ABSTRACT
Objectives
Our aim is to evaluate initial efficacy, safety, and durable response of eltrombopag in the treatment of Chinese children with chronic immune thrombocytopenia (cITP).
Methods
This was a retrospective, single-center cohort study including 30 pediatric patients with cITP administered eltrombopag between 1 July 2017 and 1 January 2019. Patients with at least 12 weeks of eltrombopag treatment and available follow-up data were included. Initial response rate, durable response rate, bleeding events, and adverse events were assessed during the follow-up period.
Results
The median duration of eltrombopag administration was 6 months (range 3–8 months). The initial response rate was 73.3%. Patients with megakaryocyte count ≥100/slide or Treg <4.5% were more likely to achieve initial response. The median follow-up period was 10 months (range 6–20 months). A total of 53.2% of pediatric patients had a durable response of up to 20 months. Patients with megakaryocyte count ≥100/slide and Treg<4.5% had more than 60% durable response rates compared with individuals with megakaryocyte count<100/slide and Treg≥4.5%, respectively. No serious bleeding events or serious adverse events occurred during the study period.
Conclusion
Eltrombopag not only shows excellent initial response but also has continued efficacy and safety. Patients with megakaryocyte count ≥100/slide and Treg<4.5% achieve increased initial response and more frequent durable response.
1. Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia (platelet count<100 × 109/L) as a result of increased platelet destruction and insufficient platelet production [Citation1]. The incidence of ITP is approximately between 5 and 10 per 100,000 children per year [Citation2]. Although ITP is a self-limiting process for the majority of children, the disease may become chronic in up to 20% of patients [Citation3]. Pediatric chronic ITP (cITP) (lasting ≥12 months) is commonly associated with limited activities and fear of bleeding that can obviously affect the quality of life [Citation4]. It is important that children with cITP receive appropriate treatment due to the bleeding risk associated with reduced platelet count. Conventional treatment options for pediatric cITP include immunosuppressive drugs such as azathioprine, cyclosporine A, cyclophosphamide, rituximab and vinca alkaloids [Citation5–7]. These therapeutics primarily aim to reduce platelet destruction, and splenectomy removes the site of platelet clearance and autoantibody production [Citation8,Citation9]. Meanwhile, a unique ITP therapy, namely applying thrombopoietin receptor agonists (TPO-RAs), improves platelet production by activating the thrombopoietin pathway [Citation10–13]. The small-molecule TPO-RAs interact with thrombopoietin receptor on megakaryocytes and hematopoietic stem cells, stimulating megakaryocytes [Citation14]. However, these molecules do not share the same sequence with endogenous thrombopoietin. Currently, the oral TPO-RA eltrombopag is the only product authorized for use in pediatric patients with chronic ITP by the US Food and Drug Administration (FDA) [Citation15]. In addition, it has a demonstrated additive, rather than antagonistic, effect to endogenous TPO. To date, ITP pathogenesis is not entirely clear. However, patients with ITP may possess autoreactive B and T cells accompanied by a breakdown of immune tolerance [Citation16,Citation17]. A subset of relatively rare T lymphocytes called regulatory T lymphocytes (Treg) representing 5-10% of the normal CD4+ T cell population, play a leading role in protecting individuals from autoimmunity, thus maintaining immune tolerance and homeostasis [Citation18]. A decrease in the number and function of Tregs has been detected in pediatric patients with ITP [Citation19]. Importantly, eltrombopag may restore the activity of both Tregs and regulatory B cells, which may help re-establish the immune tolerance to platelets [Citation20].
Being the only available treatment option for children with ITP that can improve platelet production, eltrombopag plays a central role in pediatric treatment approaches. Recently, two multicenter, double-blind, placebo-controlled trials of eltrombopag in pediatric patients with ITP lasting more than 6 months demonstrated that eltrombopag administration results in increased platelet counts and reduced bleeding symptoms, with good tolerability [Citation10,Citation21]. However, the efficacy and safety of eltrombopag in the treatment of Chinese children with cITP is largely unknown. Moreover, only scarce studies of durable response associated with eltrombopag in children have been conducted. Therefore, the primary objective of this study was to investigate the initial response, durable response and safety of eltrombopag in pediatric Chinese patients. The secondary objective was to identify the subpopulation of patients who would benefit more from the agent.
2. Materials and methods
2.1. Patients
This retrospective, single-center study included 30 children with cITP treated with eltrombopag at Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, between 1 July 2017 and 1 January 2019. Patients with at least 12 weeks of eltrombopag treatment and follow-up data were included in the analysis. Demographic and baseline ITP data regarding eltrombopag’s first administration were obtained, including age, gender, time since diagnosis (months), ITP treatments prior to starting eltrombopag, starting dose, platelet count, megakaryocyte count and Treg%. Duration of treatment, dose, platelet response and long-term outcomes (remission and relapse) were collected. The study was approved by the Ethics Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University. Informed consent was obtained from each patient or his/her legally authorized guardian. This retrospective study was not listed on Clinicaltrials.gov because of the retrospective design. Patients were followed up until the observation period ended on 30 June 2019.
2.2. Treatment
The starting dose of eltrombopag was 50 mg per day for children aged 6 years or above, and 1.5 mg/kg.d for those aged 1–6 years or weight<27 kg. Once initiated, eltrombopag dosing was adjusted to achieve a platelet count goal of 50 × 109/L, not to exceed 150 × 109/L. The dose could be escalated every 2 weeks in increments of 12.5 mg to achieve the target platelet range. The maximum dose of eltrombopag is 75 mg per day. We decreased the dose by 12.5 mg once per day at 2-week intervals with a platelet count increase of more than 150 × 109/L. In case of platelet count increase of more than 400 × 109/L, treatment was interrupted and resumed at the next lower dose based on 12.5 mg increments once the patient’s platelet count had decreased to below 150 × 109/L.
2.3. Efficacy and safety analysis
The criteria for response to treatment were as follows: (1) complete response (CR), platelet count >100 × 109/L; (2) partial response (PR), platelet count at 30–100 × 109/L, or baseline count doubling after treatment; (3) no response (NR), platelet count less than 30 × 109/L, baseline count doubling, or bleeding events after administration of an appropriate dose of eltrombopag for 12 weeks; (4) overall response rate (ORR), defined as CR rate + PR rate; (5)relapse defined as either 2 consecutive platelet counts<30 × 109/L or need for additional therapy; (6)initial response, measured at or around the 12-week mark from the first dose; (7) duration of response (DR), calculated from the date of initial response to relapse or latest follow-up (30 June 2019). Safety was assessed based on the incidence of serious bleeding events (such as mucosal bleeding) and the occurrence of any adverse events, which were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
2.4. Flow cytometric analysis of Tregs
A single-cell suspension was prepared according to a standard protocol. Cells were incubated with anti-CD4-FITC and anti-CD25-APC monoclonal antibodies at 4°C for 30 min. Cells were handled according to the manufacturer’s instructions and stained with PE anti-Foxp3 for Treg detection. Isotype controls were used to confirm antibody specificity. Stained cells were analyzed by flow cytometry on a FACScan cytometer equipped with the CellQuest software (BD Biosciences Pharmingen, San Diego, CA). CD4+ CD25+ FOXP3+ (Treg) percentage had a normal reference range of 4.5–6.2%.
2.5. Statistical analysis
Clinical characteristics were analyzed and compared by descriptive analysis, the Fisher’s exact test and the exact probability method. Durable response was determined by Kaplan–Meier analysis and single-factor analysis was performed by the Log-rank test. Factors with P < 0.05 in univariate analysis were included in multivariate analysis using the Cox regression model. Two-tailed P < 0.05 was considered statistically significant. The SPSS software (version 21.0) was used for data analysis.
3. Results
3.1. Demographic and baseline clinical data
A total of 30 pediatric patients with cITP were included. Demographic and baseline clinical data are reported in (). The median age of the patients was 7 (2.3–14.0) years. Among them, 10 patients (33.3%) were aged 1 to <6 years, 18 (60.0%) were 6–12years old, and 2 (6.7%) were aged >12 years. Baseline platelet count was 15 (7–26) × 109/L. The median duration of ITP was 2.0 (1.0–3.5) years. One patient (3.3%) underwent splenectomy and 7 (23.3%) had more than three previous immune thrombocytopenia treatments.
Table 1. Demographic and baseline clinical data of pediatric patients with cITP.
The median duration of eltrombopag administration was 6 months (range, 3–8 months). During the entire study period, 14/30 patients (46.7%) discontinued eltrombopag treatment, 8/30 (26.7%) discontinued eltrombopag permanently for a platelet count consistently <30 × 109/L within the first 12 weeks of treatment, 4 showed relapse and 2 still kept an effective level after treatment discontinuation.
3.2. Treatment response
Initial efficacy data are summarized in . Overall, 25 (83.3%) of the 30 patients achieved a platelet count of over 50 × 109/L at least once in the absence of rescue therapy. A total of 4 weeks after eltrombopag treatment initiation, the median platelet count was 55×109/L(15-146 × 109/L); 9 of the 30 patients (30%) achieved PR and 5 (17%) achieved CR. At the end of 12 weeks of treatment, the median platelet count was 78 × 109/L (20-310 × 109/L); 11 of the 30 patients (36.7%) achieved PR and 11 (36.7%) achieved CR. The initial response rate (Week 12) was 73.3%. Totally 85.0 and 50.0% of individuals with megakaryocyte count≥ 100/slide and <100/slide, respectively (P = 0.038), achieved initial response; these rates were 88.9 and 50.0% individuals with Treg<4.5% and ≥ 4.5%, respectively (P=0.034), and 70.6 and 76.9% males and females, respectively (P = 1).
Table 2. Initial efficacy data of pediatric patients with cITP treated with eltrombopag.
The follow-up period ended on June 30, 2019, with a median time of 10 months (6–20 months). Out of the 30 children treated with eltrombopag, 22 achieved initial response, among whom 4 showed relapse over time; the remaining 18 patients (53.2%) maintained a durable response without additional treatment.
3.3. Univariate analysis and multivariate analysis
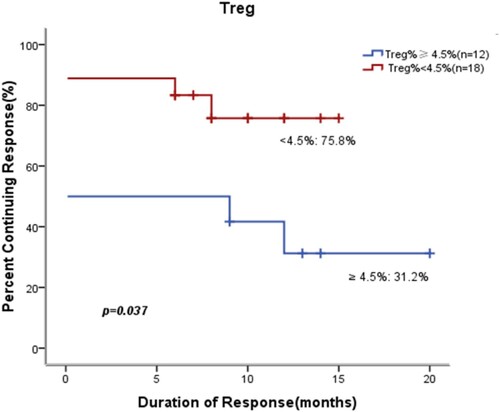
We performed univariate analysis of factors that may affect durable response in pediatric patients treated with eltrombopag such as gender, megakaryocyte count and Treg%. In the 20 patients with megakaryocyte count≥100/slide, 61.8% had a durable response to the treatment versus only 40.0% of the 10 patients with megakaryocyte count<100/slide during the follow-up period (P = 0.047) (). In the 18 patients with Treg<4.5%, 75.8% had a durable response versus only 31.2% of the 12 patients with Treg≥4.5% (P = 0.037) (). Our results indicated that megakaryocyte count≥100/slide and Treg<4.5% were significant factors leading to better prognosis in children. However, in univariate analysis, other factors, including gender, children’s age at eltrombopag treatment initiation (cutoff value, 7 years of age), duration of ITP before eltrombopag treatment (cutoff value, 2 years) and baseline platelet amounts (cutoff value, 15 × 109/L), had no significant effects on durable response to treatment (P > 0.05).
Figure 1. Effects of megakaryocyte count on prognosis after eltrombopag therapy. Patients with megakaryocyte count≥100/slide prior to treatment initiation were more likely to achieve initial response and maintain durable response.
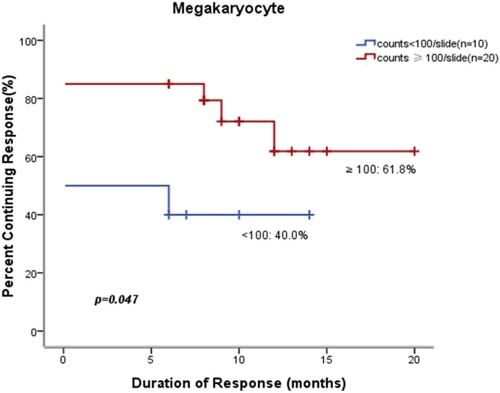
Figure 2. Kaplan-Meier curves of response rates in pediatric patients with cITP based on Treg% at baseline. Treg<4.5% not only predicted higher initial response to the therapy, but also had a significant effect on the likelihood of maintaining remission.
Cox multivariate analysis showed that megakaryocyte count≥100/slide (RR = 4.281, 95% CI 1.184–15.473, P = 0.027) and Treg<4.5% (RR = 4.355, 95% CI 1.217–15.589, P = 0.024) were independent prognostic factors affecting durable response in children ().
Table 3. Prognostic factors of durable response by multivariable analysis.
3.4 . Safety evaluation
During the treatment period, only one case (3.3%) of vomiting occurred. Headache was not observed. No treatment-related adverse events were considered to be serious. Two patients (6.7%) had mild bleeding during treatment with eltrombopag. One (3.3%) patient underwent a transitory elevation of transaminases (more than double the normal values) without clinical symptom or increased direct bilirubin. The elevations of transaminases decreased after a temporary dose reduction of eltrombopag. No patient withdrew due to adverse events. No malignancies or thromboses were reported during the study.
4 . Discussion
An initial response rate of 73.3% (22/30) for eltrombopag was observed, which is consistent with Neunert et al [Citation22]. Further information from clinical studies is needed to identify the ideal patient population for eltrombopag application in children. This study demonstrated that patients with megakaryocyte count≥100/slide and Treg<4.5% were more likely to achieve initial response. However, no association of the initial response rate to children’s age, the duration of the disease or the number of platelets at the start of the treatment was found in our study.
Recently, many investigators have reported the actual long-term outcome of adults with cITP treated with eltrombopag. Gonzá lez-López et al. [Citation23] found that 53% of adult patients with chronic ITP have a sustained remission after stopping eltrombopag at a median follow-up of 9 months. This study showed that about 50% of patients had long-term response after eltrombopag treatment, whereas 81.8% of initial responders maintained a durable response without additional treatment. Although there are differences between our data and those of Gonzá lez-López, both studies indicate that eltrombopag is an optimal long-term solution for achieving ‘cure’ in patients with cITP. Importantly, the major finding here was that the improved durable response to eltrombopag was primarily observed in specific groups of patients. Patients with megakaryocyte count≥100/slide and Treg<4.5% had higher durable response rates compared with those with megakaryocyte count<100/slide and Treg≥4.5%, respectively. Despite the limited number of children included in this study, it is clear that megakaryocyte count≥100/slide and Treg<4.5% are both indicators of long-term remission upon treatment with eltrombopag.
Eltrombopag can bind and activate the TPO-receptor to mimic TPO activity [Citation11,Citation24]. The TPO pathway is involved in the proliferation of megakaryocytes in the bone marrow. As a result, in addition to promoting platelet production from existing megakaryocytes, eltrombopag may also enhance the proliferation of megakaryocytes in the bone marrow. This is probably why patients with elevated megakaryocytes respond better to eltrombopag. Besides stimulating platelet production from megakaryocytes, eltrombopag has demonstrated additional immunomodulatory effects [Citation25]. David et al. [Citation25] and Guo et al. [Citation26] found that eltrombopag exerts direct and indirect effects on the immune system, including enhancing Treg activity, which may be associated with a permanent response in children with cITP. The above results corroborated the latter reports, as a higher durable response rate was found in patients with Treg<4.5% compared with those with Treg ≥4.5%.
Gonzalez-Porras and Bastida [Citation27] reported similar rates of adverse effects leading to study discontinuation in adults administered eltrombopag and placebo. In our study, eltrombopag appeared to be well-tolerated with minimal adverse effects. Due to its mechanism of action, eltrombopag is associated with thrombocytosis. Nevertheless, during the whole chronic period, no thrombotic events were detected in our study. Hepatotoxicity and headache are the most frequently reported side effects of eltrombopag [Citation28]. However, they were infrequent in our study.
This retrospective study was limited by small sample size, lack of randomization and relatively short follow-up. In addition, the possibility of spontaneous remission cannot be completely ruled out. Further randomized controlled trials are warranted to obtain higher quality evidence.
In conclusion, this study showed that eltrombopag appears to be efficacious and safe while waiting for a spontaneous improvement in children. Moreover, it has encouraging durable response. Finally, megakaryocyte count and Treg% at the time of eltrombopag initiation could predict response to treatment.
Acknowledgements
The work was supported by the Natural Science Foundation of Guangdong Province, China [grant number 2018A030313680], the Guangdong Basic and Applied Basic Research Foundation [grant number 2020A1515010312], the Zhuhai Technology Plan Program of Medicine and Health [grant number 20181117A010017].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chalmers S, Tarantino MD. Romiplostim as a treatmentforimmune thrombocytopenia: a review. J Blood Med. 2015;6:37–44.
- Despotovic JM, Grimes AB. Pediatric ITP: is it different from adult ITP? Hematology Am Soc Hematol Educ Program. 2018;2018(1):405–411.
- Berrueco R, Dapena JL, Sebastián E, et al. Controversies in the treatment of pediatric immune thrombocytopenia. An Pediatr (Barc). 2018;89(3):189.e1–189.e8.
- Grainger JD, Blanchette VS, Grotzinger KM, et al. Health-related quality of life in children with chronic immune thrombocytopenia treated with eltrombopag in the PETIT study. Br J Haematol. 2019;185(1):102–106.
- Kühne T. Diagnosis and management of immune thrombocytopenia in childhood. Hamostaseologie. 2017;37(1):36–44.
- Dai WJ, Zhang RR, Yang XC, et al. Efficacy of standard dose rituximab for refractory idiopathic thrombocytopenic purpura in children. Eur Rev Med Pharmacol Sci. 2015;19(13):2379–2383.
- Oved JH, Lee CSY, Bussel JB. Treatment of children with persistent and chronic idiopathic thrombocytopenic purpura: 4 infusions of rituximab and three 4-day cycles of dexamethasone. J Pediatr. 2017;191:225–231.
- Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131(11):1172–1182.
- Schifferli A, Holbro A, Chitlur M, et al. Intercontinental Cooperative ITP study group(ICIS), A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. Am J Hematol. 2018;93(6):751–759.
- Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomized, multicentre, placebo-controlled study. Lancet Haematol. 2015;2(8):e315–e325.
- Kim TO, Despotovic J, Lambert MP. Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2018;2(4):454–461.
- Guo JC, Zheng Y, Chen HT, et al. Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenia: a meta-analysis. Oncotarget. 2017;9(6):7112–7125.
- Tumaini Massaro J, Chen Y, Ke Z. Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenic purpura: meta-analysis. Platelets. 2019;30(7):828–835.
- Kühne T. Treatment of pediatric primary immune thrombocytopenia with thrombopoietin receptor agonists. Semin Hematol. 2015;52(1):25–30.
- Merli P, Strocchio L, Vinti, L, et al. Eltrombopag for treatment of thrombocytopenia-associated disorders. Expert Opin Pharmacother. 2015;16(14):2243–2256.
- Yazdanbakhsh K. Imbalanced immune homeostasis in immunethrombocytopenia. Semin Hematol. 2016;53(suppl 1):S16–S19.
- Kim TO, Grimes AB, Kirk S, et al. Association of a positive direct antiglobulin test with chronic immune thrombocytopenia and use of second line therapies in children: A multi-institutional review. Am J Hematol. 2019;94(4):461–466.
- Piconese S, Pacella I, Timperi E, et al. Divergent effects of type-I interferons on regulatory T cells. Cytokine Growth Factor Rev. 2015;26(2):133–141.
- Son BR, Kim JY. Association of CD4(+)CD25(+)FoxP3(+) regulatory T cells with natural course of childhood chronic immune thrombocytopenic purpura. Korean J Pediatr. 2015;58(5):178–182.
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16.
- Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomized, multicentre, placebo-controlled trial. Lancet. 2015;386(10004):1649–1658.
- Neunert C, Despotovic J, Haley K, et al. Pediatric ITP Consortium of North America (ICON). Thrombopoietin receptor agonist use in children: data from the pediatric ITP Consortium of North America ICON2 study. Pediatr Blood Cancer. 2016;63(8):1407–1413.
- González-López TJ, Pascual C, Álvarez-Román MT, et al. Successful discontinuation of eltrombopag after complete remission in patients with primaryimmune thrombocytopenia. Am J Hematol. 2015;90(3):E40–E43.
- Grainger JD, Thind S. A practical guide to the use of eltrombopag in children with chronic immune thrombocytopenia. Pediatr Hematol Oncol. 2017;34(2):73–89.
- Gómez-Almaguer D. Eltrombopag-based combination treatment for immune thrombocytopenia. Ther Adv Hematol. 2018;9(10):309–317.
- Guo NH, Fu X, Zi FM, et al. The potential therapeutic benefit of resveratrol on Th17/Treg imbalance in immune thrombocytopenic purpura. Int Immunopharma-col. 2019;73:181–192.
- Gonzalez-Porras JR, Bastida JM. Eltrombopag in immune thrombocytopenia: efficacy review and update on drug safety. Ther Adv Drug Saf. 2018;9(6):263–285.
- Gonzalez-Porras JR, Mingot-Castellano ME, Andrade MM, et al. Use of eltrom-bopag after romiplostim in primary immune thrombocytopenia. Br J Haematol. 2015;169(1):111–116.
