ABSTRACT
Objectives: To analyze the efficacy and safety of decitabine combined with low/reduced-dose chemotherapy in newly diagnosed acute myeloid leukemia (AML) patients unfit for intensive therapy and to investigate the early prognostic indicators for these patients.
Methods: The eligible patients treated with decitabine-based chemotherapy were retrospectively analyzed. Responses and long-term survival were calculated and their correlation with clinical characteristics was analyzed. Minimal residual disease (MRD) detected by flow cytometry (FCM) after the induction therapy was measured, and the association with prognosis was explored.
Results: Fifty-five newly diagnosed AML patients were enrolled. The overall response rate (ORR) was 80.0%, with a complete remission (CR) rate of 63.64% and partial remission (PR) rate of 16.36%. Grade 4 hematological toxicity was common, and the incidence of infections was 83.64%, with 18.18% of patients suffered from severe infections. No serious bleeding or non-hematological adverse events occurred. Treatment-related mortality was 3.64%. The median overall survival (OS) and disease-free survival (DFS) were 17.0 (13.7–20.3) months and 17.0 (10.2–23.8) months, respectively. Multivariate analysis showed that an advanced age (≥ 60 years) and higher MRD (≥ 1.34%) after induction therapy were adverse prognostic factors for patients who had achieved CR.
Conclusions: Decitabine-based chemotherapy may be a suitable therapeutic alternative for newly diagnosed AML patients who are unfit for intensive chemotherapy. An advanced age (≥ 60 years) and higher MRD (≥ 1.34%) were considered adverse prognostic factors.
Introduction
Acute myeloid leukemia (AML) is a malignant disorder with highly heterogeneous, characterized by clonal aberrant proliferation of hematopoietic stem or progenitor cells. Treated with intensive chemotherapy, 60–80% of newly diagnosed AML patients achieved complete remission (CR) and approximately 50% of patients finally relapsed. Due to aging, history of hematologic diseases, and poor cytogenetic or molecular factors, some patients are unfit or intolerable for intensive chemotherapy. Thus, there is an urgent need to explore appropriate therapeutic approaches for these patients. As epigenetics is considered to play a critical role in the pathogenesis of leukemia [Citation1], hypomethylating agents (e.g. decitabine) which could restore the expression of tumor suppressor genes silenced by hypermethylation have become a new therapeutic strategy for the treatment of AML [Citation2]. The DACO-16 study demonstrated that decitabine was superior to best supportive care or low-dose cytarabine in elderly patients ineligible for intensive chemotherapy [Citation3,Citation4]. Moreover, Quintás et al. also reported that decitabine-based chemotherapy could bring a similar survival to intensive chemotherapy in elderly AML patients [Citation5]. Both decitabine alone or in combination with other agents showed promising efficacy and tolerance in elderly and high-risk AML patients [Citation3–5], however, it is still unclear which patients will benefit from this kind of therapy. Therefore, we investigated the correlation between clinical factors and responses, and explored the prognostic impact of the MRD level after induction therapy on patients treated with decitabine-based chemotherapy.
Patients and methods
Patients
A total of 55 AML patients treated in our center from January 2014 to August 2016 were enrolled. Inclusion criteria: (1) newly diagnosed AML by morphological and immunological phenotypes, excluding acute promyelocytic leukemia (APL); (2) unfit or intolerable to intensive chemotherapy for various reasons, such as: age ≥ 60 years, poor cytogenetic or molecular factors, history of hematologic diseases (secondary to MDS, MPN, etc.), treatment-related AML and severe infection or organ dysfunction; and (3) treated with decitabine-based chemotherapy. Exclusion criteria: (1) an estimated duration of survival < 3 weeks; and (2) treated with decitabine alone. The informed consents were obtained from all enrolled patients. This study was approved by the ethics committee of Jilin University, and conducted in accordance with the Declaration of Helsinki.
Treatment
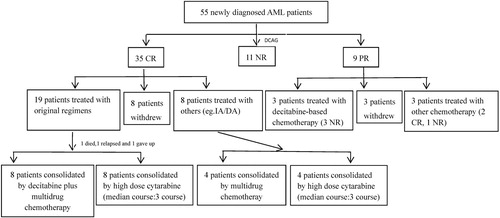
All patients were treated with decitabine-based chemotherapy. Decitabine was administrated as a dose of 20 mg/m2·d intravenously for 5 consecutive days (d1-5), and low/reduced-dose chemotherapy was administrated sequentially, as follows: (1) CAG: cytarabine (10 mg/m2, q12 h, ih, d6-12), aclarubicin (14 mg/m2, qd, ivd, d6-12) and G-CSF (300 μg, qd, ih, d6-12);(2) HAG: homoharringtonine (2 mg/m2, qd, ivd, d6-9), cytarabine (10 mg/m2, q12 h, ih, d6-12) and G-CSF (300 μg, qd, ih, d6-12); (3) DA: daunorubicin (40 mg/m2, qd, ivd, d6-8) and cytarabine (100 mg/m2, qd, ivd, d6-8); and (4) AA: aclarubicin (14 mg/m2, qd, ivd, d6-8) and cytarabine (100 mg/m2, qd, ivd, d6-8). Consolidation therapy was administrated as indicated in .
Evaluation
Response criteria were defined according to NCCN Guidelines. Overall survival (OS) was defined as the time from onset of induction to death, or last contact. Disease free survival (DFS) was defined as the time from CR to relapse, death, or last contact.
MRD monitoring
Eight-color multi-parameter flow cytometry (MP-FCM) was adopted to detect minimal residual disease (MRD). In general, (5–10)×105 cells were collected for every patient. CD117, CD34, CD33, CD13, CD38, HLA-DR, CD45, and another antibody (e.g., CD7/CD56/CD19 or CD123) were chosen as the panel of surface markers. Meanwhile, markers of leukemia-associated immunophenotypes (LAIP) (e.g., CD7/CD56/CD19) would also be investigated for MRD monitoring in those with LAIP at diagnosis. Cells with abnormal expression patterns, change of expression intensity (e.g., low expression, over expression) and aberrant expression (e.g., LAIP) were regarded as MRD.
Adverse events
Toxicities were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). All patients received antimicrobials, supportive care, and transfusion of blood products when necessary.
Statistics
All statistical analyses were conducted with the Statistical Package for Social Science (SPSS, version 23.0). χ2 or Fisher’s exact test was used to evaluate the efficacy of induction therapy. A ROC curve was applied to determine the cut-off value of MRD detected by flow cytometry after induction therapy. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test stratified by baseline age, WBC counts, BM blasts, cytogenetics, and the cut-off value for MRD amongst others. A Cox proportional hazards model was used to analyze the prognostic factors further. A P-value < .05 was considered statistically significant.
Results
Clinical characteristics
A total of 55 newly diagnosed AML patients were analyzed retrospectively, including 24 males and 31 females, the median age was 60 years (range, 23–79 years). The median WBC counts at diagnosis was 3.31×109/L (range, 0.53–54.53×109/L), and the median BM blasts was 43.5% (range, 19.0–90.5%). Fifty-one of the 55 patients obtained chromosome karyotypes, including 6 patients with favorable-risk karyotypes (4 cases with t(8;21)(q22;q22), 2 cases with inv16(p13q22)), 3 patients with poor-risk karyotypes (2 cases with complex karyotypes, 1 case with t(6;9)(p23;q34)), and 42 patients with intermediate-risk karyotypes (33 cases with normal karyotypes, 9 cases with other karyotypes). NPM1, CEBPA, TP53, FLT3-ITD, DMNT3A and IDH1/IDH2 mutations were listed in . Forty-four patients had at least 1 unfavorable factor (including age ≥ 60 years, history of hematologic diseases, treatment-related AML and poor cytogenetic or molecular factors), of which 31 patients had 1 unfavorable factor, 12 patients had 2 unfavorable factors and 1 patient had 3 unfavorable factors ().
Table 1. Characteristics of patients.
Of the 55 patients, there were 6 patients aged more than 75 years and 24 patients aged 60–74 years. In these 24 patients, 6 patients with poor cytogenetic or molecular factors, 3 patients with history of hematologic diseases or treatment-related AML, 3 patients suffered from infections or organ dysfunctions, and others with performance status > 2. For the other 25 young patients (< 60 years old), 13 patients with history of hematologic diseases, 2 patients with treatment-related AML, 8 patients suffered from severe infections and 2 patients with organ dysfunctions. Due to the above factors, all the patients were treated with decitabine-based chemotherapy. Forty-seven patients received decitabine plus CAG, 6 patients with AA, 1 patient with DA, and 1 patient with HAG.
Outcomes
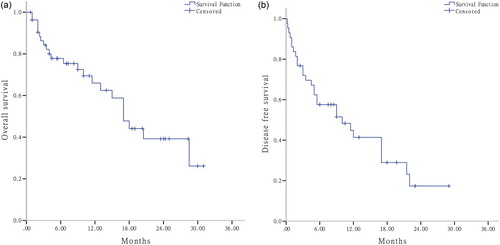
The ORR and CR rate were 80.0% (44/55) and 63.64% (35/55), respectively. ORR and CR rate were not influenced by gender, age, karyotypes, WBC counts, and BM blasts (all P > .05). Due to relatively small sample of patients in each group, the correlation between gene mutations with response rate was not analyzed. The median OS and DFS were 17.0 (13.7–20.3) months and 17.0 (10.2–23.8) months, respectively ((a,b)). Univariate analysis showed that clinical characteristics, including gender, age, karyotypes, WBC counts, BM blasts, had no significant impact on prognosis (all P > .05).
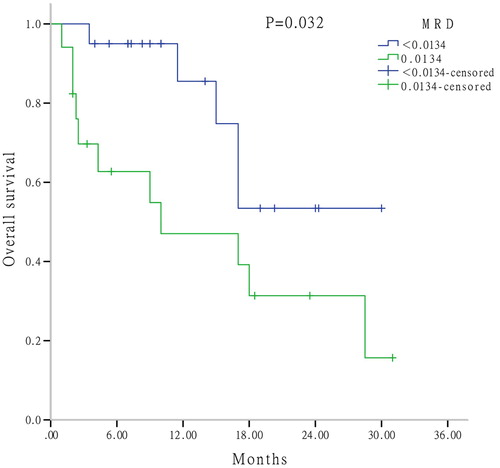
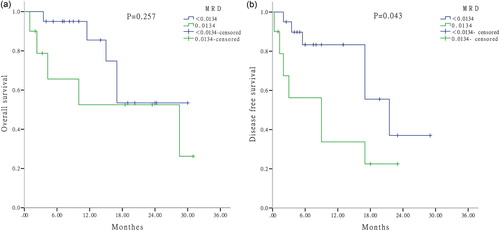
To explore the prognostic factors further, MRD was determined by FCM after one cycle of induction therapy. By applying the ROC curve, the cut-off value of MRD was 1.34% (sensitivity, 0.706; specificity, 0.600). Univariate analysis suggested that patients who responded to induction therapy (CR + PR) with higher MRD (≥ 1.34%) had a significant shorter OS than those with lower MRD (P = .032) (). For patients with CR, a significant shorter DFS was observed in patients with higher MRD than others (P = .043), however, there was no difference in OS between these two groups (P = .257) ((a,b)).
Considering the influence of subsequent chemotherapy on outcomes, we analyzed the consolidation therapy further. For CR patients, 27 of 35 cases received consolidation therapy, including 19 cases with higher MRD (≥ 1.34%) and 8 cases with lower MRD (< 1.34%). Original regimens or multidrug chemotherapy were used for consolidation. Subgroup analysis showed that there was no significant difference between patients with MRD ≥ 1.34% and < 1.34% in consolidation therapy (P = .344).
Taking the results of univariate analysis and previously reported data into account, we conducted a multivariate analysis of MRD, age, karyotypes, WBC counts, consolidation therapy (containing decitabine or not), to further clarify the impact of MRD and other clinical characteristics on survival. The results showed that an advanced age (≥ 60 years) was an adverse prognostic factor for DFS (P = .013); moreover, a higher MRD (≥ 1.34%) was an adverse prognostic factor for both OS and DFS in CR patients (P = .027 and P = .043, respectively).
Safety
During induction therapy, all patients suffered from grade 4 hematological toxicity. The median time for neutropenia and platelet < 20×109/L was 18 days (range, 0–49 days) and 11 days (range, 0–46 days), respectively. There were no serious bleeding and organ dysfunctions. The incidence of infections was 83.64% (46/55), including 27 cases with pneumonias, 5 case with sepsis, 4 cases with intra-abdominal infections, 4 cases with oral mucositis, 3 cases with cutaneous infections, and 3 cases with febrile neutropenia. According to CTCAE (version 4.0), 18.18% (10/55) of patients suffered from severe infections (grade 3 or 4). Two patients died of severe pneumonia and sepsis, and the treatment-related mortality was 3.64% (2/55).
Discussion
In recent years, the treatment of AML has significantly been improved. However, due to an advanced age, history of hematologic diseases, poor cytogenetic or molecular factors, serious infections and organ dysfunctions, some patients were unfit for intensive chemotherapy and demonstrated a poor prognosis. It has been reported that aberrant DNA methylation played a critical role in the pathogenesis of leukemia. As a specific inhibitor of DNA methylation, decitabine could inhibit DNA methyltransferases (DNMTs) and reactivate silenced tumor suppressor genes. Therefore, decitabine has become a new therapeutic approach for AML patients [Citation1,Citation6,Citation7], especially for those unfit or intolerable for intensive chemotherapy.
In 2006, decitabine was approved originally for the treatment of patients with intermediate and high-risk MDS. In 2012, European Commission approved decitabine for the treatment of newly diagnosed primary or secondary AML in elderly patients (≥ 65 years). Similarly, in 2016, NCCN Guidelines also recommended decitabine for elderly patients with AML (≥ 60 years) who were not candidates for intensive chemotherapy. Several studies reported that decitabine alone or in combination with chemotherapy in patients with MDS or AML showed a promising efficacy [Citation5,Citation8–10]. The DACO-16 study also revealed that decitabine had a better OS than palliative care and was well-tolerated in elderly patients ineligible for intensive chemotherapy [Citation3,Citation4,Citation11]. In patients with newly diagnosed or relapsed/refractory AML, the reported CR rate of decitabine-based regimens ranged from 36.5% to 83.0% [Citation12–14]. Compared with intensive chemotherapy, there was no significant difference in overall survival [Citation15,Citation16].
In our study, patients were medically unfit for intensive therapy and were treated with regimens containing decitabine and low/reduced-dose chemotherapy. Our results revealed that the ORR and CR rate were 80.0% and 63.64% respectively, similar to the rates reported for intensive chemotherapy [Citation12–14]. As we expected elderly patients to demonstrate a generally poor prognosis due to several factors, including comorbidities, decreased organ functions, poor performance status, and a higher incidence of adverse karyotypes [Citation17], however, our results showed that there was no significant difference between the elderly and young patients regarding the CR rate. In addition, our results also revealed that the remission rate of patients with hyperleukocytic AML was no worse than that of others, which was inconsistent with the results of intensive chemotherapy [Citation18]. There were fewer patients with unfavorable karyotypes in this study, and no difference of CR rate was observed in these patients compare with others. It was also inconsistent with the study of intensive chemotherapy [Citation19]. These results suggest that decitabine-based chemotherapy might overcome certain unfavorable factors, such as advanced age, history of hematologic diseases, higher WBC counts, and even unfavorable karyotypes.
For survival analysis, decitabine-based chemotherapy showed a promising prognosis with both the median OS and DFS were 17.0 months. Due to poor performance status, unfavorable cytogenetics and comorbidities, elderly AML patients often lacked consolidation and maintenance therapy after remission, which accounts for the high risk of relapse. In our study, an advanced age was identified as an unfavorable prognostic factor for DFS. The results suggested that decitabine-based chemotherapy also could not improve DFS in elderly AML patients.
It is well-known that gene mutations would influence prognosis significantly, such as FLT3-ITD mutation indicates poor prognosis in young AML patients, whereas it may be not the case in elderly patients [Citation20]. DNMT3A, TET2, IDH1/IDH2 and ASXL1 mutations are frequently observed in elderly patient, which are associated with DNA methylation [Citation1]. However, whether these mutations could predict prognosis remains unclear [Citation21–25]. It worth noting that the widely accepted prognostic implications of gene mutations mostly resulted from intensive chemotherapy. Nevertheless, the impact of these mutations on patients treated with decitabine-based chemotherapy had been less reported relatively and the results were also inconsistent [Citation26,Citation27]. Due to relatively small sample of patients in this study and the low frequencies of certain gene mutations, so we did not evaluate the prognostic impact of these mutations on the patients.
To explore the prognostic factors further, MRD was measured by FCM after one cycle of decitabine-based chemotherapy. The cut-off value of MRD was 1.34%. Higher MRD (≥ 1.34%) was identified as an unfavorable prognostic factor for OS and DFS. The influence of subsequent consolidation therapy should be taken into account in these patients. In our study, there was no significant difference in consolidation therapy between patients with MRD ≥ 1.34% and < 1.34% (P > .05), which might confirm our results further. However, due to economic or other reasons, some patients did not undergo subsequent chemotherapy as scheduled. Therefore, further clinical investigations are needed to validate these results.
During induction therapy, grade 4 hematologic toxicity commonly occurred, and the incidence of infections was 83.64%. The median time for platelet recovery was shorter than that of the intensive therapy, as the reported previously [Citation12,Citation26]. In our study, the median age of patients was 60 years old, which accounted for the higher incidence of infections; nevertheless, the rate of severe infections (grade 3 or 4) was lower relatively. Treatment-related mortality was 3.64%, which suggested that decitabine-based therapy was well-tolerated.
In summary, decitabine-based chemotherapy was well-tolerated and demonstrated promising efficacy in newly diagnosed AML patients who were not candidates for intensive chemotherapy. Furthermore, it may partially overcome certain unfavorable factors. Our results revealed that an advanced age (≥ 60 years) and higher MRD (≥ 1.34%) detected by FCM after induction therapy were adverse prognostic factors. However, considering the bias inherent in retrospective analyses and the limited number of eligible patients, these results should be explained with caution and further research is essential.
Acknowledgement
We thank Department of Hematology, The First Hospital of Jilin University, for their assistance in this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kim TK, Gore SD, Zeidan AM. Epigenetic therapy in acute myeloid leukemia: current and future directions. Semin Hematol. 2015;52(3):172–183. doi: 10.1053/j.seminhematol.2015.04.003
- Negrotto S, Ng KP, Jankowska AM, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia. 2012;26(2):244–254. doi: 10.1038/leu.2011.207
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429
- Nieto M, Demolis P, Béhanzin E, et al. The European Medicines Agency review of decitabine (Dacogen) for the treatment of adult patients with acute myeloid leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2016;21(6):692–700. doi: 10.1634/theoncologist.2015-0298
- Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120(24):4840–4845. doi: 10.1182/blood-2012-06-436055
- Bender CM, Zingg JM, Jones PA. DNA methylation as a target for drug design. Pharm Res. 1998;15(2):175–187. doi: 10.1023/A:1011946030404
- Diesch J, Zwick A, Garz AK, et al. A clinical-molecular update on azanucleoside-based therapy for the treatment of hematologic cancers. Clin Epigenetics. 2016;8(71).
- Gao S, Qiu H, Jin Z, et al. The clinical efficacy of the patients of acute myeloid leukemia and myelodysplastic syndromes treated with decitabine alone, combined with half or one couse of CAG regimen. Chin J Hematol. 2014;35(11):961–965.
- Lee JH, Jang JH, Park J, et al. A prospective multicenter observational study of decitabine treatment in Korean patients with myelodysplastic syndrome. Haematologica. 2011;96(10):1441–1447. doi: 10.3324/haematol.2011.046078
- Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–561. doi: 10.1200/JCO.2009.23.9178
- Zhu Y, Qian SX, Li JY. Interpretation of decitabine treatment for elderly patients with acute myeloid leukemia: the multicenter, open-label, phase III clinical trial (registered trial DACO-016). Chin J Hematol. 2013;34(1):83–84.
- Scandura JM, Roboz GJ, Moh M, et al. Phase I study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011;118(6):1472–1480. doi: 10.1182/blood-2010-11-320093
- Song LX, Xu L, Li X, et al. Clinical outcome of treatment with a combined regimen of decitabine and aclacinomycin/cytarabine for patients with refractory acute myeloid leukemia. Ann Hematol. 2012;91:1879–1886. doi: 10.1007/s00277-012-1550-y
- Ye XN, Zhou XP, Wei JY, et al. Epigenetic priming with decitabine followed by low-dose idarubicin/cytarabine has an increased anti-leukemic effect compared to traditional chemotherapy in high-risk myeloid neoplasms. Leuk Lymphoma. 2016;57(6):1311–1318. doi: 10.3109/10428194.2015.1091931
- Podoltsev NA, Stahl M, Zeidan AM, et al. Selecting initial treatment of acute myeloid leukemia in older adults. Blood Rev. 2017;31(2):43–62. doi: 10.1016/j.blre.2016.09.005
- Huang J, Hong M, Zhu Y, et al. Decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin is as effective as standard dose chemotherapy in the induction treatment for patients aged from 55 to 69 years old with newly diagnosed acute myeloid leukemia. Leuk Lymphoma. 2018;59(11):2570–2579. doi: 10.1080/10428194.2018.1443328
- Lin D, Zhou C, Wei H, et al. Characteristics and treatment outcomes in 822 adult patients with acute myeloid leukemia: a single center experience. Chin J Hematol. 2014;35:1058–1064.
- Marbello L, Ricci F, Nosari AM, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature. Leuk Res. 2008;32(8):1221–1227. doi: 10.1016/j.leukres.2008.01.004
- Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083.
- Prassek VV, Rothenberg-Thurley M, Sauerland MC, et al. Genetics of acute myeloid leukemia in the elderly: mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica. 2018;103(11):1853–1861. doi: 10.3324/haematol.2018.191536
- Marcucci G, Metzeler KH, Schwind S, et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J Clin Oncol, 2012, 30(7):742–750. doi: 10.1200/JCO.2011.39.2092
- Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–2433. doi: 10.1056/NEJMoa1005143
- Gaidzik VI, Schlenk RF, Paschka P, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG). Blood. 2013;121(23):4769–4777. doi: 10.1182/blood-2012-10-461624
- Virijevic M, Karan-Djurasevic T, Marjanovic I, et al. Somatic mutations of socitrate dehydrogenases 1 and 2 are prognostic and follow-up markers in patients with acute myeloid leukaemia with normal karyotype. Radiol Oncol. 2016;50(4):385–393. doi: 10.1515/raon-2016-0044
- Abdel-Wahab O, Patel J, Levine RL. Clinical implications of novel mutations in epigenetic modifiers in AML. Hematol Oncol Clin North Am. 2011:25 (6):1119–1133. doi: 10.1016/j.hoc.2011.09.013
- Li J, Chen Y, Zhu Y, et al. Efficacy and safe of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6(8):6448–6458.
- Hiller JK, Schmoor C, Gaidzik VI, et al. Evaluating the impact of genetic and epigenetic aberrations on survival and response in acute myeloid leukemia patients receiving epigenetic therapy. Ann Hematol. 2017;96(4):559–565. doi: 10.1007/s00277-016-2912-7