ABSTRACT
Objective: This meta-analysis was designed to investigate the association between interferon-gamma (IFN-γ) polymorphisms and occurrence risk of aplastic anemia.
Methods: Literature search was conducted in PubMed, Embase and Cochrane Library up to April 2018. The pooled odds ratios (ORs) and 95% confidence interval (CI) were calculated by R 3.12.
Results: Total five studies with 304 aplastic anemia patients and 588 controls were included. The statistically significant results were found in the following models: allele genetic model (T vs A: OR = 2.1749, 95% CI = 1.6825–2.8114, P < 0.01), additive genetic model (TA vs AA: OR = 2.1071, 95% CI = 1.3962–3.1799, P < 0.01; TT vs AA: OR = 4.5788, 95% CI = 2.6606–7.8797, P < 0.01), recessive genetic model (TT vs AA + TA: OR = 2.5579, 95% = 1.6680–3.9226, P < 0.01), dominant genetic model (TT + TA vs AA: OR = 2.5599, 95% = 1.7424–3.7611, P < 0.01), the results suggested that the increased occurrence risk of aplastic anemia is significantly associated with the IFN-γ polymorphism.
Conclusions: Patients with IFN-γ genotype carrying allele T have higher occurrence risk of aplastic anemia.
Introduction
Aplastic anemia is a rare hematological disease with abnormal number and functions of T lymphocytes as a main pathogenic factor and is characterized by pancytopenia and bone marrow hypoplasia which are mostly caused by immune-mediated destruction of hematopoietic stem cells [Citation1]. Currently, although there is technical development recently, the mortality rate caused by aplastic anemia is still high [Citation1–3]. The unclear pathogenesis is one of the main factor limiting the development of prevention and treatment strategy of aplastic anemia.
Previous studies have found the high levels of interferon gamma (IFN-γ) protein in peripheral blood mononuclear cells in patients with aplastic anemia [Citation4–6]. It was reported that the IFN-γ protein may be associated with the pathogenesis of aplastic anemia via T-bet/IFN-γ Signal Transduction Pathway, inflammatory factors (such as Tumor necrosis factor and interleukin) or cytokines (CD4 and CD8) [Citation6–9]. Considering the protein levels generally regulated by its gene, it was speculated that the IFN-γ gene may be also associated with the occurrence or development of aplastic anemia by regulating the expression of IFN-γ protein. Moreover, some studies have proved that the IFN-γ gene polymorphism +874(A/T) is associated with the occurrence risk of aplastic anemia [Citation10–13]. However, we could not definitely conclude the relationship between IFN-γ gene polymorphisms and occurrence risk of aplastic anemia due to small sample size and regionalism of participants in each study. Thus, we performed this meta-analysis to synthetically assess the relationship between IFN-γ gene polymorphisms and occurrence risk of aplastic anemia based on the data of recent studies.
Materials and methods
Search strategy
We conducted a literature search on databases including Embase, PubMed and Cochrane Library with the deadline of April 2018. The key words included ‘anemia, aplastic’ OR ‘Aplastic Anemia’ OR ‘aplastic’ OR ‘pure red cell aplasia’ OR ‘aplastic anemia’ AND IFN-γ AND polymorphism AND IFN-γ (interferon OR IFN) AND (polymorphi*). We also manually scanned the reference lists of some relevant reviews for obtaining additional studies.
Inclusion and exclusion criteria
The inclusion criteria of the included studies in this meta-analysis were (1) Studies were case–control studies; (2) participants were patients with aplastic anemia and controls (healthy individuals or non-aplastic anemia patients); (3) the IFN-γ polymorphism was detected; (4) the frequencies of alleles (T, A) and genotypes (TT, TA, AA) was reported or can be obtained by calculation.
Exclusion criteria were (1) non-original articles (such as letters, comments or reviews) (2) no available data, (3) duplicated publications (only the study with complete data can be included in this meta-analysis).
Quality assessment and data extraction
The quality of included studies was assessed independently by two investigators. Different evaluation results were resolved by discussion to ensure consistency of evaluation. The extracted data should be recorded using a predesigned form including first author name, publication year, study location, age, gender, sample size, method of detecting IFN-γ polymorphisms, and outcomes.
The quality of these included case–control studies was evaluated by the Newcastle-Ottawa-Scale (NOS) [Citation14], which comprised of three key domains and eight questions (Selection: total 4 questions, 1 score assigned to each question; Comparison: total 1 question, 2 scores assigned to this question; Explore: total 3 questions, 1 score assigned to each question). Thus, total of 9 scores can be obtained. The study with less than 5 scores was regarded as a low-quality study by NOS scale in this meta-analysis.
Statistical analysis
All analyses were performed using R 3.12. Hardy–Weinberg equilibrium was tested in controls by a χ2 test [Citation15]. The Chi-square and I2 tests were used to evaluate the heterogeneity across included studies. The presence of significantly statistical heterogeneity of outcomes across included studies was assessed when P < 0.05 and I2 > 50%. If there is no significant heterogeneity (P-value for heterogeneity ≥ 0.05 or I2 ≤ 50%), a fixed-effects model was chosen to pool the effect size; otherwise, a random-effects model was used. The pooled odds ratios (ORs) as well as their 95% confidence interval (CI) were used to assess the relationship between IFN-γ polymorphisms and occurrence risk of aplastic anemia. In addition, sensitivity analysis was performed by omitting one study at a time. Publication bias was assessed by Egger’s Test.
Results
Characteristics of included studies
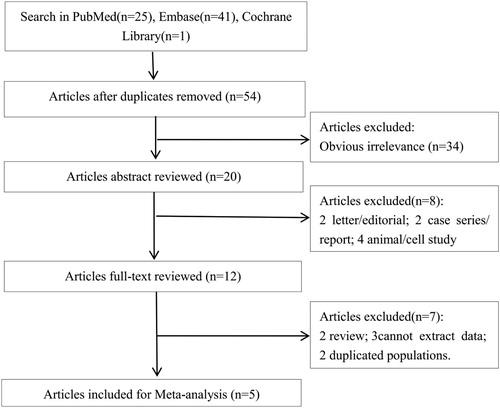
Literature search and study selection process are shown in . The initial search by literature search on databases identified 67 articles (PubMed: n = 25, from Embase: n = 41, Cochrane Library: n = 1). After excluding duplicates, 54 potentially relevant articles were remained. Afterward, 34 irrelevant articles were excluded by scanning titles. Among the 20 remaining articles, 8 studies (2 letter/editorial; 2 case series/report; 4 animal/cell study) were removed by scanning abstracts, 7 articles (2 review; 3 cannot extract data; 2 duplicated populations) were excluded. No additional studies were found by manual search. Finally, five articles were included in this meta-analysis [Citation10–13,Citation16].
The characteristics of the five included studies are listed in . The publication year ranged from 2004 to 2015. In the five included case–control studies, a total of 304 aplastic anemia patients and 588 controls were reanalyzed in this meta-analysis. All these studies detected the polymorphisms of IFN-γ by PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism), PCR-SSP (polymerase chain reaction-sequence-specific primer) or ARMS-PCR (amplification refractory mutation system-polymerase chain reaction), except one study did not describe the detection method [Citation13]. The polymorphisms of IFN-γ of the controls in each included study met the Hardy–Weinberg equilibrium, except El Mahgoub IR 2014 [Citation11] and Zayed RA 2015 [Citation16]. In addition, based on the NOS scores, no low-quality studies were included in this meta-analysis ().
Table 1. Characteristics of included studies.
Meta-analysis for relationship between polymorphisms of IFN-γ and aplastic anemia
As shown in and , no significant heterogeneity (I2 ≤ 50%, P ≥ 0.05) among studies was observed in all the analysis models, so the fixed effects model was used.
Figure 2. Overall meta-analyses for assessing relationship of IFN-γ polymorphisms with aplastic anemia risk.
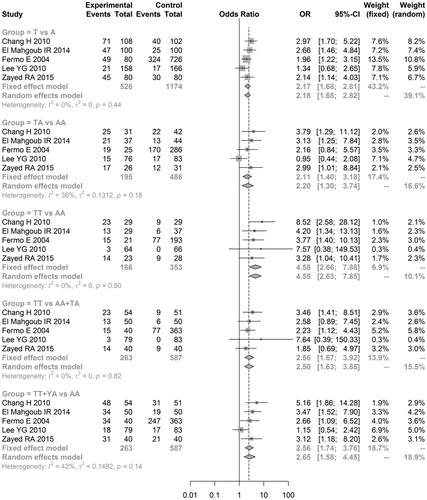
Table 2. Results of meta-analysis.
In allele genetic model, the results showed that aplastic anemia was significantly associated with the allele T (T vs A: OR = 2.1749, 95% CI = 1.6825–2.8114, P < 0.01). In additive genetic model, the pooled analyses showed that the patients with genotype TA or TT had higher occurrence risk of suffering from aplastic anemia than patients with genotype AA (TA vs AA: OR = 2.1071, 95% CI = 1.3962–3.1799, P < 0.01; TT vs AA: OR = 4.5788, 95% CI = 2.6606–7.8797, P < 0.01). In recessive genetic model, the results showed higher occurrence risk of aplastic anemia in patients with genotype TT than patients with genotype AA or TA (TT vs AA + TA: OR = 2.5579, 95% = 1.6680–3.9226, P < 0.01). Moreover, in the dominant genetic model, the analyses showed lower occurrence risk of aplastic anemia in patients with genotype AA than patients with genotype TT or TA (TT + TA vs AA: OR = 2.5599, 95% = 1.7424–3.7611, P < 0.01). Thus, we conclude that the higher occurrence risk of aplastic anemia is associated with the allele T or genotypes carrying allele T compared with the allele A or genotypes carrying allele A.
Sensitivity analyses and publication bias
In this study, no inconsistent results were found in sensitivity analyses.
Egger’s Test showed there was no significant publication bias (P > 0.05, )
Discussion
In this study, we found the significant relationship between IFN-γ polymorphisms +874(T/A) and occurrence risk of aplastic anemia. Moreover, genotypes carrying allele T was associated with the high occurrence risk of aplastic anemia.
As known, the level of IFN-γ protein is higher in peripheral blood mononuclear cells and bone marrow cells of aplastic anemia patients than healthy individuals [Citation17,Citation18]. High level of IFN-γ protein may be associated with the occurrence of aplastic anemia by disrupting lineage differentiation and altering hematopoietic stem or progenitor cell composition [Citation4]. Tumor necrosis factor alpha (TNF-α) is another higher expression protein in peripheral blood mononuclear cells and bone marrow cells from aplastic anemia patients [Citation17,Citation18]. Previous study reported that both IFN-γ protein and TNF-α protein could suppress early and late stages of hematopoiesis and induce programed cell death [Citation19]. Moreover, the gene polymorphisms of TNF-α also positively related to aplastic anemia [Citation20]. Thus, the IFN-γ/TNF-α pathway-induced suppression of human hematopoiesis may be the main pathogenesis of aplastic anemia. As shown in the previous study, the high expression of IFN-γ is associated with the genotype carrying allele T, rather than AA [Citation21]. Abnormally high expression of these genes caused by polymorphisms may be the main factor resulting in the startup of IFN-γ/TNF-α pathway, then inducing the occurrence of aplastic anemia.
Furthermore, a previous meta-analysis also analyzed the relationship between IFN-γ polymorphisms +874(T/A) and occurrence risk of aplastic anemia [Citation22]. Differently, this previous study did not find a significant relationship between occurrence risk of aplastic anemia with IFN-γ polymorphisms+874(T/A). The inconsistent result may be caused by different studies included for analysis. In this study, we included the study of Chang H 2010 [Citation10] and El Mahgoub IR 2014 [Citation11], which were not included in that previous meta-analysis. However, the study of Roderick 2013 [Citation23] was not included in this meta-analysis because the frequencies of alleles (T, A) and genotypes (TT, TA, AA) was not reported or cannot be obtained by calculation. Given the controversies and limitations in these meta-analyses, more studies should be performed to confirm the relationship between IFN-γ polymorphisms +874(T/A) and occurrence risk of aplastic anemia.
Limitations of the study
Although it was found in this meta-analysis that IFN-γ polymorphisms +874(T/A) was associated with the occurrence risk of aplastic anemia, there were still some limitations. Firstly, the covariate adjustment and meta-regression were not conducted due to the incomplete data of some studies, especially the demographic data, which might affect the results of the meta-analysis as potential confounders. Besides, the subgroup analysis was not performed due to the small total sample size and number of included studies. Secondly, it was failed to study the interactions between genes and the environment for lack of relevant data. Also, there might exist language bias because only eligible studies with English language were included. Thirdly, although no significant heterogeneity among included studies was found in this study, the impact factors of the relationship between IFN-γ polymorphisms +874(T/A) and occurrence risk of aplastic anemia must be investigated in further studies to confirm the stability of this relationship. In addition, the other gene polymorphisms (such as TNF-α) may be also associated with occurrence risk of aplastic anemia and there may be interactions between these genes [Citation24,Citation25]. Thus, the effect of these gene polymorphisms on occurrence risk of aplastic anemia should be investigated and updated based on more high-quality studies with a large sample size in further studies.
Conclusion
In conclusion, IFN-γ polymorphisms +874(T/A) is associated with the occurrence risk of aplastic anemia. More studies are needed to further confirm the results of this meta-analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15(3):162–168. doi: 10.1097/MOH.0b013e3282fa7470
- Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436. doi: 10.1182/blood-2016-08-693481
- Joshi S, Rose MJ, Stanek JR, et al. Comparison of resource utilization, cost and mortality in children treated for severe aplastic anemia. American Society of Hematology Meeting; 2016.
- Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124(25):3699–3708. doi: 10.1182/blood-2014-01-549527
- Tong Y, Li MA, Laboratory ZX-J. Expression and significance of IFN-γ and IL-4 in Th and NKT cells of patients with aplastic anemia. Chin J Immunol. 2011;27(9):840–843.
- Wang CH, Yao CJ, Tang XY, et al. Research on the change of aplastic anemia patients serum IL-2,TNF-α,IFN-γ and peripheral blood T lymphocyte subsets. Prog Mod Biomed. 2012;12(36):7077–7079.
- Wu Q, Zhang J, Shi J, et al. Increased bone marrow (BM) Plasma level of soluble CD30 and Correlations with BM Plasma level of interferon (IFN)-γ, CD4/CD8 T-cell ratio and disease Severity in aplastic anemia. PLos ONE. 2014;9(11):e110787. doi: 10.1371/journal.pone.0110787
- Xie Y. The expressions of serum IFN-γ,IL-4 and T cell subsets in aplastic anemia patients. Acta Academiae Med Zunyi. 2012;35(2):127–129.
- Zhang F, Zhao Z, Zhang X, et al. Study on T-bet/IFN-γ signal transduction pathway in aplastic anemia and Chinese medicine study development. Modern Tradi Chin Med Mater Medica-World Sci Technol. 2014;16(7):1642–1648.
- Chang H, Zeng F, Zhang JY, et al. Association of the interferon-gamma single nucleotide polymorphism + 874(T/A) with response to immunosuppressive therapy in patients with severe aplastic anemia. Blood Cells Mol Dis. 2010;45(4):313–316. doi: 10.1016/j.bcmd.2010.09.003
- Mahgoub IR E, Afify RA, Botros SK, et al. Immunoregulatory cytokines gene polymorphisms in Egyptian patients affected with acquired aplastic anemia. Ann Hematol. 2014;93(6):923–929.
- Fermo E, Bianchi P, Barcellini W, et al. Immunoregulatory cytokine polymorphisms in Italian patients affected by paroxysmal nocturnal haemoglobinuria and aplastic anaemia. Eur J Immunogenet. 2004;31(6):267–269. doi: 10.1111/j.1365-2370.2004.00480.x
- Lee YG, Kim I, Kim JH, et al. Impact of cytokine gene polymorphisms on risk and treatment outcomes of aplastic anemia. Ann Hematol. 2011;90(5):515–521. doi: 10.1007/s00277-010-1102-2
- Wells GA, Shea BJ, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of Non-Randomized studies in meta-analysis. Appl Eng Agric. 2014;18(6):727–734.
- Knapp M. Re: “biased tests of association: comparisons of allele frequencies when departing from Hardy-Weinberg proportions”. Am J Epidemiol. 1999;154(3):287–288. doi: 10.1093/aje/154.3.287
- Zayed RA, Abdel-Hamid SM, El-Lithy H. The association of cytokine genes polymorphisms and susceptibility to aplastic anemia in Egyptian patients. Hematology. 2016;21(2):106–112. doi: 10.1179/1607845415Y.0000000038
- Schultz JC, Shahidi NT. Detection of tumor necrosis factor-alpha in bone marrow plasma and peripheral blood plasma from patients with aplastic anemia. Am J Hematol. 1994;45(1):32–38. doi: 10.1002/ajh.2830450106
- Yougui YU, Wei K. The change of IFN-γ and TNF-α level in peripheral blood of mice with immune-mediated aplastic anemia. Clin Med. 2008;28(2):117–118.
- Selleri C, Sato T, Anderson S, et al. Interferon-γ and tumor necrosis factor-α suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165(3):538–546. doi: 10.1002/jcp.1041650312
- Cao FL, Liu XH, Jin-Mei LI. Study on expression levels and gene polymorphisms of TNF-α(tumor necrosis factor alpha) between them in aplastic anemia. Chin J Birth Health Heredity. 2007;15(8):27–28.
- Delaney NL, Esquenazi V, Lucas DP, et al. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. report of the ASHI minority workshops: part IV. Hum Immunol. 2004;65(12):1413–1419. doi: 10.1016/j.humimm.2004.07.240
- Alizadeh S. Interferon-gamma + 874 (T/A) polymorphism and susceptibility to aplastic anemia: a systematic review and meta-analysis. Evid Based Med. 2017;3(1):1000112. doi:10.4172/2471-9919.1000112.
- Roderick JE, Gonzalezperez G, Kuksin CA, et al. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. Rare Dis. 2013;210(1):1311–1329.
- Dirksen U, Moghadam KA, Mambetova C, et al. Glutathione S Transferase Theta 1 gene (GSTT1) Null genotype Is associated with an increased risk for acquired aplastic anemia in children. Pediatr Res. 2004;55(3):466–471. doi: 10.1203/01.PDR.0000111201.56182.FE
- Yesica B, Yamila S, Myriam A, et al. Polymorphisms in TNF and IFNG are associated with clinical characteristics of aplastic anemia in Argentinean population. Leuk Lymphoma. 2015;56(6):1793–1798. doi: 10.3109/10428194.2014.966707