ABSTRACT
Objectives: Multiple clinical trials have been conducted to investigate the therapeutic effects of blinatumomab on acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL). We did a meta-analysis including 8 clinical trials to verify the efficacy and safety of blinatumomab in patients with relapsed/refractory ALL and NHL.
Methods: We searched and investigated all relevant publications from PubMed, Web of Science, Embase, and ClinicalTrials.gov. The primary endpoint was complete remission (CR). The secondary end points were the minimal residual disease (MRD) response, and the adverse effects including cytokine release syndrome (CRS) and grade ≥ 3 neurological events.
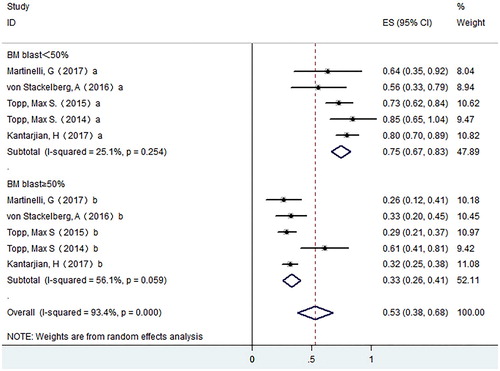
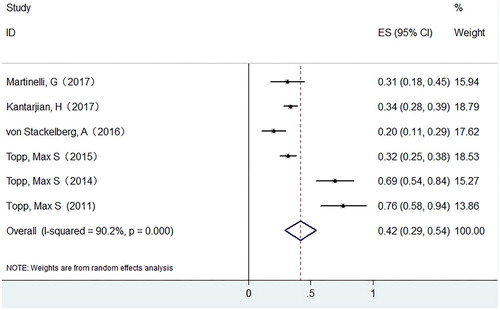
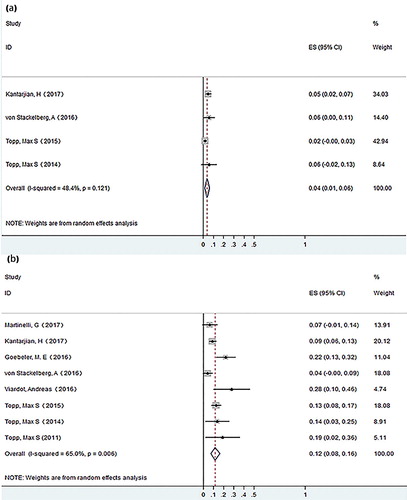
Results: Our study showed that the pooled CR rate was 0.45 (95% CI: 0.37–0.53) in ALL and 0.20 (0.12–0.27) in NHL respectively. The pooled CR rate is higher in ALL patients with BM blasts <50% than that of patients with BM blasts ≥50% (0.75 versus 0.33). A history of allo-HSCT has no effect on the CR rate. The pooled MRD response rate was 0.42 (95% CI: 0.29–0.54) in ALL. For adverse effects, the pooled occurrence rate of grade ≥3 CRS was 0.04 (95% CI: 0.01–0.06), and the pooled occurrence rate of grade ≥ 3 neurological events was 0.12 (95% CI: 0.08–0.16).
Discussion: Blinatumomab is effective in treating relapsed/refractory ALL and NHL. ALL patients manifested better therapeutic response than NHL patients and a reduced tumor load favored the clinical response. For adverse effects, severe CRS and neurological events did not happen very often.
Conclusion: Blinatumomab shows valid therapeutic effects and limited adverse response in treating relapsed/refractory ALL and NHL in our meta-analysis
Introduction
Acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL) are common and life-threatening hematological malignancies. Relapsed/refractory ALL and NHL have extremely poor prognosis. For relapsed ALL, the median survival ranges from 4 to 8 months, with less than 10% of 5-year survival [Citation1–3]. For NHL, a multi-cohort retrospective non-Hodgkin lymphoma research (SCHOLAR-1) study conducted in 636 patients with refractory DLBCL, the most common subtype of NHL, showed a CR rate of 7% to the salvage therapy with a median overall survival (OS) of 6.3 months [Citation4]. However, in recent decades, emerging immunotherapies have shown promising results. CD19 chimeric antigen receptor-modified T cells (CD19+ CAR-T cells) have been reported to induce a CR rate of 90% for ALL patients and 50%-60% for NHL patients [Citation5–8]. Inotuzumab ozogamicin, an anti-CD22 antibody conjugated to calicheamicin demonstrated encouraging result for relapsed/refractory ALL in a phase 3 randomized controlled INO-VATE trial [Citation9]. Moreover, naked monoclonal antibodies such as rituximab have been shown to prolong survival for patients with CD20+ NHL and Burkitt’s lymphoma/leukemia [Citation10].
Blinatumomab belongs to a class of bispecific T-cell engager (BiTE) antibodies. It is a 55-kDa fusion protein which simultaneously links CD3 and CD19 [Citation11]. CD19 is a cell differentiation antigen expressed on B cells during all the development stages except plasma cells. After treatment with blinatumomab, a transient cytolytic synapse is established between a cytotoxic T cell and a CD19 positive cell [Citation12]. Blinatumomab has been proved to induce complete target cell lysis in vitro, and manifest high antitumor activity in multiple animal models [Citation11]. In 2014, Blinatumomab was approved by the US Food and Drug Administration for the treatment of relapsed/refractory Philadelphia chromosome–negative ALL. Multiple clinical trials have been conducted to verify efficacy and safety of blinatumomab for relapsed/refractory lymphoid malignancies. However, the results differ greatly among different studies [Citation13–19].
Here, our meta-analysis aims to provide more comprehensive evidence on the efficacy and safety of blinatumomab for patients with relapsed/refractory ALL and NHL.
Method
Search strategy
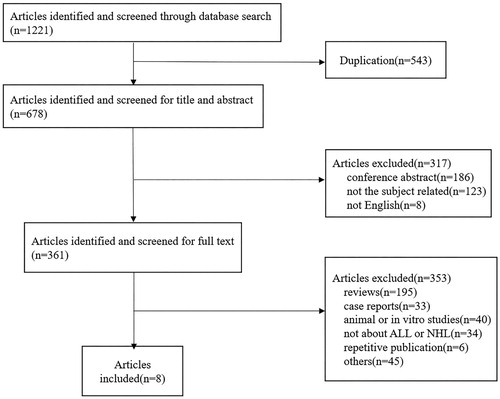
We used ‘blinatumomab’, ‘blincyto’ or ‘MT103’ as search term in the title and abstract to search for all articles in PubMed, Web of Science, Embase, and ClinicalTrials.gov until 31 October 2017. All completed clinical trials on the treatment of blinatumomab in ALL and NHL were included. After removal of duplications, 678 articles were recruited. After reviewing the titles and abstracts, 361 were recruited for full text review. Finally, 8 studies were enrolled for meta-analysis. The searching and screening process was completed independently by two authors ().
Outcome measures
The primary endpoint was complete remission (CR). The secondary end points were the minimal residual disease (MRD) response, and the occurrence of the adverse effects including cytokine release syndrome (CRS) and grade ≥ 3 neurological events.
Statistical analysis
All statistical analyses were conducted using STATA version 12.0. The heterogeneity was assessed using I2 values. Egger’s and Begg’s tests were used to evaluate the publication bias. Subgroup analysis was conducted to analyze the heterogeneity between studies. All the pooled results were calculated by the random effects model. P < .05 was considered statistically significant.
Result
Patient characteristics
A total of 8 clinical trials with 729 patients were enrolled in the systemic review [Citation13–20], with 452 (62%) of male patients. Among all the patients studied, 628 (86%) were ALL and 101 (14%) were NHL. The 101 NHL patients suffered from different subtypes of disease including diffuse large B cell lymphoma (DLBCL) (n= 39, 39%), follicular lymphoma (FL) (n = 28, 28%), mantle cell lymphoma (MCL) (n = 24, 24%), and other types of lymphoma (n = 10, 10%). For transplant history before blinatumomab treatment, 233 patients underwent allo-HSCT and 30 patients were treated with auto-HSCT. All the enrolled patients in the study had a primary refractory or relapsed disease after traditional chemotherapy or hematopoietic stem cell transplantation. Blinatumomab was given to patients through continuous intravenous infusion. All patients received glucocorticoid prophylaxis before blinatumomab administration. The characteristics of the 8 included studies were summarized in .
Table 1. Study characteristics.
Response
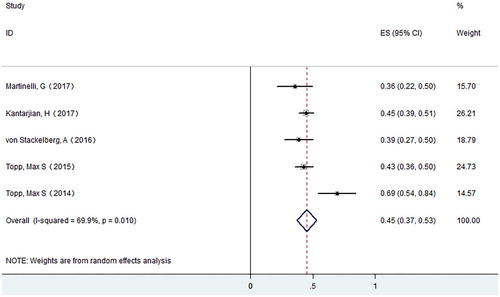
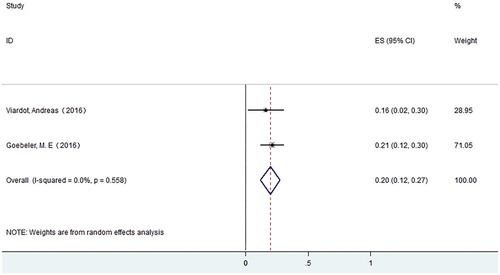
A total of 7 studies with 708 patients were enrolled for final response evaluation. For ALL, 268 patients achieved CR. The CR rate varies from 0.36–0.69, with a pooled CR rate of 0.45 (95% CI: 0.37–0.53) using the random effects model. Meanwhile, we observed substantial heterogeneity (I-squared = 69.9%, P = .01) (). For NHL, 20 patients achieved CR. The CR rate varies from 0.16–0.21, with a pooled CR rate of 0.20 (95% CI: 0.12–0.27) using the random effects model ().
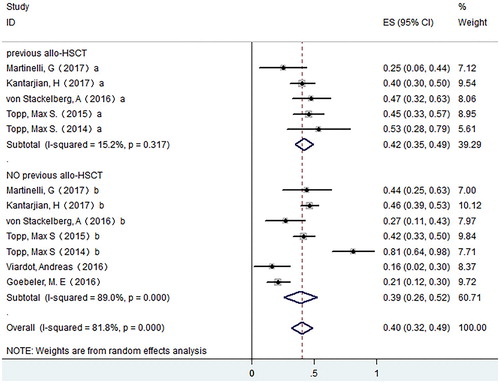
For publication bias, we used both Begg’s and Egger’s test, and no obvious publication bias was observed (P = .764 for Begg’s test; P = .705 for Egger’s test). For ALL, to analyze the source of heterogeneity for the CR rates, we conducted subgroup analysis on possible factors which may influence the CR rate. In order to investigate if previous allo-HSCT will affect the CR rate of patients receiving blinatumomab treatment, we compared CR rate between patients with allo-HSCT history and those without. We found no significant difference between the two groups, and the pooled CR rate was 0.42 (95% CI: 0.35–0.49) for patients with allo-HSCT history (I-squared = 15.2%, P = .317) and 0.39 (95% CI: 0.26–0.52) for patients without (I-squared = 89.0%, P = .000) ().
To investigate if tumor load before blinatumomab treatment impacts the CR rate in ALL, patients were divided into two groups based on the bone marrow blast right before blinatumomab administration. The pooled CR rate of patients with bone marrow blast percentage lower than 50% (0.75, 95% CI: 0.67–0.83) is higher than that of patients with percentages equal or higher than 50% (0.33,95% CI: 0.26–0.41) ().
Six studies with 628 patients were evaluated for MRD response after blinatumomab treatment, and all of them were ALL patients. The pooled MRD response rate in ALL patients was 0.42 (95% CI: 0.29–0.54) with substantial heterogeneity observed (I-squared = 90.2%, P = .0000) ().
Prognosis
Not all the studies reported OS and RFS. 6-month OS was 54% in Kantarjian et al’s study [Citation14]. Von Stackelberg et al. reported a 6-month RFS of 42% [Citation16]. Viardot et al. reported that the median progression-free survival (PFS) was 3.7 months in 25 DLBCL patients [Citation17]. summarized the reported OS and RFS in six studies.
Table 2. Summary of survival analysis.
For ALL, allogeneic HSCT information was studied in 6 studies with 628 patients. 148(23.6%) patients proceeded to allogeneic HSCT after successful blinatumomab treatment. 78 (12.4%) patients suffered from primary disease relapse. Among them, 17 relapsed without receiving allo-HSCT, 5 relapsed after receiving allo-HSCT, and the information for the remaining 56 was unknown.
For NHL, one study with 76 patients reported allogeneic HSCT information. 2 (2.6%) patients proceeded to allogeneic HSCT successfully. 3 (3.9%) patients (2 FL, 1 MCL) suffered from disease relapse after blinatumomab treatment.
Adverse effects
The common adverse effects included pyrexia, headache, neutropenia, infection, etc. Adverse events of grade ≥ 3 are summarized in Table S1 (supplementary material). Most adverse effects alleviated without intervention or with only symptom-oriented treatment. The most common severe adverse effect (grade ≥ 3) was hematological toxicity. We analyzed severe CRS and neurological events of grade ≥ 3. Two studies (97 patients) did not report the occurrence of CRS. Among the remaining 632 patients, 22 (3.5%) patients experienced grade ≥ 3 CRS. The pooled occurrence rate of grade ≥ 3 CRS was 0.04 (95% CI: 0.01–0.06), using the random effects model, with little heterogeneity observed (I-squared = 48.4%, P = .121) (). All the studies enrolled reported the occurrence of neurological events, with 88 (12.1%) patients manifesting grade ≥ 3 neurological events. The pooled occurrence rate of grade ≥ 3 neurological events was 0.12 (95% CI: 0.08–0.12) using the random effects model, with substantial heterogeneity observed (I-squared = 65%, P = .006) ().
Discussion
In our analysis, the pooled CR rate of blinatumomab treatment was 0.45 in ALL and 0.20 in NHL, which indicated the effectiveness of blinatumomab in treating relapsed/refractory ALL and NHL. The pooled MRD response rate in ALL was 0.42, revealing blinatumomab’s capacity to eliminate minimal residual disease. Allo-HSCT is the sole curative method for many relapsed/refractory hematological malignancies. However, due to an unsatisfied primary disease control, many patients have no opportunity to proceed to HSCT. Moreover, disease status at transplantation is closely related to the long-term prognosis after transplantation, and leukemia patients who did not reach CR for their primary disease before allo-HSCT are more likely to suffer from disease recurrence after transplantation [Citation21,Citation22]. In our analysis, a total of 148 (23.6%) ALL and 2 (2.6%) NHL patients successfully proceeded to allogeneic HSCT after blinatumomab treatment, which demonstrated that blinatumomab could build a good bridge to hematopoietic stem cell transplantation.
ALL with Philadelphia chromosome (Ph) often indicates a dismal prognosis, and the treatment failure is frequently associated with TKI resistance substitutions in the ABL kinase domain, particularly within the T315I mutation [Citation23]. Blinatumomab has shown its efficacy in Ph+ ALL patients who were refractory or relapsed after TKI therapy in some studies. For example, in Martinelli et al’s study, a 35.6% CR rate was obtained in Ph+ ALL. For those with T315I mutation, the CR rate was 40% [Citation13]. Although the preliminary data is encouraging, more studies are required to determine the role of blinatumomab for treatment of Ph+ ALL.
Interestingly, we have found a higher CR rate in ALL patients than that of NHL patients receiving blinatumomab, which is consistent with the results found for anti-CD19 CAR-T therapy [Citation24]. This conclusion, however, needs to be confirmed by more studies since only two clinical trials included NHL patients, with a relatively small sample size. Moreover, ALL patients whose BM blasts <50% reached a higher CR rate than that of patients with BM blasts ≥50%, which implies that heavy tumor load is related with worse response. These results suggest that pretreatments to reduce tumor load, such as chemotherapy are necessary before blinatumomab treatment in ALL patients. For NHL patients, more studies are required to determine the relationship between efficacy and tumor load.
In the studies enrolled, single-agent blinatumomab reached a median OS of 6–10 months and a median RFS of 5–8 months for ALL patients. In a phase 3 study, blinatumomab was shown to nearly double the median OS compared with the salvage chemotherapy group (7.7 months vs 4.0 months) [Citation14]. Several studies have found that achieving a complete MRD response was associated with prolonged OS and RFS compared with no MRD response [Citation16,Citation18], indicating that treatment with blinatumomab may prolong ALL patients’ overall survival as compared with chemotherapy.
CRS and neurological events are the most common causes to treatment-related death or premature discontinuation of trial. In our analysis, the occurrence rate of grade ≥3 CRS after blinatumomab treatment was obviously lower than that of anti-CD19 CART therapy [Citation5]. Two reasons may explain the low incidence and mildness of CRS during blinatumomab therapy. One reason is that blinatumomab has been administrated in a stepwise rather than a flat dosing manner. A relatively lower serum cytokine levels and lower body temperature were observed in patients treated with stepwise dosing than flat dosing [Citation25]. The other reason is probably the glucocorticoid prophylaxis before administration of blinatumomab. In an in-vitro experiment, dexamethasone was proven to be able to reduce cytokine levels and did not inhibit the T cell cytotoxic activity of CD19/CD3-bispecific single-chain antibody [Citation26]. In Shahram Mori’s study, methyl-prednisolone was given to two patients who developed symptoms of CRS such as pyrexia and ferritin increase. Afterwards both patients achieved complete remission of CRS [Citation27]. The other important adverse effects are neurological events. Transient neuroinflammatory irritation is considered to be associated with blinatumomab-associated neurological events, though the detailed pathogenesis is not entirely comprehended [Citation28]. The pooled occurrence rate of grade ≥ 3 neurological events is 0.12 in our study, which is also lower than that of anti-CD19 CART therapy [Citation6–8]. Moreover, all neurologic events are fully reversible and manageable. In addition, since pharmacodynamics and pharmacokinetics studies have shown a short half-life of only several hours for blinatumomab [Citation29], we speculate that blinatumomab related adverse effects may relieve if the drug is withdrawn timely.
Our study is the first meta-analysis evaluating the efficacy and safety of blinatumomab used for the treatment of relapsed/refractory ALL and NHL. However, there are limitations in our study. Firstly, the sample size is relatively small, especially that there are only two studies on NHL patients. Secondly, the random effects model we use in this study may minimize the inherent variances. Finally, we did not make analysis on patients with Ph+ ALL due to the limited numbers of patients.
In conclusion, blinatumomab is effective and safe in treating relapsed/refractory ALL or NHL in our meta-analysis. We also find that ALL patients respond better to this drug than NHL patients, and lower tumor load favors the therapeutic effect. For adverse effects, severe CRS and neurological events do not happen very often. In the future, more clinical trials are needed to explore the therapeutic potential and safety of blinatumomab in hematologic malignancies.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available for containing information that could compromise the privacy of research participants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Goekbuget N, Dombret H, Ribera J-M, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 2016;101(12):1524–1533. doi: 10.3324/haematol.2016.144311
- Gokbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012;120(10):2032–2041. doi: 10.1182/blood-2011-12-399287
- Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA study group. Haematologica 2010;95(4):589–596. doi: 10.3324/haematol.2009.014274
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129(25):3322–3331.
- Pan J, Yang JF, Deng BP, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31(12):2587–2593. doi: 10.1038/leu.2017.145
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447
- Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. doi: 10.1200/JCO.2016.71.3024
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277
- Hoelzer D, Walewski J, Dohner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood 2014;124(26):3870–3879. doi: 10.1182/blood-2014-03-563627
- Nagorsen D, Kufer P, Baeuerle PA, et al. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136(3):334–342. doi: 10.1016/j.pharmthera.2012.07.013
- Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011;317(9):1255–1260. doi: 10.1016/j.yexcr.2011.03.010
- Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with Blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531
- Kantarjian H, Stein A, Goekbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783
- Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct Blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–1111. doi: 10.1200/JCO.2014.59.1586
- von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II study of Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi: 10.1200/JCO.2016.67.3301
- Viardot A, Goebeler M-E, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016;127(11):1410–1416. doi: 10.1182/blood-2015-06-651380
- Topp MS, Goekbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukemia: a multicenter, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2
- Topp MS, Gokbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. doi: 10.1200/JCO.2014.56.3247
- Topp MS, Kufer P, Goekbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270
- Krauter J, Wagner K, Stadler M, et al. Prognostic factors in allo-SCT of elderly patients with AML. Bone Marrow Transplant. 2011;46(4):545–551. doi: 10.1038/bmt.2010.145
- Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32(8):835–842. doi: 10.1038/sj.bmt.1704223
- Maino E, Sancetta R, Viero P, et al. Current and future management of Ph/BCR-ABL positive ALL. Expert Rev Anticancer Ther. 2014;14(6):723–740. doi: 10.1586/14737140.2014.895669
- Zhang T, Cao L, Xie J, et al. Efficiency of CD19 chimeric antigen receptor-modified T cells for treatment of B cell malignancies in phase I clinical trials: a meta-analysis. Oncotarget. 2015;6(32):33961–33971.
- Nägele V, Kratzer A, Zugmaier G, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. 2017;6:14. doi: 10.1186/s40164-017-0074-5
- Brandl C, Haas C, d’Argouges S, et al. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother. 2007;56(10):1551–1563. doi: 10.1007/s00262-007-0298-z
- Mori S, Nelson RJ, Patel RD, et al. Low dose steroids can alleviate blinatumomab-associated toxicities without negatively impacting treatment efficacy. 57th ASH Annual Meeting and Exposition; 2015 Dec 05-08; Orlando, FL.
- Goebeler ME, Bargou R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma.2016;57(5):1021–1032. doi: 10.3109/10428194.2016.1161185
- Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–6233. doi: 10.1182/blood-2012-01-400515