ABSTRACT
Objective
To assess clinical outcomes of newly diagnosed extranodal NK/T-cell lymphoma nasal-type (ENKTL) patients treated with L-asparaginase-containing chemotherapy as first-line chemotherapy.
Materials and Methods
We retrospectively reviewed data of new ENKTL patients in Siriraj Hospital, Thailand during 2012–2016.
Results
Among 27 patients, 16 were men and 11 were women, and their median age was 52 (range, 25–83) years. The primary sites were the aero-nasal area (23) and skin (4). Clinical stages of ENKTL were I, II, III and IV in 10, 8, 1 and 8 patients, respectively. Patients classified according to the NK cell-lymphoma prognostic index with PINK (27) and PINK-E (19) as low, intermediate, and high were 14, 8, 5, respectively and 11, 4, and 4, respectively. Chemotherapy regimens used were SMILE (24) and AspaMetDex (3). Fourteen patients received post- chemotherapy local radiation. The overall response rate for 26 assessable patients was 69%, with complete response in 14 patients (52%). Median progression-free and overall survival were 32 and 33 months, respectively. The most common L-asparaginase regimen related complications were hypofibrinogenemia (24) and hypersensitivity (9). The overall mortality at follow-up was 14/27 (52%) owing to sepsis (11) and disease progression (3).
Discussion
L-asparaginase-based regimens showed similar results as other study results. including those in which hematopoietic stem cell transplantation was performed, suggesting that transplantation can be avoided if it is unaffordable. Thrombosis or bleeding, the regimen side effects, should be carefully monitored during treatment.
Conclusions
L-asparaginase-based regimens offer a good outcome as a front-line treatment for ENKTL.
Introduction
According to the World Health Organization (WHO) classification of tumors of lymphoid tissues [Citation1], extranodal natural killer/T-cell lymphoma, nasal-type (ENKTL) is classified in the same group of aggressive subtypes of non-Hodgkin lymphoma or mature T and NK neoplasms, which are strongly associated with the Epstein–Barr virus (EBV) infection. ENKTL has a poor prognosis, with a 5-year overall survival rate of only 54% for nasal type, and 34% for extra nasal type [Citation2]. The most commonly involved sites are the extranodal regions, such as the upper respiratory or gastrointestinal tract [Citation3]. The patients are predominantly male (2.4:1), with a median age of around 44 [Citation4]. The incidence is low in North America, and in Europe, it is about 0.3% of total lymphoma cases. However, it is much more common in Southeast Asia, where ENKTL comprises 5.2% of all lymphoma cases [Citation5].
Because of its low incidence, there has been no randomized controlled study for this disease, and its treatment regimens are either institutional or according to local guidelines The treatment regimen depends on the disease stage and the performance status of the patient According to a previous 7-year retrospective study at Siriraj Hospital in Bangkok, Thailand, CHOP, the conventional lymphoma chemotherapy regimen, with or without radiotherapy, achieved a complete response (CR) rate of only 16%, with a median overall survival of 6 months [Citation6] Because of the overexpression of p-glycoprotein in lymphoma cells, multidrug resistance has occurred L-asparaginase is not affected by a multidrug-resistance gene [Citation7]; therefore, it is applied to chemotherapy regimens such as the AspaMetDex regimen (CR rate 61%; median overall survival of 12.2 months [Citation8]), the LVP regimen (CR rate 83% and 3-year overall survival rate of 862% [Citation9]), and the SMILE regimen (CR rate 45%, an overall response rate of 79%, and a 1-year overall survival rate of 55% [Citation10]), for treating ENKTL. L-asparaginase is an important drug for treating ENKTL; however, it can have either minor or life-threatening side effects, such as hypofibrinogenemia, pancreatitis, bleeding, thrombosis, and anaphylaxis.
In Thailand, a 2-year retrospective study of outcomes from the SMILE regimen at Siriraj Hospital revealed a CR rate of 36% and an overall response rate of 64% [Citation11]. Nevertheless, there is no data regarding the outcome of all L-asparaginase-based chemotherapy protocols in Thailand This study aimed to assess the clinical responses of first-line L-asparaginase containing regimen in newly diagnosed ENKTL patients and also examine the survival outcomes of these patients.
Materials and methods
This study included all newly diagnosed ENKTL patients at Siriraj Hospital from January 2012 to December 2016 We reviewed patient charts retrospectively All patients had a biopsy-proven diagnosis with immunohistochemistry and/or EBV-encoded small nuclear RNA by Epstein–Barr encoding region in situ hybridization method Hematopathologists at the Siriraj Hospital reviewed biopsy results from other hospitals.
We reviewed patient charts for clinical data, including age, sex, site of presentation, characteristic lesions detected before treatment, Eastern Cooperative Oncology Group (ECOG) performance status, laboratory results, images, the first chemotherapy regimen, the second chemotherapy regimen, the number of cycles, the outcomes, the radiation therapy, and the survival rate Lastly, we measured EBV DNA in peripheral blood by real-time polymerase chain reaction (RT–PCR) and reported the positive or negative results. The cut-off date of the data was December 30, 2019.
Response assessment with computerized tomography scan was based on the Lugano response criteria for non-Hodgkin lymphoma [Citation12]. In brief, CR was defined as complete disappearance or regression less than or equal to 1.5 cm in longest transverse diameter of the lesion. Partial response (PR) was defined as at least a 50% reduction in the lesion. Stable disease (SD) was defined as a lesion that decreased in size by less than 50%. Progressive disease (PD) was defined as an increase in the lesion’s transvers and/or perpendicular diameter.
The SMILE [Citation10] and AspaMetDex [Citation8] regimens are shown in .
Table 1. L-asparaginase-based regimen.
End points
The primary end point was progression-free survival (PFS). The secondary end points were overall survival (OS) and response rates. We defined PFS as the time from the first treatment to disease progression or death from any cause, and OS as the time from the first treatment to death from any cause.
Statistical analysis
We analyzed the demographic data for frequency or percentage distribution. We conducted the survival analysis using the Kaplan-Meier method to calculate PFS and OS, and compared survival curves using log-rank test with univariate analysis. The log-rank tests were used to assess the relationship between the variables and survival outcomes. Multivariate analysis was performed on the relative factors of PFS and OS by using the Cox regression analysis. Statistical significance was set at p < 0.05 (two-sided), and we used SPSS 16.0 for all statistical calculations.
Ethical approval
The Siriraj Institutional Review Board, Faculty of Medicine, Siriraj Hospital, Mahidol University approved this research.
Results
Patient characteristics
From January 2012 to December 2016, 52 newly diagnosed ENKTL patients were treated at Siriraj Hospital The baseline and demographic characteristics of all patients are summarized in Their median age was 52 years (range, 24–83 years). Twelve patients had extra nasal disease involving the lymph nodes, skin, ileum, bone marrow, and adrenal glands Eighteen patients (35%) had cervical lymphadenopathy, and 14 had bone marrow involvement.
Table 2. Clinical characteristics of patients with newly diagnosed ENKTL.
Twenty-five patients were excluded, as they were undergoing regimens without L-asparaginase (15), receiving radiation alone (1), and due to palliation (9). This group received a non-L-asparaginase based regimen because they were in conditions not suitable for L-asparaginase-based regimens, such as poor performance status at diagnosis, abnormal liver function test, and severe infection. The remaining 27 patients were 16 male patients and 11 female patients, and their median age was 52 years (range, 25–83 years). The patients mostly presented with aero-nasal-associated symptoms (nasal congestion, epistaxis, or sinusitis) or prolonged fever (30%). The primary sites were the aero-nasal area (23) and the skin (4). The patients were in clinical stages I, II, III and IV (10, 8, 1, and 8 patients, respectively) Positive EBV DNA was found in 9/19 patients All 27 (included) patients were classified by the prognostic index of natural killer lymphoma (PINK) into low, intermediate, and high groups (14, 8, and 5 patients, respectively), while 19 patients were classified by the prognostic index of natural killer lymphoma EBV (PINK-E) into low, intermediate, and high groups (11, 4, and 4 patients, respectively).
Laboratory data revealed a mean hemoglobin level of 12.2 g/dL (range, 6.9–15.7), a mean WBC count of 7410 /µL (range, 1070–16480), a mean platelet count of 245,769 /µL (range, 76,000–390,000), a mean of uric acid at 4.5 mg/dL (range, 1.7–10.2), and a mean of LDH at 545 U/L (range, 220–2162, normal range 240–480 U/L).
Treatment
The physicians decided which L-asparaginase-based regimen was used for their patients. Twenty-four patients were treated with the SMILE regimen, and the remaining 3 patients received the AspaMetDex regimen The number of cycles used in the SMILE regimen ranged from 1 to 6, with a median of 3. For AspaMetDex regimen, 4 cycles were used in 2 patients, while 1 patient received 3 cycles of treatment.
Fourteen patients in the limited stage received post-chemotherapy local radiation (sequential chemoradiation).
Efficacy and response rates
The treatment outcomes for the 26 assessable patients were as follows: 14 had a CR, 4 had a partial response (PR), and 8 had a progressive disease (PD) One patient died before the evaluation of disease status. The overall response rate (ORR) was 69%. Compared to a L-asparaginase-based regimen, the ORR of non-L-asparaginase-based regimen was only 27% (CR in 4 patients, SD in 3 patients, PD in 8 patients and without chemotherapy treatment in 10 patients). Regarding patients who achieved CR, the PINK score was classified as low risk for 11 patients, intermediate risk for 2 patients, and high risk for 1 patient. Most of their disease stages were in the limited stage (8 in stage I; 4 in stage II, 2 in stage IV), and all except 3 patients received post-chemotherapy radiation
All 8 patients who achieved PD received a second regimen with ESHAP (4), HyperCVAD (1), gemcitabine with radiotherapy (1), AspaMetDex (1), and pembrolizumab (1). None of the patients received autologous hematopoietic stem cell transplantation. Patients who achieved PR and PD died.
Survival
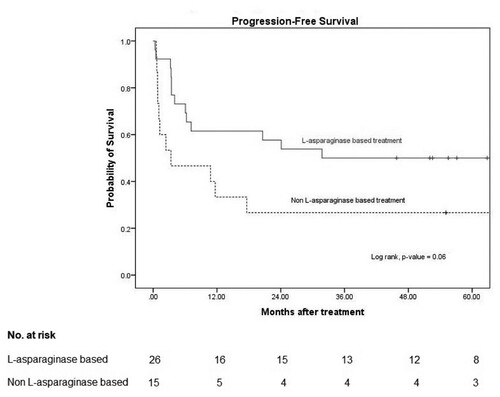
The median follow-up time was 21 months (interquartile range, 8–67 months). In the L-asparaginase-based regimen group, the median PFS was 32 months and the 3-year PFS was 50% (95% confidence interval [CI]: 30–67). In the non-L-asparaginase-based regimen group, the median PFS was 3 months (95% CI: 0–15) and the 3-year PFS was 27% (95% CI: 8–50). The p value was 0.06 ().
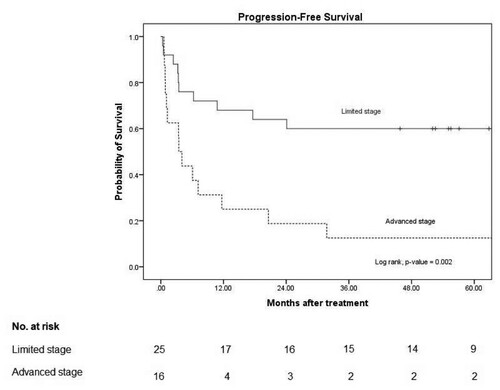
Figure 1. Progression-free survival.
Note: 3-year PFS in L-asparaginase based and non L-asparaginase based were 50% (95% CI: 30–67) and 27% (95% CI: 8–50), respectively with p-value 0.06.
The results of the Cox multivariate proportional hazards analysis showed that the independent prognostic factors for PFS among all newly diagnosed ENKTL patients were a limited stage of disease (adjusted hazard ratio [HR] 3.32, 95% CI: 1.45–7.6, p = 0.004) ().
Table 3. Multivariate hazard analysis.
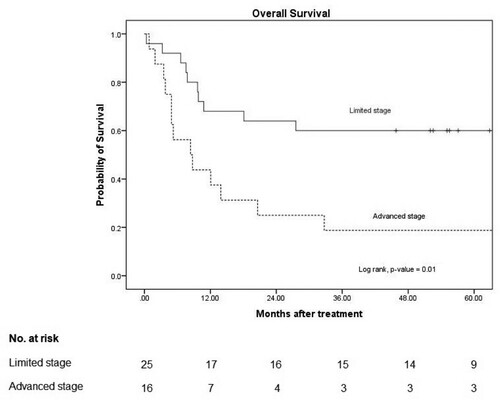
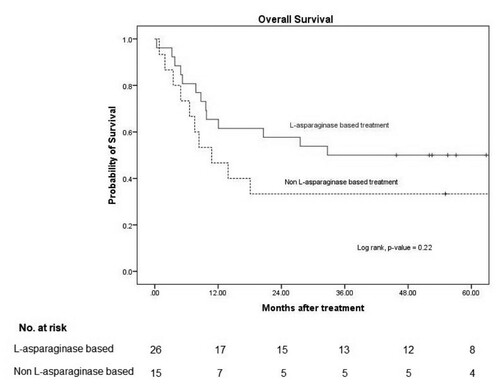
The overall mortality rate was 52% (14/27) due to sepsis or septic shock (11) and PD (3). None of the patients died during disease remission The median OS and 3-year OS in the patients with an L-asparaginase-based regimen was 33 months and 50% (95% CI: 30–67), respectively, and it was 11 months (95% CI: 3–19) and 33% (95% CI: 12–56) in the non-L-asparaginase-based regimen, with a p-value of 0.22 ().
Figure 2. Overall survival.
Note: 3-year OS in L-asparaginase based and non L-asparaginase based were 50% (95% CI: 30–67) and 33% (95% CI: 12–57), respectively with p-value 0.22.
The Cox multivariate proportional hazards analysis showed that the independent prognostic factors for OS among all newly diagnosed ENKTL patients included an ECOG score of 0–1 (adjusted HR 23.18, 95%CI: 3.01–178.27, p = 0.03) and a limited stage of the disease (adjusted HR 3.05, 95% CI: 1.32–7.03, p = 0.01) ().
The median PFS in the limited stage was not reached, and that in the advanced stage was 3 months (95% CI: 2–5), with a p-value of 0.002. The median OS in the limited stage was not reached, and that in the advanced stage was 8 months (95% CI 1–15), with a p-value of 0.01 ( and ).
Safety and toxicity
Complications related to L-asparaginase use were hypofibrinogenemia in 24 patients, with a median lowest fibrinogen level during treatment of 1047 mg/dL (range, 67.6–200.4). There was hypersensitivity in 9 patients, with a successful de-sensitization in 3 patients, and cessation of L-asparaginase in 1 patient. Five patients had a hepatocellular injury, and one had hyperglycemia Thrombosis, hemorrhage, and acute pancreatitis were not identified in this study.
Discussion
Extranodal NK/T-cell lymphoma is a rare subtype of lymphoid malignancy, more common in Asia than in Europe or North America Most treatment data on ENKTL are derived from a single medical center studies or resulted from cooperation amongst Asian countries A combination of chemotherapy and radiotherapy is associated with an improvement in the survival rate and disease progression
This retrospective review is the first study in Thailand that has demonstrated the clinical characteristics of Thai patients with ENKTL patients, their treatment outcomes, and treatment-related complications. Our findings indicate that L-asparaginase-based chemotherapy is quite effective for the treatment of newly diagnosed extranodal NK/T-cell lymphoma The overall response rate was 69%. The median PFS and OS were 32 and 33 months, respectively
Compared with previous studies, our outcomes were comparable The5-year OS and median survival of patients with ENKTL reported in previous studies ranged from 20–65% and 13–42 months, respectively [Citation13–16]. Yamagushi et al. reported a phase II study outcome involving a SMILE regimen in a setting of 38 patients in relapsed or refractory ENKTL stage IV with ORR 79% and a one-year overall survival rate of 55% [Citation10] Jaccard et al. showed 61.1% ORR in 19 relapsed or refractory patients treated using AsapaMetDex regimen, with a median survival time of 122 months [Citation8]
None of the patients in our study received an upfront hematopoietic stem cell transplantation in accordance with institutional treatment guidelines. However, compared to the retrospective data of newly diagnosed ENKTL patients who underwent autologous hematopoietic stem cell transplantation after the first remission (Ho-Young Yhim and colleagues), the 3-year PFS and OS were 52.4 and 60%, respectively [Citation17] The outcome of our study is comparable with data in upfront autologous stem cell transplantation studies. As mentioned above, autologous hematopoietic stem cell transplantation after the first remission might be omitted in countries with limited resources.
Factors that may be associate with a better survival outcome are good performance status, and limited stage disease Moreover, our study showed that 79% of patients who achieved CR were classified in the low-risk PINK score, 86% of them were in the limited stage, and 79% of them received post-chemotherapy radiation
Regarding the safety of L-asparaginase, hypofibrinogenemia associated with bleeding or thrombosis should be carefully monitored during treatment To avoid a severe adverse effect, we usually monitored clinical information related to bleeding, thrombosis, pancreatitis, hypersensitivity, and fibrinogen level. We administered fresh frozen plasma to our patients if their fibrinogen level were severely low as it contains fibrinogen, coagulation factors, and natural anticoagulant proteins, which were low in patients treated with L-asparaginase. This procedure balanced the risk of bleeding and thrombosis that could have occurred after patients received L-asparaginase. We also performed a liver biochemistry test, fasting blood sugar Discontinuation of L-asparaginase is suggested if a severe side-effect is suspected
The major limitation of our study is that it uses retrospective data from a single medical center. Our patients only received treatment at our institution Another limiting factor in our report is that nearly half (48%) of the newly diagnosed ENKTL patients were not treated with L-asparaginase-based regimen. A better method to determine statistically significant results is to conduct a multicenter prospective study to obtain an increased number of patients.
According to these findings, L-asparaginase-based regimens offer a good outcome as a front-line treatment for newly diagnosed ENKTL patients Hypofibrinogenemia is the most frequent side effect, although it is manageable
Conclusion
Our retrospective data confirm that the L-asparaginase- based regimen is effective and safe, as it provides a better overall response rate and survival outcome Combined with radiotherapy, it is recommended in the limited stage Careful monitoring is crucial because of potential toxicity The outcome of refractory cases is dismal
Acknowledgements
This study was supported by a research grant from the Thai Society of Hematology IRB/IEC Certification: Siriraj Institutional Review Board, Protocol number 800/2559(EC4).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chan JKC, Quintanilla-Martinez L, Ferry JA. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdkow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC;2017.p. 368–371.
- Fox CP, Civallero M, Ko Y-H, et al. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell project. The Lancet Haematology. 2020;7(4):e284–ee94.
- Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005.
- Chen SY, Yang Y, Qi SN, et al. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making. Leukemia. 2020; (published online March 9).
- Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the international non-Hodgkin lymphoma classification project. Haematologica. 2016;101(10):1244–1250.
- Thamprasert W, Khuhapinant A. A 7-year retrospective clinical study of patients diagnosed of extranodal NK/T-cell lymphoma, nasal type in Siriraj Hospital. Abstract book of the 41st Annual Meeting of the Thai Society of Hematology, Controversies in Hematology. 2012;62–4.
- Yong W, Zheng W, Zhu J, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009;88(7):647–652.
- Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839.
- Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for Improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027–6032.
- Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-cell tumor study group study. J Clin Oncol. 2011;29(33):4410–4416.
- Charoenwut M, Utchariyaprasit A, Khuhapinant A. A 2-year retrospective study of SMILE regimen as a first-line treatment of patients diagnosed of extranodal NK/T-cell lymphoma, nasal type in Siriraj Hospital. Abstract from 2013 annual meeting of the Thai Society of Hematology book. 2013;33–5.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068.
- Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood. 2009;113(17):3931–3937.
- Wu X, Li P, Zhao J, et al. A clinical study of 115 patients with extranodal natural killer/T-cell lymphoma, nasal type. Clin Oncol (R Coll Radiol). 2008;20(8):619–625.
- Harabuchi Y, Takahara M, Kishibe K, et al. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol. 2009;14(3):181–190.
- Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004;17(9):1097–1107.
- Yhim HY, Kim JS, Mun YC, et al. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/T cell lymphoma. Biol Blood Marrow Transplant. 2015;21(9):1597–1604.