ABSTRACT
Objectives: To analyze the clinical characteristics and prognostic factors in Chinese patients with classical Hodgkin’s lymphoma (cHL) involving extranodal sites.
Methods: Clinical features and outcomes of 68 patients diagnosed with cHL involving extranodal sites from April 2003 to November 2017 were analyzed retrospectively. The data was compared with that of 76 cHL patients without extranodal involvement in the same period.
Results: (1) Extranodal involvement was common in Chinese cHL patients. The most common sites were lung (44.1%) and bone (33.8%), followed by bone marrow, liver, pericardium, pleura and other sites. (2) With a median follow-up period of 4.58 years, the 5-year overall survival (OS) of 68 patients with extranodal involvement was significantly poorer than that of 76 patients with only nodal involvement (81.4% vs. 92.8%, p = 0.018). (3) In univariate analysis, lymphocytopenia (p = 0.027), elevated lactate dehydrogenase (LDH) (p = 0.026) and involved lymph node region (LNR) ≥4 (p = 0.044) predicted inferior freedom from progression (FFP) with significant difference. Elder age (p = 0.010), elevated LDH (p = 0.013), elevated platelet (p = 0.044), involved LNR ≥ 4 (p = 0.047) were also statistically significant in OS. Extranodal sites and number of extranodal sites showed no significant difference in FFP and OS. Factors with p-value smaller than 0.100 were evaluated in multivariate analysis, turning out that lymphocytopenia was the only independent adverse prognostic factor in FFP (p = 0.039; HR = 2.595) and OS (p = 0.028; HR = 4.993).
Conclusion: Extranodal involvement was frequent in Chinese cHL patients, with lung to be the most commonly involved site. Lymphocytopenia was the only independent adverse prognostic factor.
1. Background
Hodgkin’s lymphoma (HL) is a group of malignant tumor originating from B lymphocyte [Citation1], the histologic type of which including nodular lymphocyte-predominant HL (NLPHL) and classical HL(cHL). The latter is further divided into four subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte rich (LR) and lymphocyte-depletion (LD) [Citation2]. Although HL patients have a good prognosis with a cure rate of 80–90% [Citation3], there are still a minority of patients having recurrence or dying early. Thus, identifying prognostic factors accurately is important for individualized risk-adapted therapy.
There is some difference of HL existing in China and western countries, including but not limited to peaking age of diagnosis, pathological type [Citation4]. Extranodal involvement is rare in western countries, and most studies are case reports or very small series of cases. While in China, 29.0−32.8% [Citation4,Citation5] had extranodal involvement, with one-third primary extranodal HL [Citation6,Citation7]. So far, there are at least 4 studies about HL involving extranodal sites in China, with the biggest study including no more than 30 patients [Citation5]. Thus, we conducted a retrospective study to analyze clinical characteristics and prognostic factors of classical HL involving extranodal sites in our hospital. The study follows the principles of the Declaration of Helsinki.
2. Methods
2.1. Patients
From April 2003 to November 2017, there were totally 182 patients diagnosed with untreated HL in our hospital, including 144 patients with cHL. Among them, 68 patients had extranodal involvement at diagnosis, including 4 patients diagnosed with NLPHL, 68 with cHL and 14 with unclassified. All these patients were pathologically diagnosed by at least three experienced pathologists. Extranodal involvement was confirmed through biopsy combined with imaging, including contrast-enhanced Computed Tomography (CT), magnetic resonance imaging, radionuclide bone scan or Positron Emission Tomography-CT, and then judged by clinicians. Spleen was treated as a nodal organ. Patients’ baseline clinical and laboratory parameters, treatment and evaluation were collected. The study mainly analyzed patients with cHL involving extranodal sites and compared the results with those who without extranodal involvement.
2.2. Efficacy assessment
The response of treatment was assessed in the middle of chemotherapy (after two to four cycles of chemotherapy), after chemotherapy and after consolidation therapy. Assessment was based on the 2007 revised response criteria for malignant lymphoma [Citation8]. Overall response rate (ORR) was defined as the rate of complete remission (CR) plus partial remission (PR). Patients were followed up until March 2019. Freedom from progression (FFP) of disease was defined as the interval from the date of diagnosis to the first recurrence (progression or relapse) of disease; deaths not caused by disease progression/relapse during remission or loss to follow-up were censored. Overall survival (OS) defined as the interval from the date of diagnosis to the death from any cause.
2.3. Statistical analysis
All statistical analyses were performed using SPSS statistical software (Version 20.0; IBM Corp.; Armonk, NY, USA). Differences of clinical characteristics between patients with and without extranodal involvement were analyzed with the χ2 test. The Cox proportional hazard model was used for the univariate and multivariate analyses to evaluate the prognostic effect of parameters for survival, which was expressed as hazard ratio (HR) with a 95% confidence interval (CI). All p-values reported were two-sided with p < 0.05 considered statistically significant.
3. Results
3.1. Clinical characteristics
Sixty-eight (47.2%) of 144 cHL patients had extranodal involvement at diagnosis. Characteristics of 68 Patients with extranodal involvement and 76 patients with only nodal involvement are shown in . Of the 68 patients with extranodal involvement, 42 (61.8%) were male. The median age was 31 (15−79) years, with 16 (23.5%) patients older than 45 years. Five patients presented with bulky disease and 44 with B symptoms. Almost all patients (61 of 68, 89.7%) were at advanced stage (stage II with bulky disease, stage III and IV). Serum lactate dehydrogenase (LDH) levels were elevated in 21 (30.9%) patients, and 21 had lymphocytopenia. Based on the international prognostic score (IPS) [Citation9], 27 (39.7%) patients were classified into a low-risk group (IPS < 3) and 41 (60.3%) into a high-risk group (IPS ≥ 3). Most (44 of 68, 66.2%) patients had ≥4 lymph node regions (LNR) involved (definition of lymph node regions was based on Ann Arbor Stage [Citation10]). Three patients were Hepatitis B surface antigen positive and none was human immunodeficiency virus positive. Of the 30 patients detecting Epstein–Barr virus DNA, 8 had positive results. In patients with extranodal involvement, advanced stage, B symptoms, low serum albumin, lymphocytopenia and ≥4 LNR involved were more common than those in patients with only nodal involvement. Other clinical characteristics of patients with extranodal involvement, such as age, gender and pathological type, showed no significant difference in comparison with those of patients with only nodal involvement.
Table 1. Characteristics of patients with and without extranodal involvement at diagnosis.
The most common involved extranodal sites were lung (44.1%) and bone (33.8%), followed by bone marrow (14.7%), liver (17.6%), pericardium (10.3%), pleura (11.8%), skin and soft tissue (8.9%). Thyroid, thymus gland, pancreas, great vessels, kidney, adrenal gland, intestine and spinal cord involvement was also found in this study. The extranodal lesion sites are shown in . Of the 68 patients, most (41 0f 68, 60.3%) had extranodal involvement at one site, 16 at two sites, and 11 at more than three sites ().
Table 2. Pathologic types of different extranodal sites.
Pathologically, the most common type was NS (42 of 68, 61.8%), followed by MC, LR and no LD (). In most extranodal sites, NS was the most common histologic type, while in liver, NS and MC were equally common ().
3.2. Treatment and outcomes
Except one old female patient died before treatment, all the other 67 patients underwent chemotherapy with ABVD regimen (doxorubicin, bleomycin, vinblastine, dacarbazine). As to consolidation radiotherapy, 11 patients received radiotherapy (RT), 6 received autologous stem cell transplantation (ASCT), and 5 received both RT and ASCT. In 61 patients with available efficacy assessment when finishing the whole therapy, 42 (72.1%) achieved CR, 1 (1.7%) had PR, while the other 16 (26.2%) had progressed disease during the treatment.
The 5-year FFP (p = 0.715) and 5-year OS (p = 0.987) of those who achieved CR in the middle of chemotherapy, after therapy and after consolidation therapy showed no significant difference. (Not shown).
4. Survival and prognostic factors analyses
4.1. Survival of patients with and without extranodal involvement
Median follow-up time was 4.58 (0.02–12.7) years for 68 patients with extranodal involvement, and 4.15 (1.33–12.7) years for 44 patients who achieved CR. There were 11 of 68 patients dying during the follow-up, including one 48-year-old man dying of pulmonary interstitial fibrosis about 2 months after CR.
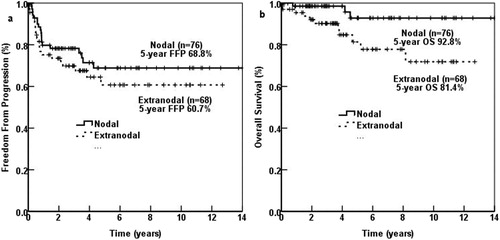
The 5-year OS of 68 patients with extranodal involvement was significantly poorer than that of 76 patients with only nodal involvement (81.4% vs. 92.8%, p = 0.018). The 5-year FFP of patients with extranodal involvement was also poorer (60.7% vs. 68.8%, p = 0.325), but without significant difference. ()
4.2. Prognostic factors analyses
The 5-year OS and 5-year FFP were higher in the low-risk group than in the high-risk group based on IPS (5-year OS: 83.1% vs. 81.4%, p = 0.152; 5-year FFP: 70.9% vs. 52.2%, p = 0.262), but no significant difference was found.
The prognostic significance of baseline parameters for FFP and OS evaluated by the Kaplan–Meier method and the Cox proportional hazard regression analysis was shown in and . In univariate analysis, only one factor of IPS predicted inferior FFP with significant difference, which was lymphocytopenia (p = 0.027). Elevated LDH (p = 0.026), LNR ≥ 4 (p = 0.044) were also statistically significant, which were not part of IPS. B symptom (p = 0.058) and advanced stage (p = 0.054) predicted inferior FFP, with non-significant p-values. The only factor of IPS predicting poorer OS with significant difference was elder age (p = 0.010). Elevated LDH (p = 0.013), elevated platelet (PLT) (p = 0.044), LNR ≥ 4 (p = 0.047) were also statistically significant in OS. Lymphocytopenia (p = 0.054) and HGB < 105 g/L (p = 0.058) also predicted inferior OS, but with non-significant p-values. Extranodal sites, number of extranodal sites and other factors showed no significant difference in FFP and OS. Those factors with p-value smaller than 0.100 were all evaluated in multivariate analysis, showing that lymphocytopenia was the only independent adverse prognostic factor in FFP (p = 0.039; HR = 2.595) and OS (p = 0.028; HR = 4.993).
Table 3. Results of the univariate and multivariate analysis of freedom from progression.
Table 4. Results of the univariate and multivariate analysis of overall survival.
5. Discussion
There is some difference of HL existing in China and western countries, including peaking age of diagnosis, pathological type [Citation4], especially that extranodal involvement are more common in Chinese patients [Citation7]. Therefore, we analyzed the clinical characteristics and prognostic factors of classical HL involving extranodal sites in our hospital. Extranodal involvement in western countries are mostly case reports or reports of a very small series of cases, while in China, 29.0−32.8% [Citation4,Citation5] had extranodal involvement. In our study, 47.2% cHL patients had extranodal involvement, with an incidence higher than other studies. Extranodal involvement mainly happened in young male patients in an advanced stage and the most common type was NS and MC, similarly to other studies in China [Citation6,Citation7,Citation11] and an American study about HL involving extranodal and nodal head and neck sites [Citation12]. Like other studies, the most common involved extranodal sites were lung, bone and liver, but with more involvement in bone marrow and less in intestine. Multiple (≥2) extranodal involvement was also more common than other studies (39.7% vs. 3.8−31.8%) [Citation6,Citation7,Citation11]. In most extranodal sites, NS was the most common histologic type, while in liver, NS and MC were equally common.
Data about the survival of HL patients with extranodal involvement was limited to Chinese patients, whose 5-year OS was 86.8−89.3% and 5-year DFS was 76.5−78.9% [Citation11,Citation13]. In this study, 5-year OS and 5-year FFP were 81.4% and 60.7%, respectively, for patients with cHL involving extranodal sites. The survival was a little poorer than other studies, maybe due to the high incidence of multiple extranodal involvement. Compared with the 5-year OS and FFP of 76 patients with only nodal involvement, those of patients with extranodal involvement were poorer, possibly because of more patients with advanced stages, B symptoms, hypoalbuminemia, lymphocytopenia or LNR ≥ 4. Multiple extranodal involvement was linked to poor outcome in a study about diffuse large B-cell lymphoma [Citation14], while in our study, extranodal involvement is a poor prognostic indicator in the Chinese cHL patients. The FFP difference between nodal and extranodal groups is smaller than the OS difference, maybe as some patients died of other diseases, instead of disease progression.
Compared with the survival of advanced HL with 5-year OS of 83−91% in clinical studies in western countries [Citation15,Citation16], the survival was poorer in our study. Extranodal involvement, advanced age, complications of pulmonary disease, cardia-cerebrovascular disease or hepatitis B and other diseases may explain the difference. The difference indicated a unique biological characteristic of cHL involving extranodal sites in some way.
In western countries, patients with early unfavorable HL after two cycles of ABVD would receive intensified chemotherapy with two cycles of BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone) to improve outcome [Citation17]. In China, procarbazine was not available, and some patients didn’t evaluate the outcome in early therapy due to economic reasons, so there was no dose-intensification in early unfavorable patients. Those who didn’t have CR in middle evaluation or had extranodal involvement would receive radiotherapy and ASCT as consolidation therapies based on economic ability. Patients achieving CR had similar FFP and OS, ignoring the time when achieving CR, which suggested that radiotherapy and ASCT might make up the loss of dose-intensification for early unfavorable patients.
Since the IPS developed based on the outcome of more than 5000 patients with advanced HL in 1998, it was widely used in the prognosis analysis of advanced HL [Citation9]. However, along with the advance of accurate diagnosis, chemotherapy, related supportive treatment, and the use of ASCT, the outcome of patients was becoming better, and the prognostic value of IPS in advanced HL gradually decreased [Citation18], including patients with extranodal involvement [Citation6,Citation7,Citation11]. In our study, IPS didn’t differentiate 5-year OS and 5-year FFP in the high-risk group well from those in low-risk group, although the former was lower than the latter. Many studies were trying to seek for potent prognostic factors in the era of individualized therapy [Citation19–21].
Studies about HL with exranodal involvement found that, hypoalbuminemia, advanced stage, pathological type [Citation7], elevated LDH and elevated PLT [Citation6] were adverse prognostic factors. But these studies including a small group of patients and didn’t analyze cHL alone. Some other studies also found anemia [Citation22], eosinophilia [Citation23], elevated β2-microglubin [Citation19] and elevated serum ferritin [Citation24] to be adverse factors. Thus, our study analyzed all the factors above that could be collected, and then included factor with p-value < 0.100 into multivariate analysis. The result revealed that lymphocytopenia was the only independent adverse prognostic factor of OS (p = 0.028) and FFP (p = 0.039). The percent and absolute count of lymphocyte were cheap and easy to obtain, making the evaluation of prognosis easy and quick.
In the background of cHL exist plentiful inflammatory cells, including lymphocytes, neutrophils, macrophages, eosinophils and plasma cells. High level of T helper 2 cells [Citation25], low level of cytotoxic cells [Citation26] were reported to have positive prognostic value. Peripheral lymphocytopenia might have a correlation with the immune cells in tumor tissue, which could explain the adverse prognostic value of lymphocytopenia. On the other hand, lymphocytopenia represents a state of immunodeficiency. Whether it leads to HL cells immigrating to extranodal sites and impairs the ability of eliminating tumor cells still need to be illuminated. The mechanism of immune factors in tumor is drawing more and more attention and the successful use of immunomodifier, like programmed cell death-1 (PD-1) blockade and the CD30 monoclonal antibody, in oncotherapy proved the importance of immune factors in tumor [Citation27]. Whether patients with lymocytopenia are better candidates for PD-1 blockade, the CD30 antibody and other immunomodifiers still needs further research.
In conclusion, extranodal involvement was frequent in Chinese cHL patients, with lung to be the most commonly involved site. Classical HL involving extranodal sites had its unique biological characteristics from those who without extranodal involvement. Lymphocytopenia was the only independent adverse prognostic factor in our study, which might suggest immunodeficiency and good response to immunomodifier, like PD-1 blockade and the CD30 monoclonal antibody. Recognizing high-risk patients accurately and early was important to guide individualized therapy. However, there still exist some limitations, like the retrospective study and the relatively small number of cases. A well-designed prospective study including more patients is needed to make a more convincing conclusion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dong-Mei Zou is a doctoral candidate in Peking Union Medical College Hospital (PUMCH), majoring in hematology, who is also a resident doctor.
Dao-Bin Zhou is the chief physician in the department of hematology in PUMCH, skilled in the diagnosis and treatment of hematologic malignancies and hematopoietic stem cell transplantation. He is also a doctoral supervisor.
Yan Zhang graduated from Peking Union Medical College in 2009 and is the visiting physician in PUMCH now.
Wei Wang graduated from Peking Union Medical College and is the visiting physician in PUMCH now.
Wei Zhang is the chief physician in the department of hematology in PUMCH, skilled in the diagnosis and treatment of hematologic malignancies and hematopoietic stem cell transplantation.
ORCID
Yan Zhang http://orcid.org/0000-0003-0244-218X
Wei Wang http://orcid.org/0000-0002-1192-4896
Wei Zhang http://orcid.org/0000-0003-0063-6490
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Eberle FC, Mani H, Jaffe ES. Histopathology of Hodgkin’s lymphoma. Cancer J. 2009;15(2):129–137. doi: 10.1097/PPO.0b013e31819e31cf
- Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569
- Broeckelmann PJ, Engert A. The GHSG approach to treating Hodgkin’s lymphoma. Curr Hematol Malig Rep. 2015;10(3):256–265. doi: 10.1007/s11899-015-0262-5
- Zhu YJ, et al. Clinical characteristics and prognostic factors in Chinese patients with Hodgkin’s lymphoma. Med Oncol. 2012;29(2):1127–1133. doi: 10.1007/s12032-011-9902-3
- Wan WL, et al. Clinical characteristics and prognostic factors of 62 cases with Hodgkin lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2013;34(7):618–621.
- Yang M, et al. Clinical characteristics and prognostic factors of primary extranodal classical Hodgkin lymphoma: a retrospective study. Hematology. 2019;24(1):413–419. doi: 10.1080/16078454.2019.1598678
- Ma J, et al. Clinical characteristics of 26 patients with primary extranodal Hodgkin lymphoma. Int J Clin Exp Pathol. 2014;7(8):5045–5050.
- Cheson BD, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403
- Hasenclever D, Diehl V. D. Int prognostic factors project Adv Hodgkins, a prognostic score for advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104
- Lister TA, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–1636. doi: 10.1200/JCO.1989.7.11.1630
- Li ZM, et al. Clinical characteristics of the patients with Hodgkin’s lymphoma involving extranodal sites. Chin J Cancer. 2012;31(7):342–347. doi: 10.5732/cjc.012.10008
- Iyengar P, et al. Hodgkin lymphoma involving extranodal and nodal head and neck sites: characteristics and outcomes. Cancer. 2010;116(16):3825–3829. doi: 10.1002/cncr.25138
- Li YF, et al. Clinical characteristics of 30 Hodgkin’s lymphoma patients with extranodal involvement. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(3):712–716.
- Yao S, et al. Extranodal involvement in young patients with diffuse large B-cell lymphoma: distribution, prognostic value and treatment options. Chin J Cancer Res. 2017;29(1):57–65. doi: 10.21147/j.issn.1000-9604.2017.01.07
- Diehl V, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–2395. doi: 10.1056/NEJMoa022473
- Aleman BMP, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348(24):2396–2406. doi: 10.1056/NEJMoa022628
- von Tresckow B, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol. 2012;30(9):907–913. doi: 10.1200/JCO.2011.38.5807
- Moccia AA, et al. International prognostic score in advanced-stage Hodgkin’s lymphoma: altered utility in the Modern Era. J Clin Oncol. 2012;30(27):3383–3388. doi: 10.1200/JCO.2011.41.0910
- Wang Q, et al. Prognostic value of pretreatment serum beta-2 microglobulin level in advanced classical Hodgkin lymphoma treated in the modern era. Oncotarget. 2016;7(44):72219–72228.
- Vassilakopoulos TP, et al. Prognostic implication of the absolute lymphocyte to absolute monocyte count ratio in patients with classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine or equivalent regimens. Oncologist. 2016;21(3):343–353. doi: 10.1634/theoncologist.2015-0251
- Alonso-Alvarez S, et al. The number of tumor infiltrating T-cell subsets in lymph nodes from patients with Hodgkin lymphoma is associated with the outcome after first line ABVD therapy. Leuk Lymphoma. 2017;58(5):1144–1152. doi: 10.1080/10428194.2016.1239263
- Wang Q, et al. Decreased prognostic value of international prognostic score in Chinese advanced Hodgkin lymphoma patients treated in the contemporary era. Chin Med J. 2016;129(23):2780–2785. doi: 10.4103/0366-6999.194661
- Glimelius I, et al. Effect of eosinophil cationic protein (ECP) on Hodgkin lymphoma cell lines. Exp Hematol. 2011;39(8):850–858. doi: 10.1016/j.exphem.2011.05.006
- Fernandez-Alvarez R, et al. Serum ferritin as prognostic marker in classical Hodgkin lymphoma treated with ABVD-based therapy. Leuk Lymphoma. 2015;56(11):3096–3102. doi: 10.3109/10428194.2015.1038709
- Schreck S, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27(1):31–39. doi: 10.1002/hon.878
- Alvaro-Naranjo T, et al. Tumor-infiltrating cells as a prognostic factor in Hodgkin’s lymphoma: a quantitative tissue microarray study in a large retrospective cohort of 267 patients. Leuk Lymphoma. 2005;46(11):1581–1591. doi: 10.1080/10428190500220654
- Aoki T, Steidl C. Novel biomarker approaches in classic Hodgkin lymphoma. Cancer J. 2018;24(5):206–214. doi: 10.1097/PPO.0000000000000334