LNS, had a slightly better outcome. The beneficial effects of SQ-LNS observed in the Tswaka-RCT were not sustained at age 18 months in the two previously exposed groups, suggesting that provision of SQ-LNS should be continued after age 12 months and also indicates the vulnerability of children at this age.
Studies to determine the optimum supplementary period to achieve sustainable benefits of SQ-LNS on linear growth and iron status in children are warranted. Incorporating other interventions may be necessary as intervening with SQ-LNS alone may not have been sufficient for children in our study. We could have combined point-of-use fortification with SQ-LNS with other strategies that concern the health of the family and many other factors that may reduce the risk of stunting.Citation34 In future we should make sure we ensure good early childhood development, by making an intervention that encourages caregiver activities with children, and educating them about diverse diet. Introduction of the SQ-LNS from age 6 to12 months did not have sustained benefits on Hb and anaemia and LAZ at age 18 months compared with provision of the SQ-LNS from age 12–18 months. Therefore, it is possible that SQ-LNS alone are not sufficient; incorporating other interventions that aim to improve children’s well-being and existing stunting reduction interventions may have helped in improving the outcome of our study. We could have possibly increased the dosage of SQ-LNS as children grew older.
Author contributions
IR was involved in supervising field data collection and data quality control, data analysis and interpretation of results, and drafted the paper. CL analysed the data, and provided guidance on statistical analysis and interpretation. TMM and MR were study coordinators and they contributed by supervising field data collection and quality control of the data. CMS and MF were the principal investigators, initiated the study and provided training, gave guidance on data collection, quality control and analysis, academic input and review of the paper. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank all the members of the Tswaka team, parents/guardians of the young children who participated in the study and the fieldworkers who helped with data collection. To the administrative units of Matlosana Municipality as well as Department of Health and local clinics, the authors appreciate their understanding and support.
Disclosure statement
CMS received traveling support from Unilever, DSM, and Sight and Life
Additional information
Funding
References
- UNICEF/WHO/The World Bank Group joint child malnutrition estimates: levels and trends in child malnutrition: key findings of the 2020 edition. 2020. Available from: https://www.who.int/publications-detail/jme-2020-edition
- National Department of Health (NDoH), South Africa (Stats SA), South African Medical Research Council (SAMRC), ICF. South Africa Demographic and Health Survey 2016. Key indicators report. Pretoria: Statistics South Africa, 2017. Available from: https://www.gov.za/sites/default/files/gcis_document/201409/sadhs-complete0.pdf
- Shisana O, Labadarios D, Rehle T, et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: the health and nutritional status of the nation. HSRC Press; 2014. Available from: http://repository.hsrc.ac.za/handle/20.500.11910/2864
- Bentley ME, Johnson SL, Wasser H, et al. Formative research methods for designing culturally appropriate, integrated child nutrition and development interventions: an overview. Ann NY Acad Sci. 2014;1308:54–67.
- Casale D, Desmond C. Recovery from stunting and cognitive outcomes in young children: evidence from the South African Birth to Twenty Cohort Study. J Dev Orig Health Dis. 2016;7(2):163–71.
- Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70.
- Mireku MO, Cot M, Massougbodji A, et al. Relationship between stunting, wasting, underweight and geophagy and cognitive function of children. J Trop Pediatr. 2020: fmaa009. https://doi.org/http://doi.org/10.1093/tropej/fmaa009
- Crookston BT, Schott W, Cueto S, et al. Postinfancy growth, schooling, and cognitive achievement: young lives. Am J Clin Nutr. 2013;98(6):1555–63.
- Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(s3):5–18.
- Adu-Afarwuah S, Lartey A, Dewey KG. Meeting nutritional needs in the first 1000 days: a place for small-quantity lipid-based nutrient supplements: nutrient needs during first 1000 days. Ann NY Acad Sci. 2017;1392(1):18–29.
- Piwoz E, Sundberg S, Rooke J. Promoting healthy growth: what are the priorities for research and action? Adv Nutr. 2012;3(2):234–41.
- Faber M, Laubscher R, Berti C. Poor dietary diversity and low nutrient density of the complementary diet for 6- to 24-month-old children in urban and rural KwaZulu-Natal, South Africa. Matern Child Nutr. 2016;12(3):528–45.
- Arimond M, Zeilani M, Jungjohann S, et al. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern Child Nutr. 2015;11(S4):31–61.
- Dewey KG, Arimond M. Lipid-based nutrient supplements: how can they combat child malnutrition? PLoS Med. 2012;9(9):e1001314.
- Hess SY, Peerson JM, Becquey E, et al. Differing growth responses to nutritional supplements in neighboring health districts of Burkina Faso are likely due to benefits of small-quantity lipid-based nutrient supplements (LNS). PLoS One. 2017;12(8):e0181770.
- Hess SY, Abbeddou S, Jimenez EY, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS One. 2015;10(3):e0122242.
- Maleta KM, Phuka J, Alho L, et al. Provision of 10–40 g/d lipid-based nutrient supplements from 6 to 18 months of age does not prevent linear growth faltering in Malawi. J Nutr. 2015;145(8):1909–15.
- Mangani C, Maleta K, Phuka J, et al. Effect of complementary feeding with lipid-based nutrient supplements and corn–soy blend on the incidence of stunting and linear growth among 6- to 18-month-old infants and children in rural Malawi. Matern Child Nutr. 2015;11(S4):132–43.
- Phuka JC, Maleta K, Thakwalakwa C, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6-to 18-month-old rural Malawians. Arc Pediatr Adolesc Med. 2008;162(7):619–26.
- Rothman M, Berti C, Smuts CM, et al. Acceptability of novel small-quantity lipid-based nutrient supplements for complementary feeding in a peri-urban South African community. Food Nutr Bull. 2015;36(4):455–66.
- Smuts CM, Matsungo TM, Malan L, et al. Effect of small-quantity lipid-based nutrient supplements on growth, psychomotor development, iron status, and morbidity among 6-to 12-mo-old infants in South Africa: a randomized controlled trial. Am J Clin Nutr. 2019;109(1):55–68.
- WHO (World Health Organization). Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. Geneva: World Health Organization; 1995.
- De Onis M. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006.
- Sabanathan S, Wills B, Gladstone M. Child development assessment tools in low-income and middle-income countries: how can we use them more appropriately? Arch Dis Childh. 2015;100(5):482–8.
- Kvalsvig JD, Govender K, Taylor M. Research on the age validation of NELDS related to the cognitive development of children between 0 and 4 years of ages. Report to UNICEF and the Department of Education. Available from: http://www.education.gpg.gov.za/Documents/NELDSguidelines8Jan.pdf
- WHO (World Health Organization). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization; 2011. Available from: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf
- Adu-Afarwuah S, Lartey A, Okronipa H, et al. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr. 2016;104(3):797–08.
- Ramakrishnan U, Nguyen P, Martorell R. Effects of micronutrients on growth of children under 5 y of age: meta-analyses of single and multiple nutrient interventions. Am J Clin Nutr. 2009;89(1):191–203.
- Iannotti LL, Dulience SJL, Green J, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid-based nutrient supplement. Am J Clin Nutr. 2014;99(1):198–208.
- Prado EL, Adu-Afarwuah S, Lartey A, et al. Effects of pre- and post-natal lipid-based nutrient supplements on infant development in a randomized trial in Ghana. Early Hum Dev. 2016;99:43–51.
- Phuka JC, Gladstone M, Maleta K, et al. Developmental outcomes among 18-month-old Malawians after a year of complementary feeding with lipid-based nutrient supplements or corn-soy flour. Matern Child Nutr. 2012;8(2):239–48.
- Prado EL, Maleta K, Ashorn P, et al. Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi. Am J Clin Nutr. 2016;103(3):784–93.
- Matias SL, Mridha MK, Tofail F, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr. 2017;105(4):958–69.
- De Onis M, Dewey KG, Borghi E, et al. The World Health Organization’s global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr. 2013;9(S2):6–26.
- Larson LM, Young MF, Bauer PJ, et al. Effectiveness of a home fortification programme with multiple micronutrients on infant and young child development: a cluster-randomised trial in rural Bihar, India. Br J Nutr. 2018;120(2):176–87.
- Kuusipalo H, Maleta K, Briend A, et al. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. J Ped Gastro Nutr. 2006;43(4):525–32.
- Osei AK, Pandey P, Spiro D, et al. Adding multiple micronutrient powders to a homestead food production programme yields marginally significant benefit on anaemia reduction among young children in Nepal. Matern Child Nutr. 2015;11(S4):188–202.
- Bauserman M, Lokangaka A, Gado J, et al. A cluster-randomized trial determining the efficacy of caterpillar cereal as a locally available and sustainable complementary food to prevent stunting and anaemia. Public Health Nutr. 2015;18(10):1785–92.
- Onis M, Borghi E, Arimond M, et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2019;22(1):175–79.
- Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90.
- Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–77.