ABSTRACT
Objectives: Although complement inhibition is highly effective, patients with paroxysmal nocturnal hemoglobinuria (PNH) may experience intravascular breakthrough hemolysis (BTH). Underlying causes may include elevated free C5, pregnancy, or non-pregnancy complement-activating conditions (e.g. infections). This study compared BTH-related resource utilization and costs in PNH patients treated with eculizumab versus ravulizumab.
Methods: A cost model was developed using data from a targeted literature review and a survey of experienced clinicians. Costs associated with BTH episodes were calculated by cause and weighted by the proportion attributed to each cause and the cost of treating each episode. The model captured direct medical costs in 2018 US dollars. Annual BTH-related healthcare resource utilization was also calculated.
Results: BTH episodes in the literature were commonly associated with elevated lactate dehydrogenase and aspartate aminotransferase, hemoglobinuria, transfusion needs, and/or recurrence of PNH symptoms. The majority of BTH management costs in eculizumab-treated patients related to changing from the approved dosing regimen following an episode of BTH, rather than acute management. No ongoing dosing changes were expected for ravulizumab-treated patients following episodes of BTH, substantially reducing its ongoing management costs. Resource utilization was greater for eculizumab-treated patients than ravulizumab-treated patients due to higher incidence of BTH, and risk of elevated free C5-related BTH. Total incremental cost was substantially lower for ravulizumab- vs eculizumab-treated patients ($407 vs $9379); results were consistent when pregnant women were not included ($386 vs $3472).
Conclusion: Overall resource use and costs for BTH are estimated to be lower for PNH patients receiving ravulizumab compared with eculizumab.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, life-threatening hematologic disorder characterized by uncontrolled activation of the terminal complement pathway [Citation1,Citation2]. This leads to intravascular hemolysis, thrombosis, bone marrow failure, impaired health-related quality of life (HRQoL), and premature mortality in patients with PNH [Citation1,Citation3].
In 2007, eculizumab, a humanized anti-C5 monoclonal antibody, became the first complement-targeting therapy to be granted marketing approval for the treatment of PNH [Citation1,Citation2]. Eculizumab provides symptomatic relief and extends patient survival compared with placebo [Citation4–8]; the safety of eculizumab has also been established during more than 10 years of real-world experience [Citation9].
However, approximately 11–27% of patients experience breakthrough hemolysis (BTH) on approved dosages of eculizumab [Citation7,Citation10,Citation11] when serum drug concentrations fall below the threshold needed to provide complement inhibition [Citation10]. In addition, patients who are pregnant and those with non-pregnancy complement-amplifying conditions (CACs), including, but not limited to surgery, trauma, and infections, have an increased risk of BTH [Citation8,Citation10].
BTH, defined in clinical studies as ≥1 new or worsening sign or symptom of intravascular hemolysis in the presence of lactate dehydrogenase (LDH) ≥2x upper limit of normal (ULN) after prior on-treatment reduction of LDH to <1.5x ULN [Citation12,Citation13], represents a loss of disease control, which may include thrombosis. Therefore, cases of BTH require further patient monitoring and may necessitate blood transfusions or additional doses of complement-inhibitor therapy to re-establish disease control. Use of blood transfusions in PNH may put strain on blood supplies as an ever-increasing need for transfusions exists [Citation14]. In some cases, physicians may alter eculizumab maintenance dosing, either increasing dosage or dosing frequency compared with the approved regimen [Citation1,Citation2,Citation15]. Therefore, BTH not only impacts patients’ clinical outcomes and HRQoL, but its management may also require substantial healthcare resource utilization.
Ravulizumab is the first long-acting C5 inhibitor with an increase in half-life of approximately four-fold compared with eculizumab [Citation16]. In two large phase 3 clinical studies in patients with PNH, ravulizumab was shown to be non-inferior to eculizumab for all primary endpoints and key secondary endpoints, including the proportion of patients with BTH [Citation12,Citation13]. During these clinical trials, ravulizumab was associated with numerically fewer BTH episodes than eculizumab [Citation12,Citation13].
This study aimed to characterize healthcare resource utilization and direct medical costs associated with the management of BTH in patients with PNH receiving eculizumab versus ravulizumab and to compare annual healthcare resource utilization and costs between individuals being treated with each drug, which may influence medical management decisions.
Materials and methods
Definition of BTH
The definition of BTH that was used in the two ravulizumab phase 3 PNH clinical studies was used for the current analysis. In both studies, BTH was defined as one or more new or worsening symptoms or signs of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin <10 g/dL], major adverse vascular event [MAVE; including thrombosis], dysphagia, or erectile dysfunction) in the presence of elevated lactate dehydrogenase (LDH) ≥2 times the upper limit of normal (ULN) after prior LDH reduction to <1.5× ULN while on therapy [Citation17].
Data sources
The burden of BTH among patients with PNH was estimated, and a cost model was developed, using data on the causes and management of BTH derived from a targeted literature review and a survey of 10 clinicians from various countries expert in treating PNH. The clinicians were part of their respective national referral centers for PNH, collectively manage ∼500 patients with PNH, and at the time, were among the few with experience with both eculizumab and ravulizumab because they had participated in the clinical trials of ravulizumab. The range of patients with PNH treated among the ten individual physicians was between 10 and 200. The literature review was performed in MEDLINE, MEDLINE in-process, and Embase (via OVID SP) on March 26, 2018. The survey, conducted in October 2018, asked the clinicians to estimate rates and causes of BTH in patients treated with eculizumab or ravulizumab and define medical management strategies (see Supplementary Appendix 1).
Model for costs associated with BTH
Costs associated with an episode of BTH were calculated by cause (elevated free C5 levels, pregnancy or non-pregnancy CACs) and weighted by the proportion of episodes attributed to each cause (px) and the cost (Cx) for treating each episode (). The costs for treating each BTH episode were dependent on whether a patient received eculizumab or ravulizumab, the only therapies granted regulatory approval for the treatment of PNH at the time the study was conducted. The model captured direct medical costs, defined as hospitalizations, physician visits, medications, and increased dosing of eculizumab or ravulizumab, in 2018 US dollars. Note that even though the majority of direct medical costs are similar between eculizumab and ravulizumab, the cost implications of changes in dosage and dosing frequency can vary based on the PNH treatment received (). Information on additional medical management due to BTH was derived from clinical expert survey responses (). Unit costs for medical management were identified from the published literature and gray literature sources ().
Figure 1. BTH in patients with PNH cost model design schematic. BTH, breakthrough hemolysis; CAC, complement-amplifying condition; Cx, cost for treating each episode; PNH, paroxysmal nocturnal hemoglobinuria; px, proportion of episodes.
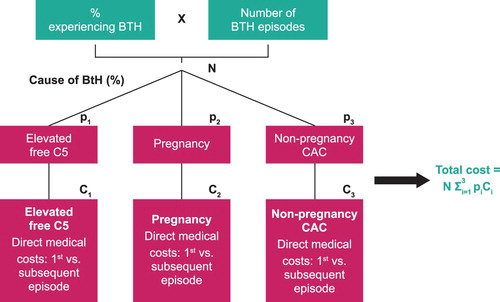
Table 1. Expected management strategy in response to BTH according to a survey of 10 clinical experts in the treatment of PNH.
Table 2. Additional medical management requirements for an episode of BTH according to a survey of 10 clinical experts in the treatment of PNHa.
Table 3. Costs attributed to medical management of BTH in patients with PNH.
Search strategy
A search strategy was developed using medical subject headings to identify indexed terms, as well as free-text search terms, for the population (i.e. PNH patients) and outcome (breakthrough hemolysis) (see Supplementary Appendix 2). Study design filters were not applied because the number of hits was expected to be low and additional filters would limit the search results unnecessarily. Articles were reviewed for relevance and limited to reports of BTH from observational (cohort, case–control, or cross-sectional) studies, randomized controlled studies, and case reports or series from patients with PNH treated with eculizumab.
Model inputs
According to clinical trial data, BTH prevalence was 10.7% and 4.0% for complement-inhibitor naive patients with PNH treated with eculizumab and ravulizumab, respectively [Citation17,Citation25]. The distribution of underlying causes was informed by the survey of PNH expert clinicians, which indicated that the causes of BTH in patients treated with eculizumab were free C5 elevations (39%), pregnancy (55%), and non-pregnancy CAC (6%). The anticipated distribution of causes of BTH for patients treated with ravulizumab, as indicated by the survey of PNH expert clinicians, were pregnancy (5%) and non-pregnancy CAC (95%); risk of elevated free C5 levels was negligible in this population, which is similar to the findings from the ravulizumab clinical trial program [Citation12,Citation13].
Base-case assumptions
The base-case model assumed that 100% of the costs for blood transfusions and 50% of the costs for hospitalization were applicable to BTH. Furthermore, it was assumed that 50% of patients with PNH would receive eculizumab infusions in a home-based setting and 50% in a medical clinic, and all patients treated with eculizumab would receive a standard dosing regimen (900 mg every 14 days). For the assignment of annual costs to BTH, an episode was assumed to occur at the midpoint of the year for any ongoing costs. Thus, 50% of ongoing annual costs were assigned to the year in which an episode occurred. Costs were calculated from the perspective of hospital/clinic costs associated with managing incidents of BTH in patients with PNH.
Statistical analysis
Descriptive statistics are provided for all analyses.
Results
Targeted literature review
In total, 37 relevant abstracts were identified in the literature review. Of those, 14 were excluded, primarily because BTH was not reported as an outcome. Thirteen articles (56.5%) were conference abstracts and eight (34.8%) were case reports or case series. In addition, two randomized, open-label phase 3 clinical trials were included – one in patients who were naive to complement-inhibitor therapy and one in patients who had previously been treated with eculizumab for at least 6 months. The sample sizes ranged from 1 to 195 patients in each article.
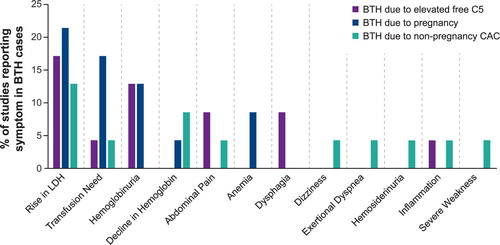
Eleven (47.8%) articles reported pregnancy as the source of BTH (21/49 [42.9%] patients experienced BTH) and 8 (34.8%) articles reported elevated free C5-related reasons (45/641 [7.0%] patients experienced BTH). Four articles (17.3%; 45 patients) reported BTH due to non-pregnancy CACs (infection, unknown, or not reported; 16/45 [35.6%] patients experienced BTH).
However, given that reports of BTH in the literature were only available for 82 patients, including possible duplicate case reporting, the results of the targeted literature review were not considered sufficient to systematically describe the underlying causes of BTH in a general population. Therefore, a survey was developed and administered to a group of 10 clinicians experienced in treating PNH to estimate the parameters to be included in the pharmacoeconomic evaluation of costs related to managing BTH.
Characteristics and management of BTH
Episodes of BTH reported in the literature were most commonly characterized by elevated LDH, hemoglobinuria, and increased transfusion needs, which were managed by increasing eculizumab dosage and/or dosing frequency, or a blood transfusion (). The most frequently reported management strategies for BTH in the literature were to increase the dose of eculizumab (e.g. to 1200 mg every 2 weeks) and/or reduce the dosing interval (e.g. from 14 to 12 days). Survey results from expert clinicians also indicated that BTH cases are frequently managed with an increased dose or dosing frequency in the real-world setting ().
Costs associated with BTH
The majority of costs associated with an episode of BTH in patients treated with eculizumab are related to ongoing management, rather than the acute treatment of the episode (). These ongoing costs largely reflect costs related to the increased dosing frequency and/or dose of future eculizumab prophylaxis. In contrast, the one-time costs of treating a BTH episode in patients treated with ravulizumab are higher than for eculizumab due to a higher cost per dose (i.e. a one-time increase in ravulizumab dose represents 8 weeks’ treatment [3300 mg dose] compared with 2 weeks’ for eculizumab [900 mg dose], so even as single-dose costs are higher for ravulizumab, overall annual costs are lower) () [Citation25,Citation26]. Ongoing management costs are substantially lower for patients treated with ravulizumab compared with eculizumab because treating physicians are unlikely to increase the frequency of ravulizumab dosing, and in the 10% of cases where a dose increase may be considered, increasing the dosing frequency to every 7 weeks (versus the approved 8-week dosing period) represented a modest increase in dosing frequency from 6–7 times to 7–8 times per year that would not substantially alter the cost of managing BTH.
Table 4. Costs associated with an episode of BTH by cause.
Healthcare resource utilization
Annual healthcare resource utilization related to BTH, including the number of blood transfusions, hospital days, and dosing changes experienced by a cohort of patients treated with eculizumab and ravulizumab over 1 year were calculated on the basis of the frequency of BTH episodes and the distribution of causes of BTH. Values represent averages across the entire cohort, including those who did not experience BTH. Results are reported for a hypothetical individual patient with PNH and hypothetical cohorts of 10 and 20 patients.
For a cohort of 20 patients treated with eculizumab, the estimated number of BTH episodes annually, based on the total number of events observed in trial data and the distribution of causes reported by clinicians, is 0.96 due to elevated free C5, 1.36 due to pregnancy, and 0.15 due to non-pregnancy CAC compared with 0 due to elevated free C5, 0.04 due to pregnancy, and 0.76 due to non-pregnancy CAC in patients treated with ravulizumab, respectively (). Overall resource use is estimated to be higher for patients treated with eculizumab than ravulizumab due to a higher incidence of BTH episodes, and in particular because the risk of elevated free C5-related BTH applies continuously to all treated patients versus the limited population at risk of BTH during pregnancy or due to non-pregnancy CAC-related causes.
Table 5. Annual resource implications of BTH given episode frequency and resources used per episode
Absolute and incremental cost of BTH
The cost of managing BTH in a patient receiving eculizumab is substantial at $9379 annually ($3472 excluding pregnancy-related BTH) compared with $407 annually per patient treated with ravulizumab ($386 excluding pregnancy-related BTH) (). The total incremental cost of managing BTH is therefore substantially lower for patients treated with ravulizumab compared with eculizumab, including when pregnant patients are excluded from the analysis. In particular, patients treated with ravulizumab incur no costs due to elevated free C5-related BTH compared with $3267 annually for a patient treated with eculizumab. For non-pregnancy CAC-related BTH, the incremental cost of ravulizumab is $181 per patient more than for patients treated with eculizumab due to a slightly higher number of cases associated with ravulizumab, although this is heavily outweighed by the relative number of BTH episodes related to elevated free C5 and pregnancy and the associated cost of these episodes.
Table 6. Annual costs of BTH for patients with PNH.
Discussion
Data on the incidence of BTH in patients with PNH are limited because of the very low incidence of PNH. Since eculizumab was granted marketing approval for the treatment of PNH in 2007, more than 10 years of real-world treatment experience has shown that approximately 11–27% of patients treated with eculizumab are potentially at risk of BTH [Citation1,Citation2,Citation7,Citation10,Citation11]. Combined with the low prevalence of PNH within the general population [Citation8] published data on real-world management strategies are limited and primarily comprise case reports in pregnant women. However, the body of medical literature and expert clinical opinion indicate that increasing the dose or frequency of eculizumab are the preferred methods for managing BTH in patients treated with eculizumab.
However, deviations from the approved eculizumab regimen by adjusting the dosage or dosing frequency to prevent BTH is associated with significant increases in ongoing drug costs, which may be incurred indefinitely, particularly in the case of individuals with a history of multiple BTH episodes. This additional cost is likely to remain even if therapeutic drug monitoring is instituted for patients treated with eculizumab experiencing BTH [Citation27,Citation28]. In contrast, ravulizumab provides immediate, complete, and sustained control of free C5 levels when given according to the approved weight-based dosing regimen. The improved pharmacokinetic/pharmacodynamic profile of ravulizumab greatly reduces the risk of BTH related to elevated free C5. Consequently, our cost model considered a short-term management approach comprising a possible additional dose to manage the acute episode with no ongoing adjustments to the ravulizumab dosing regimen. Therefore, although one-time costs are expected to be greater when managing patients with BTH treated with ravulizumab, ongoing costs are expected to be substantially lower. Accordingly, the incremental costs of managing BTH are expected to be lower with ravulizumab than with eculizumab.
This difference is driven by the absence of elevated free C5-related BTH for ravulizumab in the present model and substantial savings in managing pregnancy-related BTH. However, even after excluding pregnancy-related BTH, the total incremental cost benefit of ravulizumab versus eculizumab was apparent because the incremental annual cost of non-pregnancy CAC-related BTH is $181 per patient treated with ravulizumab. This is negligible when compared to the cost savings from avoiding elevated free C5-related BTH for patients treated with eculizumab.
The present model is limited by a number of assumptions, most notably that the management strategies are stratified only by the underlying cause of BTH and PNH therapy. However, BTH episodes are heterogeneous in nature with corresponding variation in management requirements [Citation8,Citation10,Citation11]. Due to the rareness of PNH and difficulty in obtaining real-world data, rates and causes of BTH were estimated from a targeted literature review, the ravulizumab clinical trials, and a clinician survey and used in the current model development. In addition, ravulizumab experience was clinical trial-based and prior to its approval and use in a real-world setting. A dedicated costing study to more precisely understand use of resources across BTH episodes would be valuable in refining the data imputed into the model. The feasibility of such a study is limited by the paucity of data generated by the small patient population with PNH and the even smaller proportion of patients who either experience BTH in the setting of a clinical study or are considered of sufficient clinical interest to motivate a case report published in a peer-reviewed medical journal. The opinions provided by the panel of PNH experts from multiple countries enlisted here can be interpreted as a suitable estimate reflecting typical BTH presentation and management in real-world clinical practice.
In conclusion, resource use and costs for BTH are estimated to be lower for patients with PNH receiving ravulizumab compared with eculizumab and these costs should be accounted for in any economic comparison of therapies for PNH.
Data sharing statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development.
Link to Data Request Form: https://alexion.com/contact-alexion/medical-information
Supplemental Material
Download MS Word (598.3 KB)Supplemental Material
Download MS Word (50.9 KB)Acknowledgments
The authors thank all the patients and investigators who participated in and contributed to this study. Medical writing and editorial support were provided by ApotheCom (Yardley, PA, USA) and was funded by Alexion Pharmaceuticals, Inc. (Boston, MA, USA). The content of this article, the ultimate data interpretation, and the decision to submit it for publication in Hematology were made by the authors. Editorial support/critical review was provided by Gabriela Marcheva, PharmD, at Alexion Pharmaceuticals, Inc.
Disclosure statement
IT and JRS are employees and shareholders of Alexion Pharmaceuticals, Inc. KJ and AC are employees of Broadstreet HEOR, which received payment from Alexion Pharmaceuticals, Inc. to conduct this study. RB has received grant funding from Alexion Pharmaceuticals, Inc. and has been an investigator in Alexion-sponsored clinical trials. IW has participated in the Alexion Pharmaceuticals, Inc. speakers’ bureaus for and been an investigator in Alexion-sponsored clinical trials.
Additional information
Funding
References
- Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi: 10.1182/blood-2014-02-522128
- Kelly R, Richards S, Hillmen P, et al. The pathophysiology of paroxysmal nocturnal hemoglobinuria and treatment with eculizumab. Ther Clin Risk Manag. 2009;5:911–921. doi: 10.2147/TCRM.S3334
- Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry. Ann Hematol. 2020;99:1505–1514. doi: 10.1007/s00277-020-04052-z
- Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. doi: 10.1056/NEJMoa061648
- Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–1847. doi: 10.1182/blood-2007-06-094136
- Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–6792. doi: 10.1182/blood-2011-02-333997
- Hillmen P, Muus P, Roth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73. doi: 10.1111/bjh.12347
- Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Prim. 2017;3:17028. doi: 10.1038/nrdp.2017.28
- Socie G, Caby-Tosi MP, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185(2):297–310. doi: 10.1111/bjh.15790
- Nakayama H, Usuki K, Echizen H, et al. Eculizumab dosing intervals longer than 17 days may be associated with greater risk of breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria. Biol Pharm Bull. 2016;39(2):285–288. doi: 10.1248/bpb.b15-00703
- Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125(5):775–783. doi: 10.1182/blood-2014-03-560540
- Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–549. doi: 10.1182/blood-2018-09-876805
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–539. doi: 10.1182/blood-2018-09-876136
- Roberts N, James S, Delaney M, et al. The global need and availability of blood products: a modelling study. Lancet Haematol. 2019;6(12):e606–e615. doi: 10.1016/S2352-3026(19)30200-5
- Sharma R, Keyzner A, Liu J, et al. Successful pregnancy outcome in paroxysmal nocturnal hemoglobinuria (PNH) following escalated eculizumab dosing to control breakthrough hemolysis. Leuk Res Rep. 2015;4(1):36–38.
- Sheridan D, Yu ZX, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One. 2018;13(4):e0195909. doi: 10.1371/journal.pone.0195909
- Brodsky RA, Peffault De Latour R, Rottinghaus ST, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematology. 2019: 236877.
- Soliris. Red Book Online®, Micromedex Solutions: IBM Watson Health; 2017. https://www.micromedexsolutions.com. Accessed September 18, 2019.
- US Securities and Exchange Commission. Form 8-K—current Report: Pursuant to Section 13 or 15(D) of The Securities Exchange Act of 1934. Washington (DC): Alexion Pharmaceuticals Inc.; US Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/899866/000089986618000137/alexion8-kultomirisapproval.htm. Published December 21, 2018. Accessed November 15, 2019.
- Centers for Medicare & Medicaid Services. ASC Payment Rates- Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/11_Addenda_Updates.html. Last updated October 16, 2019. Accessed November 15, 2019.
- Health Care Cost Institute. 2016 Health Care Cost and Utilization Report. https://www.healthcostinstitute.org/research/annual-reports/entry/2016-health-care-cost-and-utilization-report. Published January 2018. Accessed November 15, 2019.
- Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy, and costs in the United States: a methodological review. Crit Care Med. 2015;43(11):2452–2459. doi: 10.1097/CCM.0000000000001227
- US Renal Data System. USRDS Annual Data Report 2018. Chapter 9: Healthcare Expenditures for Persons with ESRD. In: Volume 2: End-Stage Renal Disease in the United States https://www.usrds.org/2018/download/v2_c09_ESRD_Costs_18_usrds.pdf. Accessed November 15, 2019.
- Levy AR, Tomazos I, Patel Y, et al. Comparison of lost productivity due to eculizumab and ravulizumab treatments for paroxysmal nocturnal hemoglobinuria in the United States. Value Health. 2019;22(suppl 2):S377). Abstract PSY15. doi: 10.1016/j.jval.2019.04.1840
- Ultomiris [prescribing information]. Boston (MA): Alexion Pharmaceuticals, Inc.; 2019.
- Soliris [prescribing information]. New Haven (CT): Alexion Pharmaceuticals, Inc.; 2019.
- Wijnsma KL, Ter Heine R, Moes D, et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin Pharmacokinet. 2019;58(7):859–874. doi: 10.1007/s40262-019-00742-8
- Gatault P, Brachet G, Ternant D, et al. Therapeutic drug monitoring of eculizumab: Rationale for an individualized dosing schedule. MAbs. 2015;7(6):1205–1211. doi: 10.1080/19420862.2015.1086049