ABSTRACT
Objectives: The 8p11 myeloproliferative syndrome (EMS) is an extremely rare, generally aggressive haematologic malignancies. This study provided the clinical outcomes and therapeutic strategies for EMS patients confirmed with CEP110-FGFR1 fusion.
Methods: We report here a case of translocation (8;9) (p12;q33)/CEP110-FGFR1 who received allo-HSCT and achieved molecular remission. We searched the PubMed database for relevant medical literatures published between 1992 and 2018. We generalized the laboratory results, clinical features, therapeutic outcomes for EMS with confirmed CEP110-FGFR1 fusion.
Results: We identified 16 EMS cases with CEP110-FGFR1 fusions including our patient. The observed common syndrome features were characterized as follows: a male predominance, fatigue (35.7%), tonsil hypertrophy (41.7%), lymphadenopathy (53.8%), hepatosplenomegaly (54.5%). leukocytosis (greater than 20.0 × 109/L, 71.4%), coexisting of eosinophilia and monocytosis (93.3%), and frequent progression to acute leukaemia. High incidence of tonsil hypertrophy and monocytosis may be a feature of EMS with CEP110/FGFR1 fusions. The CR rate for EMS was 23.1%. One patient treated with highly selective FGFR kinase inhibitor, INCB054828, achieved complete molecular remission rapidly. Allo-HSCT was performed in 8 patients. The median survival time for those patients was 9.0 (95%CI 5.599–12.601) months, with a range between 5 and 27 months. Allogeneic HSCT could improve survival in selected patients.
Conclusion: FGFR1 and RUNX1 may be potential therapeutic targets for clinical trials. More accumulation of cases is also needed to determine whether allo-HSCT could be an optimal approach.
Introduction
The 8p11 myeloproliferative syndrome (EMS) is a rare and distinct clinical pathologic entity characterized by reciprocal translocations involving the fibroblast growth factor receptor 1 (FGFR1) tyrosine kinase gene on chromosome 8p11-12 [Citation1]. Recently, this entity of neoplasms was listed in the 2016 World Health Organization classification of myeloid/lymphoid neoplasm with FGFR1 rearrangement [Citation2]. Currently, at least 14 FGFR1 partner genes have been identified in EMS. The various partner genes appear to foster activation of FGFR1 tyrosine kinases, and result in activation of downstream pathways involved in oncogenesis [Citation3,Citation4].
A literature review of 45 cases of EMS reported by Umino K demonstrated that the one-year overall survival (OS) from diagnosis was only 43.1% [Citation3]. The clinical manifestations of EMS are related with different partner genes and depend in part on whether or not the neoplasm is in chronic or acute phase. For example, patients with t(8;13) have T-cell non-Hodgkin’s lymphoma in addition to myeloproliferative neoplasm (MPN). By contrast, patients with t(8;22) are clinically and haematologically very similar to patients with BCR/ABL-positive chronic myelocytic leukaemia (CML) [Citation1,Citation5,Citation6]. Jackson et al. recently searched the literature for cases of EMS and only 65 cases are reported in the literature [Citation1]. A case analysis limited to confirmed CEP110-FGFR1 translocation has not yet been reported.
We here describe a case harbouring CEP110-FGFR1 fusions who received allo-HSCT and achieved molecular remission. Moreover, we collected a case series harbouring CEP110-FGFR1 fusions by reviewing the related literature to identify the laboratory characteristics and evaluate the therapeutic outcomes.
Patients and methods
Case report
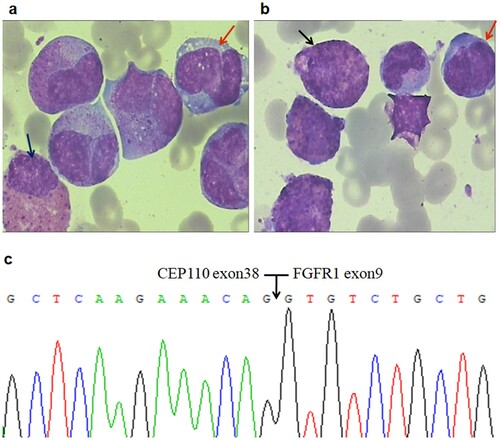
A 35-year-old female was presented with fatigue and purpura of 2-week duration in June 2016. She had no organomegaly. Initial investigations showed a leukocyte count of 62.99 × 109/L (neutrophils 30.87%, lymphocytes 7.0%, monocytes 5.0%, eosinophils 17.0%, and basophils 0%), haemoglobin 117 g/L, platelets 113 × 109/L. A high lactate dehydrogenase (LDH) was observed (540U/L; normal, <240 U/L). Bone marrow(BM) specimen showed hypercellularity with hypereosinophilia (28.0%). However, no definite blasts were found ((a,b)). There was no evidence of parasitic allergic, or other known causes of eosinophilia. Testing for 4q12 PDGFRA, 5q33 PDGFRB, or 9q34 ABL1 gene rearrangements by fluorescence in situ hybridization (FISH) was also negative. Bone marrow cytogenetics revealed the translocation 46,XY,t(8;9) (p11;q33) in 15/17 metaphases, whereas FISH identified rearrangement of the FGFR1. Analysis by reverse transcriptase polymerase chain reaction analysis (RT–PCR) and sequencing revealed an in-frame CEP110-FGFR1 fusion transcript with a breakpoint at exon 38 of the CEP110 gene and exon 9 of the FGFR1 gene ((c)). We detected the molecular mutations in the patient using next-generation sequencing (NGS) and DNA-based PCR Sanger sequencing of 51 genes known or suspected to have a role in myeloid malignancies, and identified one mutation, RUNX1p.Pro359fs, in BM samples at diagnosis. A definitive diagnosis of EMS was reached.
Figure 1. Morphologic changes, FISH analysis and RT-PCR of CEP110-FGFR1 fusion in bone marrow cells at the diagnosis. (a/b) Initial bone marrow showed hypercellular with eosinophilia (black arrow) and monocytosis (red arrow); no definite blasts were found in this specimen (WrighteGiemsa, 1000). (c) Nucleotide sequence analysis of the PCR product showing CEP110/FGFR1 fusion transcript with a breakpoint at exon 38 of the CEP110 gene and at exon 9 of the FGFR1 gene.
Initial treatment was started with hydroxyurea and Interferon alpha (IFNα), but complete response (CR) was not achieved. The patient subsequently underwent unrelated allogeneic haematopoietic stem cell transplantation (Allo-HSCT) 7 months later. At her last follow-up on 28 September 2018, she remained in complete haematological and molecular remission with normal blood parameters and negative RT-PCR results.
Case selection
The relevant medical literatures in the PubMed database in English were retrieved with terms including the disease ‘leukaemia’ OR ‘lymphoma’ OR ‘myeloproliferative’ and the words ‘FGFR1’ OR ‘8p11’. The PubMed databases were searched under the identification strategy described above for articles published between 1992 and 2018. The following laboratory results and clinical features were obtained from the literature. The study was approved by the local Ethics Committee and conducted in approach with the Declaration of Helsinki.
Statistical analysis
Statistical evaluation of the data was carried out using the Statistical Package for Social Sciences (SPSS version 17.0; SPSS Institute, Chicago, IL, U.S.A.). Overall survival time was calculated by the Kaplan-Meier method and compared statistically using the Log rank test. Statistical significance was considered to be present if the P < 0.05.
Results
Review of the literature
As a result, 395 reports were identified from PubMed. The following inclusion criteria must have been met: (1) Cases must be clinically consistent with the characteristics of EMS; (2) The conventional karyotyping revealed 8p11 and 9q33 abnormalities on bone marrow cells at diagnosis; (3) FGFR1 rearrangements and CEP110-FGFR1 fusions were confirmed by fluorescence in situ hybridization (FISH) or reverse transcription-polymerase chain reaction (RT-PCR). Finally, we identified 15 EMS cases with CEP110-FGFR1 fusions. Finally, 16 cases including our patients were included in the following analyses [Citation7–21].
Clinical presentation
EMS patients with CEP110-FGFR1 fusions can occur at any age, with a reported range from 7 months to 69 years of age. The median age at diagnosis was 37 years. Two patients were older than 60 years [Citation7,Citation8], and other 2 male infant patients [Citation9,Citation10] were younger than 1 year. In our review of the cases, we identified 10 males and 6 females, with a male predominance (female to male ratio 1.67:1). The case reported by Sohal et al. was not documented by clinical or biological data [Citation19]. In the other patients with clinical data, including the here-described case, fatigue (35.7%), fever (21.4%), and weight loss (14.3%) were the most common symptoms at initial presentation. Notably, both infant EMS patients presented with diffuse rash [Citation9,Citation10]. Swelling of tonsils presented in 41.7%, lymphadenopathy and hepatosplenomegaly presented in 53.8% and 54.5%, respectively, at the time of diagnosis.
leukocyte counts have ranged from 4.3 to 335 × 109/L (median, 44.5 × 109/L). In 71.4% of all patients, leukocytosis was greater than 20.0 × 109/L. Co-existing of eosinophilia and monocytosis were noted in 93.3% (14/15) cases, whereas basophils are rarely increased. Haemoglobin levels have ranged from 7.9 to 12.7 g/dL (median, 10.4 g/dL). Platelet counts have ranged from 30 to 349 × 109/L, with a median of 82.0 × 109/L; 63.6% had a count of less than 100 × 109/L and only one patient had a platelet count of greater than 300 × 109/L.
All of 16 cases, the initial BM aspirate was interpreted as hypercellular or slightly hypercellular. Additional chromosome 21 abnormalities were observed in two patients. The primary diagnosis was myeloproliferative neoplasm (MPN, n = 2), acute myeloid leukaemia (AML, n = 2), biphenotypic acute leukaemia (n = 1), Ph− CML (atypical chronic myelogenous leukemia, n = 1), eosinophilic chronic leukaemia (ECL, n = 1), T-cell lymphoma associated with hypereosinophilic syndrome (n = 1), and EMS (n = 8). shows the clinical manifestations of patients with CEP110-FGFR1fusions.
Table 1. Clinical and laboratory features of 16 Cases with CEP110-FGFR1 fusions.
Therapy and outcome analysis
Therapy and follow-up data are available in the literature for 15 patients with CEP110-FGFR1. Twelve patients with EMS (n = 8), or MPN (n = 2), or Ph− CML (n = 1), or ECL (n = 1) were treated with hydroxyurea (HU) (n = 4), interferon (n = 3), prednisolone (n = 3), or imatinib (n = 2). Three patients, including AML-M0 (n = 1), acute myelomonocytic leukaemia (AMMoL, n = 1), and biphenotypic acute leukaemia (B + Mono, n = 1), were administrated with conventional chemotherapy. The remaining 1 was observed without any treatment [Citation11]. Twelve of the above cases, transformation to blast phase (BP) was observed in 5 patients with a frequency at 1 year of 41.7%. Induction therapy data were available for 13 patients, CR was achieved only in 3 of 13 patients (23.1%) [Citation12–14]. Notably, the success of targeted therapy has been achieved in one patient, by INCB054828, a novel, highly selective FGFR kinase inhibitor [Citation13]. In another patient who remained complete haematological and molecular remission for two years after continuous single-agent treatment of low dose Interferon alpha (IFNα) [Citation14].
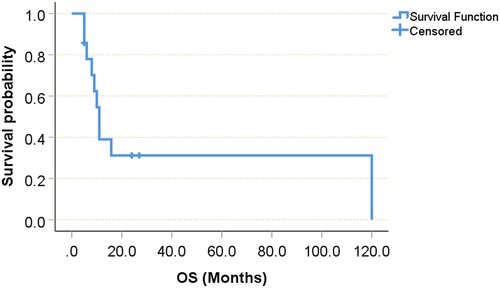
Allo-HSCT was performed in 8 patients: 2 with MPN, 4 with EMS, and 2 with acute leukaemia. Follow-up ranged from 0 to about 10 years. Survival time ranged from 5 months to 10 years, with some patients succumbing to complications of therapy. The median survival time for those patients who received HSCT was 9.0 (95%CI 5.599–12.601) months, with a range between 5 and 27 months. By contrast, the median survival time is 10.0 (95%CI 4.868–15.132) months for those who did not receive HSCT (log rank test, P = 0.741), with a range between 5 months to 10 years. Of the 15 EMS patients with follow-up, 71.4% died of disease, 6.7% are alive with disease, and 26.7% are alive without disease. Overall survival is 10.0 (95% CI 7.389–12.611) months. So far, almost all of the patients who survive long are treated with allo-HSCT. Disease-free survival was more than 24 months for our and another patient who received transplant and never underwent transformation [Citation13]. However, both patients aged younger 1 year died of graft-versus-host disease (GVHD) [Citation9,Citation10]. The Kaplan–Meyer curve with the indication of a median overall survival was illustrated in .
Discussion
The diagnosis of FGFR1 abnormalities remains poorly understood and often missed because the clinical and haematologic manifestations are heterogeneous. The variable manifestations of neoplasms associated with FGFR1 gene fusions suggest a need for nomenclature that incorporates the clinicopathologic features that may better characterize and treat this neoplasm. Here, we describe an EMS patient with CEP110-FGFR1 fusion who underwent allo-HSCT and achieved molecular remission. Moreover, we conducted a retrospective review of a case series from the published literature to evaluate the clinical features and therapeutic outcomes for EMS with confirmed CEP110-FGFR1 fusion.
The observed common syndrome features at presentation were characterized as follows: a male predominance, fatigue (35.7%), tonsil hypertrophy (41.7%), lymphadenopathy (53.8%), hepatosplenomegaly (54.5%). leukocytosis (greater than 20.0 × 109/L, 71.4%), coexisting of eosinophilia and monocytosis (93.3%), and frequent progression to acute leukaemia. High incidence of tonsil hypertrophy and monocytosis may be a feature of EMS with CEP110/FGFR1 fusions, which differ from EMS with other FGFR1 related fusions [Citation1,Citation3].
There is no established therapy for EMS patients and therefore various treatments, including protocols for acute lymphoblastic leukaemia (ALL), AML and for MPN, were used in this cohort. Our study demonstrated that the CR rate for EMS was extremely low (23.1%). Our findings suggested that FGFR1 rearrangement may be associated with chemoresistance in EMS. Novel therapeutic strategies are urgently required to improve the currently unfavourable outcome of EMS patients.
FGFR1 inhibitors are being developed for the treatment of solid tumours and more recently also for this neoplasm. Inhibitors of FGFR1 including ponatinib and dovitinib have shown in vitro activity against cells harbouring FGFR1 rearrangements [Citation22]. Prior reports have demonstrated ponatinib could lead to a complete morphologic remission and maintenance of a major cytogenetic response before the patient proceeded to HSCT [Citation23]. However, ponatinib alone may be insufficient to produce a deep remission. In our series, treatment of CEP110-FGFR1 fusion-associated EMS with a novel, highly selective FGFR kinase inhibitor, INCB054828, elicited complete molecular remission, but the patient had treatment interrupted for an unrelated issue and his disease progressed rapidly to AML. Subsequently, the patient underwent treatment with intensive chemotherapy followed by allogeneic HSCT. Therefore, INCB054828 may represent a promising therapeutic option for elderly patients or patients not eligible for an HSCT [Citation13], but more accumulation of cases is needed to verify the effect of INCB054828. Remarkably, WEHRLI M et al. reported on a patient with relapsed, imatinib-refractory EMS, who has been treated successfully with dasatinib to achieve a haematological remission for more than 9 months [Citation8]. It suggests that the oral multi-TKI dasatinib may provide a therapeutic option for elderly and frail EMS patients who cannot be offered allo-HCT. In addition, we also observed that one EMS case in China remained complete haematological and molecular remission for two years after continuous single agent treatment of low dose Interferon alpha [Citation14]. But such effect was not seen in other patients treated with Interferon alpha.
In this study, our case underwent allogeneic HSCT before blast phase achieved relative long-term remission. The result suggests that allo-HSCT may improve the poor prognosis of patients with CEP110-FGFR1 fusions, when performed before blast phase transformation. Finally, we did not find a significant difference between allo-HSCT and non-allo-HSCT group. Disease-free survival was more than 24 months for 2 patients who received transplant, but 2 infant cases died of GVHD. It suggests that the importance of HSCT as the only potentially curative option for its ‘right’ patients.
We observed that the present patient had a concomitant mutation in RUNX1, a gene encoding the alpha subunit of the heterodimeric transcription factor core binding factor, whose mutation was a marker of poor prognosis in AML [Citation24]. Strati P and co-workers performed a retrospective analysis of 17 EMS patients with FGFR1 rearrangement, the study revealed that 78% of tested patients with ZMYM2-FGFR1 or BCR-FGFR1 had a RUNX1 mutation, of whom all had acute leukaemia [Citation25]. Our case and a child patient with CEP110-FGFR1 described by Lv H et al. were also positive for RUNX1 mutations [Citation12]. High incidence of RUNX1 mutation in FGFR1 rearranged (FGFR1r) cases suggests the FGFR1 rearrangement and RUNX1 mutation may act cooperatively in EMS and RUNX1 may be a therapeutic target for novel small molecule inhibitors.
Here we describe an EMS patient with CEP110-FGFR1 fusion who underwent allogeneic HSCT and achieved molecular remission. Moreover, the available literature points to that these patients share the common syndrome features: fatigue, tonsil hypertrophy, leukocytosis, coexisting of eosinophilia and monocytosis, lymphadenopathy, hepatosplenomegaly, and frequent progression to acute leukaemia. Allogeneic HSCT could improve survival in selected patients. However, fatal complications of HSCT suggested that more accumulation of cases is needed to determine whether allo-HSCT could be an optimal approach. Additionally, FGFR1 and RUNX1 may be potential therapeutic targets for clinical trials.
Acknowledgements
This work was partly supported by grants from the National Science funds (no. 81500103), Natural Science Foundation of Jiangsu Province, China (no. BK-20160283), Changzhou Sci Tech Program (grant no. 20180033), Young Scientists Foundation of Changzhou No. 2 People’s Hospital (grant no. 2019K002).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Human Pathol. 2010;41:461–476. DOI:https://doi.org/10.1016/j.humpath.2009.11.003.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the WorldHealth Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. DOI:https://doi.org/10.1182/blood-2016-03-643544.
- Umino K, Fujiwara SI, Ikeda T, et al. Clinical outcomes of myeloid/lymphoid neoplasms with fibroblast growth factor receptor-1 (FGFR1) rearrangement. Hematology. 2018;23:470–477. DOI:https://doi.org/10.1080/10245332.2018.1446279.
- Xiao S, McCarthy JG, Aster JC, et al. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood. 2000;96:699–704.
- Macdonald D, Reiter A, Cross NC. The 8p11 myeloproliferative syndrome: a distinct clinical entity caused by constitutive activation of FGFR1. Acta Haematol. 2002;107:101–107. DOI:https://doi.org/10.1159/000046639.
- Lee SG, Park TS, Lee ST, et al. Rare translocations involving chromosome band 8p11 in myeloid neoplasms. Cancer Genet Cytogenet. 2008;186:127–129. DOI:https://doi.org/10.1016/j.cancergencyto.2008.07.002.
- Mozziconacci MJ, Carbuccia N, Prebet T, et al. Common features of myeloproliferative disorders with t(8;9)(p12;q33) and CEP110–FGFR1 fusion: report of a new case and review of the literature. Leuk Res. 2008;32:1304–1308. DOI:https://doi.org/10.1016/j.leukres.2007.11.012.
- Wehrli M, Oppliger Leibundgut E, Gattiker HH, et al. Response to tyrosine kinase inhibitors in myeloproliferative neoplasia with 8p11 translocation and CEP110-FGFR1 rearrangement. Oncologist. 2017;22:480–483. DOI:https://doi.org/10.1634/theoncologist.2016-0354.
- Sarthy JF, Reddivalla N, Radhi M, et al. Pediatric 8p11 eosinophilic myeloproliferative syndrome (EMS): a case report and review of the literature. Pediatr Blood Cancer. 2017;64(5). DOI:https://doi.org/10.1002/pbc.26310.
- Li W, Cooley LD. Unusual infant eosinophilia: myeloid neoplasm with FGFR1 abnormality. Blood. 2016;128:1440. DOI:https://doi.org/10.1182/blood-2016-06-722231.
- Hu S, He Y, Zhu X, et al. Myeloproliferative disorders with t(8;9)(p12;q33): a case report and review of the literature. Pediatr Hematol Oncol. 2011;28:140–146. DOI:https://doi.org/10.3109/08880018.2010.528170.
- Lv H, Hu S, Lu J, et al. Precursor T-lymphoblastic lymphoma associated with t(8;9)(p11.2;q33): a case report and review of the literature. Acta Haematol. 2018;139:176–182. DOI:https://doi.org/10.1159/000481392.
- Verstovsek S, Subbiah V, Masarova L, et al. Treatment of the myeloid/lymphoid neoplasm with FGFR1 rearrangement with FGFR1 inhibitor. Ann Oncol. 2018;29:1880–1882. DOI: https://doi.org/10.1093/annonc/mdy173.
- Zhou L, Fu W, Yuan Z, et al. Complete molecular remission after interferon alpha treatment in a case of 8p11 myeloproliferative syndrome. Leuk Res. 2010;34:e306–e307. DOI: https://doi.org/10.1016/j.leukres.2010.06.027.
- Park TS, Song J, Kim JS, et al. 8p11 myeloproliferative syndrome preceded by t(8;9)(p11;q33), CEP110/FGFR1 fusion transcript: morphologic, molecular, and cytogenetic characterization of myeloid neoplasms associated with eosinophilia and FGFR1 abnormality. Cancer Genet Cytogenet. 2008;181:93–99. DOI:https://doi.org/10.1016/j.cancergencyto.2007.11.011.
- Yamamoto K, Kawano H, Nishikawa S, et al. A biphenotypic transformation of 8p11 myeloproliferative syndrome with CEP1/FGFR1 fusion gene. Eur J Haematol. 2006;77:349–354. DOI:https://doi.org/10.1111/j.1600-0609.2006.00723.x.
- Lee H, Kim M, Lim J, et al. Acute myeloid leukemia associated with FGFR1 abnormalities. Int J Hematol. 2013;97:808–812. DOI:https://doi.org/10.1007/s12185-013-1337-5.
- Guasch G, Mack GJ, Popovici C, et al. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33). Blood. 2000;95:1788–1796.
- Sohal J, Chase A, Mould S, et al. Identification of four New translocations involving FGFR1 in myeloid disorders. Genes Chromosomes Cancer. 2001;32:155–163.
- Yamamoto S, Ebihara Y, Mochizuki S, et al. Quantitative polymerase chain reaction detection of CEP110-FGFR1 fusion gene in a patient with 8p11 myeloproliferative syndrome. Leuk Lymphoma. 2013;54:2068–2069. DOI:https://doi.org/10.3109/10428194.2013.767455.
- Sarahx OJ, Anthony AO, Titilope AA, et al. The 8p12 myeloproliferative syndrome. Niger Med J. 2014;55:176–179. DOI:https://doi.org/10.4103/0300-1652.129669.
- Landberg N, Dreimane A, Rissler M, et al. Primary cells in BCR/FGFR1-positive 8p11 myeloproliferative syndrome are sensitive to dovitinib, ponatinib, and dasatinib. Eur J Haematol. 2017;99:442–448. DOI:https://doi.org/10.1111/ejh.12957.
- Khodadoust MS, Luo B, Medeiros BC, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed-phenotype acute leukemia. Leukemia. 2016;30:947–950. DOI:https://doi.org/10.1038/leu.2015.136.
- Gaidzik VI, Teleanu V, Papaemmanuil E, et al. RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leukemia. 2016;30:2160–2168. DOI:https://doi.org/10.1038/leu.2016.126.
- Strati P, Tang G, Duose DY, et al. Myeloid/lymphoid neoplasms with FGFR1 rearrangement. Leuk Lymphoma. 2018;59:1672–1676. DOI:https://doi.org/10.1080/10428194.2017.1397663.