ABSTRACT
Background: Various subsets of diffuse large B-cell lymphoma(DLBCL) are distinguished based on molecular and immunohistochemical features. As we know, CD5 is a pan-T-cell surface marker and is seldom expressed in DLBCL. Large-scale studies of de novo CD5+ DLBCL are lacking in Chinese patients.
Method: A total of 139 patients with DLBCL (30 CD5+ DLBCL and 109 CD5− DLBCL) who were immunophenotyped and treated with chemotherapy were subjected to this analysis. There were 85 males and 54 females. Their age ranged from 17 to 84 years old, and the median age was 58 years old.
Results: In this study CD5+ DLBCL was associated with higher IPI scores (>2), bone marrow involvement, higher probability of >1 ECOG performance status, non-germinal center B-cell like(non-GCB), BCL2 overexpression, whereas seldom expressed CD10 or BCL6, and unconspicuous higher expression of Ki67. With standard chemotherapy, CD5+ DLBCL patients had significantly worse overall survival (OS, median, 29.5 months vs. not reached, P = 0.0004) and progression-free survival (PFS, median, 18.0 months vs. not reached, P = 0.0002) than CD5− DLBCL patients, which had independent prognostic significance of the International Prognostic Index (IPI), and subtype of the non-GCB DLBCL. For CD5+ DLBCL, the addition of rituximab to chemotherapy may not significantly improve the OS (median, 14 months vs. 29.5 months, P = 0.72) and PFS (median, 10 months vs. 12 months, P = 0.92).
Conclusion: CD5+ DLBCL patients have the distinctive clinical and biological features, they should be provided with clinic individualized treatment and important pathways with therapeutic implications should be underscored.
Introduction
DLBCL is one of the most common subtypes of mature B-cell neoplasms, which constitutes about 40% of the Non-Hodgkin lymphoma(NHL) [Citation1]. DLBCL is a distinct group with many subtypes and entities based on morphology, immunophenotypic characteristics and clinical presentation. The phenomenon of CD5 expression in DLBCL evolving primary, which is not as a result of the transformation of chronic lymphocytic leukemia or mantle cell lymphoma, was firstly described by Matolcsy et al. in 1995 [Citation2]. Since then, accumulating studies have gradually clarified that de novo CD5 positive (CD5+) DLBCL constitutes a unique subgroup which is different from non-GCB subtype and the germinal center B-cell-like (GCB) subtype on immunophenotypic features [Citation3].
Gene-expression profiling (GEP) has demonstrated that B-cell receptor signaling dysfunction and microenvironment alterations are important mechanisms underlying the clinical impact of CD5 expression. CD5 inhibits signaling downstream of the BCR pathway, including the calcium response and interleukin-2 (IL2) production whereas augments BCR-mediated IL10 productions, an anti-inflammatory cytokine and a survival factor for B-cells [Citation4–6]. This molecular basis may explain in part why de novo CD5+ DLBCL shows more aggressive clinical features than CD5− DLBCL.
To date large-scale studies of de novo CD5+ DLBCL have been conducted in Japan, with a reported frequency of 5% to 10% of all DLBCL [Citation3,Citation7–10]. Compared with patients with CD5− DLBCL, CD5+ DLBCL patients are reportedly more often elderly, female, and having >1 ECOG performance status, elevated serum lactate dehydrogenase (LDH) level, advanced stage disease, >1 extranodal sites, B-symptoms, and high IPI at diagnosis [Citation3,Citation7,Citation11]. Pathologically, CD5+ DLBCL is associated with active B-cell (ABC) subtype and BCL2 overexpression [Citation9,Citation11,Citation12].
Most studies from Japan have shown that the clinical outcomes of CD5+ DLBCL patients treated with standard CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy with or without rituximab are poor [Citation13,Citation14]. So far, some other salvage therapy has also been reported. Alinari L et al. [Citation15] reported on 102 patients with de novo CD5+ DLBCL treated with rituximab-containing therapy at nine different institutions. Their study confirms the poor prognosis of de novo CD5+ DLBCL in a large multi-center cohort despite initial rituximab-containing chemotherapy and suggests that stem cell transplantation fails to salvage the majority of these patients.
In China, a few cases of de novo CD5+ DLBCL have been reported [Citation12,Citation16], and a morphologic and immunophenotypic study of 33 cases of de novo CD5+ DLBCL showed its clinical and pathological features by the Cancer Institute and Hospital of Tianjin Medical University [Citation17]. Few large-scale study of CD5+ DLBCL in China has been performed with attention focused on the clinicopathologic features and clinical response to rituximab-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone). The purpose of this study is to assess the clinicopathologic and biological features of de novo CD5+ DLBCL and to evaluate the prognostic significance of CD5 expression in DLBCL treated with R-CHOP in Chinese patients.
Design and methods
Patients
We selected 30 patients with de novo CD5+ DLBCL and homochromous 109 patients with CD5− DLBCL. There were 85 males and 54 females. Their age ranged from 17 to 84 years old, and the median age was 58 years old. All patients were diagnosed between April 2011 and October 2015 with DLBCL according to the 2008 WHO classification [Citation18]. Patients with diffuse large B cell lymphoma not otherwise specified (DLBCL, NOS) were selected as the subjects of the study, exclusive of primary mediastinum (thymus) B cell lymphoma, ALK positive large B cell lymphoma, plasma cell lymphoma, lymphomatoid granulomatosis, intravascular large B-cell lymphoma, primary exudative lymphoma and large B cell lymphoma produced by HHV8-related multicenter Castleman disease. The results of immunohistochemistry include CD5 markers in patients who qualify for the group. Clinical information was obtained from the hospital records.
Immunohistochemistry
The pathological sections of all CD5+ DLBCL patients were reviewed by at least 2 pathologists. The tissue section was immunohistochemically stained using the EnVision method. And the pathological data of CD5 positive patients were provided by the pathologists. We use a cutoff of 10% or more of tumor cells with dim to expression for CD5 to be considered positive. According to the Hans algorithm [Citation19], based on immunohistochemical results of CD10, BCL6 and MUM1, GCB subtypes were classified as CD10(+)/BCL6(±)/MUM1(±) or CD10(−)/BCL6(+)/MUM1(−), and the rest is classified as non-GCB subtypes. The cutoffs for positivity were ≥30% for CD10, BCL6, and MUM1 to classify these tumors as germinal center or non-germinal center cell-like immunophenotype. The positive cutoff for BCL2 was ≥50%.
Statistical analysis
Correlations between the two groups were examined with the χ2 test and Fisher’s exact test. In this study, the OS was used as the main terminal marker. The last follow-up period was September 30, 2017. OS was calculated from the time of diagnosis to death from any cause or the last follow-up. PFS was calculated from the time of diagnosis to disease progression, relapse, or death from any cause. Patients who were alive and/or had no disease progression were censored at the last follow-up. Survival analysis was performed using the Kaplan–Meier method with GraphPad Prism 7 (GraphPad Software), and differences were compared using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazards regression model with the SPSS statistics software program (version 23.0; IBM Corporation). All differences with P ≤ 0.05 were considered statistically significant.
Results
Associated clinicopathologic features of CD5 expression in DLBCL
Comparison of the clinical characteristics of CD5+ vs. CD5− DLBCL patients in this study showed that CD5+ DLBCL patients were more frequently with higher IPI scores (>2), bone marrow involvement, higher probability of >1 ECOG performance status (P < 0.05), and it seemed to be the tendency of women to dominate (). Moreover, there was no significant difference between the two groups in age, sex, B-symptoms, LDH level, number of extranodal site and central nervous system (CNS) involvement, which were inconsistent to previous reports.
Table 1. Clinical features of patients between CD5+ and CD5-de novo DLBCL, and patients between CD5+ and CD5− Non-GCB DLBCL.
Pathological features of CD5+ vs. CD5– DLBCL patients were characterized by comparing their protein expression profiles (). CD10 was positive in 1 (3.3%) of the 30 patients with CD5+ DLBCL and in 25 (43.1%) of the 109 patients with CD5− DLBCL. The CD10 positive rate was significantly lower in the CD5+ DLBCL group than that in the CD5− DLBCL group (P < 0.0001). BCL-2 was positive in 89.3% of the patients with CD5+ DLBCL and in 55.4% of the patients with CD5− DLBCL, showing a significant difference (P = 0.001). BCL-6 was positive in 62.1% of the patients with CD5+ DLBCL and in 80.7% of the patients with CD5− DLBCL, showing that BCL6 positive rate was significantly lower in the CD5+ DLBCL group than that in the CD5− DLBCL group (P = 0.043). The MUM-1 was positive in 82.1% of the patients with CD5+ DLBCL and in 77.9% of the patients with CD5− DLBCL (No significance). Similarly, there was no significant difference in the expression of EBER in CD5+ DLBCL and CD5− DLBCL. However, Chuang WY et al. [Citation20] have collected 174 DLBCL cases in Taiwan and performed immunophenotyping and detection of Epstein–Barr virus (EBV)-encoded RNA (EBER) by in situ hybridization. They indicated that EBER positivity was associated with IPI > 2, stage III–IV, and B symptoms which was not an independent adverse prognostic factor, but its effect was likely to be due to accompanying adverse clinical parameters.
Table 2. Pathological features of patients with CD5+ and CD5− de novo DLBCL.
Likewise, the high expression of Ki67(≥80%) was not significantly different between the two groups. The results appear to be different from those reported in the literature. Thakral B et al. [Citation21] showed a higher rate of central nervous system relapse (33.3% vs 15.6%; P < 0.01) and a higher Ki67 proliferative index compared with CD5− patients. When the 30 CD5 positive DLBCL patients were divided into the GCB group and the non-GCB group, the non-GCB group consisted of 25 patients (83.3%) and the GCB group consisted of 2 patients (6.7%). The proportion of patients with the non-GCB type was significantly higher in the CD5 positive DLBCL group than that in the CD5 negative DLBCL group (P = 0.014). The present study, which includes the largest reported number of patients with CD5+ DLBCL, confirms that most CD5+ DLBCLs are non-GCB DLBCLs, suggesting that therapeutic strategies for non-GCB DLBCL may be effective for the treatment of CD5+ DLBCL [Citation22].
Multivariate analysis of OS and PFS, and therapeutic response in patients with de novo CD5+ DLBCL and overall DLBCL
CD5+ DLBCL patients had significantly poorer OS (median OS: 29.5 months vs. not reached, hazard ratio [HR]: 2.64, 95% confidence interval [CI] of rate: 1.28–5.47, P = 0.0004) and PFS (median PFS: 18 months vs. not reached, HR: 2.75, 95% CI: 1.32–5.75, P = 0.0002) regardless of cell-of-origin (COO). The expression of CD5 in non-GCB DLBCL is still significant for OS and PFS. CD5+ non-GCB DLBCL patients had significantly worse OS (median OS: 22 months vs. not reached, P = 0.0018) and PFS (median PFS: 17 months vs. not reached, P = 0.0008) than CD5− non-GCB DLBCL patients (A–D). Between CD5+ patients with GCB and non-GCB DLBCL there was no significant difference in OS or PFS (P = 0.13 for OS, and P = 0.17 for PFS). These may indicate that CD5 expression has a stronger effect on prognosis than cell-of –origin.
Figure 1. Prognostic significance of CD5 in de novo DLBCL. (A–D) CD5 expression was correlated with significantly poor OS and PFS in the overall, and non-GCB DLBCL cohorts.
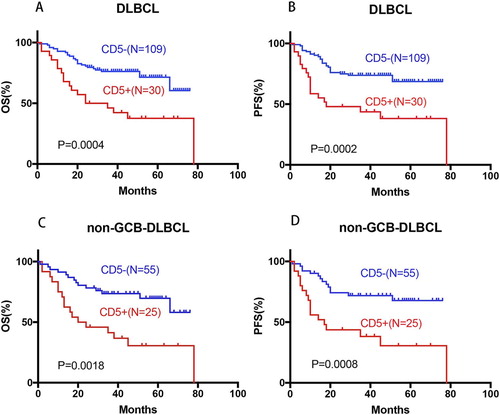
B-symptoms significantly impacted OS (P = 0.048) and unconsciously influenced PFS (P = 0.052) of overall DLBCL patients (E–F). No significant statistical difference about B-symptoms was found in the OS and PFS of non-GCB DLBCL. Signally, non-GCB subtypes significantly affected the OS (P = 0.0018) and PFS (P = 0.0031) of all DLBCL patients (G–H). Multivariate analysis of clinical and pathological factors including IPI (defined by age, stage, serum LDH, performance status, and extranodal sites), sex, B-symptoms and CD5+, BCL2+, and COO confirmed that CD5 expression independently predicted significantly poorer OS (P = 0.0004) and PFS (P = 0.0002) in DLBCL, in addition to the known unfavorable prognostic factors IPI >2 () (I–L). Moreover, Xu-Monette ZY et al. [Citation23] have reported the effect on BCL2 expression and prognostic independence of CD5 expression, and BCL2+ remained an independent prognostic factor for both OS (P < 0.0001) and PFS (P < 0.0001) in overall DLBCLs. Comprehensive above in our study, CD5+, non-GCB subtype and IPI > 2 are independent factors affecting the prognosis of DLBCL patients. As it is known, the addition of rituximab improves the OS of DLBCL patients [Citation10,Citation24]. However, when it is restricted to CD5 positive DLBCL, the prognosis is not statistically different between the patients treated with and without rituximab, the addition of rituximab can not improve OS (HR: 1.214, 95%CI: 0.49–3.75, P = 0.72) and PFS (HR: 1.05, 95%CI: 0.35–3.17, P = 0.92) of DLBCL patients (M–N).
Figure 2. Prognostic significance of B-symptoms and cell-of-origin(COO) in de novo DLBCL. (E–F) B-symptoms showed significant influence on the OS and PFS in the overall DLBCL; (G–H) non-GCB subtype significantly affected the OS and PFS of all DLBCL patients;
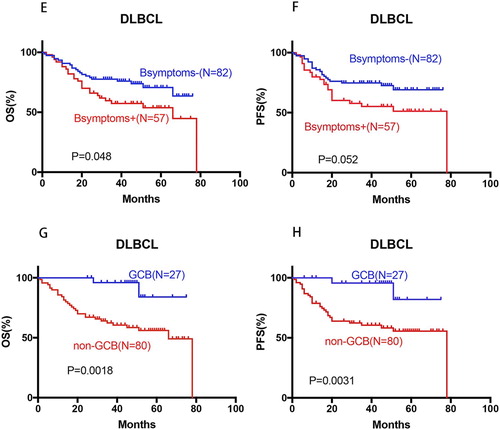
Figure 3. Prognosis significance of IPI in de novo DLBCL, OS and PFS of CD5-positive DLBCL patients treated with or without rituximab. (I–L) IPI > 2 was correlated with significantly poor OS and PFS in the overall, and non-GCB- DLBCL cohorts; (M–N) no beneficial change was observed for CD5-positive DLBCL patients after the introduction of rituximab.
Table 3. Multivariate analysis in the overall and non-GCB DLBCL cohort of patients.
Discussion
De novo CD5+ DLBCL is a unique subset of DLBCL, and CD5 is a cell surface glycoprotein which is mainly expressed by T cells and a small subset of normal B cells. In general, CD5 is observed in the lymphoma cells of patients with CLL, MCL and Richter’s transformation of CLL [Citation25]. In this study, we compared the clinicopathologic characteristics and prognosis of patients with CD5+ and CD5− DLBCL. We found that DLBCL patients with CD5 expression tended to be stage III-IV disease at diagnosis with a female preponderance. Furthermore, these patients were typically present with higher IPI scores (>2), bone marrow involvement, higher probability of >1 ECOG performance status, non-GCB, BCL2 overexpression, whereas seldom expressed CD10 or BCL6, and unconspicuous higher expression of Ki67. CD5 expression independently predicted significantly poorer OS and PFS in DLBCL.
There was no significant difference between the two groups in CNS involvement. The reason may be that the number of cases received was too small to analysis, and the short follow-up time resulted in low diagnostic rate of CNS. While Miyazaki K et al. [Citation14] have reported that this disease is characterized by a high incidence (12.7%) of central nervous system (CNS) relapse, compared with an incidence of approximately 5% for the whole DLBCL, and they reported that LDH levels plays an important role in monitoring CNS involment [Citation26,Citation27]. Patients with higher LDH levels are prone to CNS involvement and should be treated timely. Some patients are more likely to have central nervous system recurrence, mainly including who have paranasal sinus, testis, breast, bone marrow involvement and elevated LDH or more than 2 extranodal lesions. The current guidelines recommend the use of chemotherapy in central nervous system invasion in these patients. Prophylactic treatment, including 4–8 intrathecal injections or methotrexate 3–3.5 g/m2 systemic chemotherapy, and early identification of central nervous system invasion are essential for prognosis and long-term survival.
Our findings as well as those of Ennishi et al. [Citation28] showed the prognosis of CD5 positive patients was poor with rituximab treatment. In addition to this, our study showed CD5 positive DLBCL frequently expresses BCL2, which is consistent with the literature findings that the expression of BCL2 is significantly more frequent in CD5 positive DLBCL than that in CD5 negative DLBCL [Citation9,Citation28]. BCL2 protein expression has been associated with poor prognosis in DLBCLs. Since many related literatures have reported that the induction of rituximab improves the poor prognosis of BCL2 positive DLBCL [Citation29,Citation30], we naturally assume that rituximab is beneficial to improve the prognosis and survival of CD5+ DLBCLs. However, when it comes to CD5+ DLBCL patients with BCL2 positive, the prognosis did not improve after supplementation with rituximab. These findings suggest that the favorable outcome of rituximab containing chemotherapy is not mediated by BCL2. Or maybe there are some action mechanism targets that interfere with the role of rituximab in BCL2 with CD5+ DLBCLs.
As we know, dose-increased chemotherapy such as ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone) is superior to standard CHOP with regard to both event-free survival (EFS) and OS for poor-prognosis aggressive NHL [Citation31]. Compared with standard R-CHOP, intensified immunochemotherapy with R-ACVBP significantly improves survival of DLBCLs with low-intermediate risk according to the IPI, and its hematological toxic effects of the intensive regimen were raised but manageable [Citation32]. And rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R- EPOCH) is a dose- adjusted infusional regimen that has shown improved outcome (vs R- CHOP) in untreated patients with aggressive DLBCL. Nevertheless, when it comes to CD5+ DLBCL, the R- EPOCH regimen can’t improve the poor prognosis or reduce central nervous system relapse. Thakral B et al. [Citation21] concluded that addition of rituximab to the chemotherapy regimens improved PFS in patients with CD5+ DLBCL, but not CNS relapse rate and OS. CD5+ DLBCL has poor prognosis, when treated with R-CHOP and more intensive regimen such as R-ACVBP and R-EPOCH do not seem to improve the inferior survival associated with CD5+ DLBCL.
Interestingly, Zhang Y et al. [Citation25] reported a patient with CD5+ DLBCL who was resistant to R-CHOP as first-line chemotherapy and the intensive R-Hyper CVAD regimen (rituximab plus cyclophosphamide, vincristine, doxorubicin, and dexamethasone), but who quickly achieved a complete response after the administration of additional lenalidomide to R-GDP (rituximab plus gemcitabine, dexamethasone, cisplatin). The case report indicates that the clinical benefit of CD5+ DLBCL may depend on selection of a combined regimen including agents with a novel mechanism of action rather than dose-intensified chemotherapy.
Alinari L et al. [Citation15] retrospectively reviewed the clinical features and outcomes of 102 patients with de novo CD5+ DLBCL treated with rituximab-containing therapy as front-line chemotherapy, twenty of 28 (71%) recurrent transplanted patients with autologous, allogeneic, or both, relapsed once again. This study confirms the poor prognosis of de novo CD5+ DLBCL in a large multi-center cohort despite initial rituximab-containing chemotherapy and suggests that stem cell transplantation fails to salvage the majority of these patients. However, Tokuhira M et al. [Citation33] reported a successful treatment with autologous peripheral blood stem cell transplantation (APBSCT), they described the clinical course of a young Japanese man with de novo CD5+ DLBCL who showed marked elevation of the PB basophil count, and documented the clinical results and suggested that some salvage therapy, including APBSCT, may have the potential to cure the condition. From all parts mentioned above, it is worthy of note that de novo CD5+ DLBCL is associated with higher proliferative activity and a poorer prognosis. Novel effective therapy is needed for this subset of patients with DLBCL.
In conclusion, CD5 positivity should be routinely evaluated at diagnosis and utilized for risk stratification in future DLBCL clinical trials. Prospective studies are needed to determine the appropriate immunohistochemistry (IHC) cut-offs to better define which cases are CD5+ and the correlate degree of CD5 expression with outcomes. Furthermore, as our understanding of genetics and pathogenesis of DLBCL is increasing, specific focus on CD5+ DLBCL may guide us to regard the use of novel agents in clinical trials [Citation34]. With the development of our research in distinct genotypic, epigenetic, and clinical characteristics, special attention to CD5+ DLBCL may guide us to provide a potential nosology for precision-medicine strategies and individualized treatment in clinical trials with these patients. Hence, CD5 expression of DLBCL could be a new subtype with profound clinical significance.
Available data and materials
All data generated or analyzed during this study are included in this published article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050
- Matolcsy A, Chadburn A, Knowles DM. De novo CD5-positive and Richter’s syndrome-associated diffuse large B cell lymphomas are genotypically distinct. Am J Pathol. 1995;147(1):207–216.
- Yamaguchi M, Seto M, Okamoto M, et al. De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood. 2002;99(3):815–821. doi: 10.1182/blood.V99.3.815
- Jevremovic D, Dronca RS, Morice WG, et al. CD5+ B-cell lymphoproliferative disorders: beyond chronic lymphocytic leukemia and mantle cell lymphoma. Leuk Res. 2010;34(9):1235–1238. doi: 10.1016/j.leukres.2010.03.020
- Gary-Gouy H, Bruhns P, Schmitt C, et al. The pseudo-immunoreceptor tyrosine-based activation motif of CD5 mediates its inhibitory action on B-cell receptor signaling. J Biol Chem. 2000;275(1):548–556. doi: 10.1074/jbc.275.1.548
- Gary-Gouy H, Harriague J, Bismuth G, et al. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100(13):4537–4543. doi: 10.1182/blood-2002-05-1525
- Yamaguchi M, Ohno T, Oka K, et al. De novo CD5-positive diffuse large B-cell lymphoma: clinical characteristics and therapeutic outcome. Br J Haematol. 1999;105(4):1133–1139. doi: 10.1046/j.1365-2141.1999.01513.x
- Harada S, Suzuki R, Uehira K, et al. Molecular and immunological dissection of diffuse large B cell lymphoma: CD5+, and CD5− with CD10+ groups may constitute clinically relevant subtypes. Leukemia. 1999;13(9):1441–1447. doi: 10.1038/sj.leu.2401487
- Yamaguchi M, Nakamura N, Suzuki R, et al. De novo CD5+ diffuse large B-cell lymphoma: results of a detailed clinicopathological review in 120 patients. Haematologica. 2008;93(8):1195–1202. doi: 10.3324/haematol.12810
- Hyo R, Tomita N, Takeuchi K, et al. The therapeutic effect of rituximab on CD5-positive and CD5-negative diffuse large B-cell lymphoma. Hematol Oncol. 2010;28(1):27–32.
- Niitsu N, Okamoto M, Tamaru JI, et al. Clinicopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large B-cell lymphoma. Ann Oncol. 2010;21(10):2069–2074. doi: 10.1093/annonc/mdq057
- Zhang Q, Zhang HY, Zhong FL, et al. Clinical analysis of 10 patients with de novo CD5 positive diffuse large B cell lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(2):399–402.
- Tzankov A, Leu N, Muenst S, et al. Multiparameter analysis of homogeneously R-CHOP-treated diffuse large B cell lymphomas identifies CD5 and FOXP1 as relevant prognostic biomarkers: report of the prospective SAKK 38/07 study. J Hematol Oncol. 2015;8(70).
- Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22(7):1601–1607. doi: 10.1093/annonc/mdq627
- Alinari L, Gru A, Quinion C, et al. De novo CD5+ diffuse large B-cell lymphoma: adverse outcomes with and without stem cell transplantation in a large, multicenter, rituximab treated cohort. Am J Hematol. 2016;91(4):395–399. doi: 10.1002/ajh.24299
- Zheng Y, Ma XB, Jiang J, et al. CD5 expression is an adverse prognostic factor in diffuse large B-cell lymphoma. Zhonghua Bing Li Xue Za Zhi. 2012;41(3):156–160.
- Zang L, Zhang HY, Zhong FL, et al. Clinical analysis of CD5 + diffuse large B-cell lymphoma. Shandong Med J. 2015;55(37):12–14.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009: 523–531. doi: 10.1182/asheducation-2009.1.523
- Hans CP., Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545
- Chuang WY, Chang H, Shih LH, et al. CD5 positivity is an independent adverse prognostic factor in elderly patients with diffuse large B cell lymphoma. Virchows Arch. 2015;467(5):571–582. doi: 10.1007/s00428-015-1845-1
- Thakral B, Medeiros LJ, Desai P, et al. Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol. 2017;98(4):415–421. doi: 10.1111/ejh.12847
- Miyazaki K, Yamaguchi M, Imai H, et al. Gene expression profiling of diffuse large B-cell lymphomas supervised by CD5 expression. Int J Hematol. 2015;102(2):188–194. doi: 10.1007/s12185-015-1812-2
- Xu-Monette Z, Tu M, Jabbar KJ, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015;6(8):5615–5633. doi: 10.18632/oncotarget.3479
- Jain P, Fayad LE, Rosenwald A, et al. Recent advances in de novo CD5+ diffuse large B cell lymphoma. Am J Hematol. 2013;88(9):798–802. doi: 10.1002/ajh.23467
- Zhang Y, Wang X, Liu Y, et al. Lenalidomide combined with R-GDP in a patient with refractory CD5-positive diffuse large B-cell lymphoma: a promising response and review. Cancer Biol Ther. 2018;19(7):549–553. doi: 10.1080/15384047.2018.1449609
- Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma–a survey of 1693 patients treated in protocols of the German high-grade non-Hodgkin’s lymphoma study group (DSHNHL). Ann Oncol. 2007;18(1):149–157. doi: 10.1093/annonc/mdl327
- Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol. 2004;15(1):129–133. doi: 10.1093/annonc/mdh013
- Ennishi D, Takeuchi K, Yokoyama M, et al. CD5 expression is potentially predictive of poor outcome among biomarkers in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP therapy. Ann Oncol. 2008;19(11):1921–1926. doi: 10.1093/annonc/mdn392
- Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2–associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003;101(11):4279–4284. doi: 10.1182/blood-2002-11-3442
- Wilson KS, Sehn LH, Berry B, et al. CHOP-R therapy overcomes the adverse prognostic influence of BCL-2 expression in diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48(6):1102–1109. doi: 10.1080/10428190701344881
- Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–4289. doi: 10.1182/blood-2003-02-0542
- Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial.
- Tokuhira M, Watanabe R, Iizuka A, et al. De novo CD5+ diffuse large B cell lymphoma with basophilia in the peripheral blood: successful treatment with autologous peripheral blood stem cell transplantation. Am J Hematol. 2007;82(2):162–167. doi: 10.1002/ajh.20786
- Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445
