ABSTRACT
Background
The Democratic Republic of the Congo (DRC) is the third most affected country worldwide by sickle cell disease (SCD). However, this disease is still orphaned in the country; large-scale control actions are rare, and little is known about its management.
Objective
To assess current practices in the management of SCD in Kisangani, DRC.
Methods
This cross-sectional study was conducted in six health facilities in Kisangani.
It involved 198 presumed sickle cell patients attending the above health facilities. The study focused on the sociodemographic and clinical data of the participants, obtained through a clinical examination and their medical records. Diagnostic confirmation of SCD was made by high-performance liquid chromatography coupled to mass spectrometry. Data were analyzed using SPSS 20.0.
Results
The diagnosis of SCD was confirmed in 194 (98.0%; 95% CI: 94.9–99.2) participants, while it was not confirmed in 4 (2.0%; 95% CI: 0.8–5.1) participants. The diagnosis was mainly made by the Emmel test (42.9%). 45.8% of participants had previously been transfused with the blood of their parents. Folic acid was taken by 48.5% of participants and the previous intake of hydroxyurea was reported in 5.1% of participants. The participants vaccinated against Pneumococcus were 13.6% and against Haemophilus influenzae type b 28.3%. Penicillin prophylaxis was received by only 1.5% and malaria prophylaxis by 11.6% of participants.
Conclusion
Standard-care practices for SCD patients in Kisangani are insufficient. The Congolese government should regard this disease as a health priority and consider actions to improve its management.
Introduction
Sickle cell disease (SCD), the most common inherited genetic disease worldwide, is currently a major global health concern, especially in sub-Saharan Africa (SSA). Indeed, it is estimated that more than 300,000 babies with SCD are born annually in this region, representing approximately 75-85% of all births of children with SCD worldwide [Citation1–4]. Moreover, according to the World Health Organization (WHO), 50-80% of SCD children who are born in SSA die before their 5th birthday, usually from an infection or severe anemia [Citation3–5]. Without a good management, the disease causes complications in multiple body organs. Some of the most common associated morbidities include chronic pain and intermittent painful episodes, anemia, musculoskeletal problems, stroke, acute chest syndrome and recurrent infections. These complications often co-exist, affecting the quality of life for patients, and if not adequately managed, may lead to death.
Keith Wailoo’s study [Citation6] which provided a background on the evolution of the life expectancy in persons with SCD from 1910 to 2000, showed that specific interventions in the United States of America since the 1970s have increased the life expectancy of SCD patients from less than 15 years before 1970s to over 40 years in 2000. The importance of specific interventions was also highlighted in the study by Rahimy et al. [Citation7] in Benin, who observed that early neonatal screening for SCD followed by enrollment in a follow-up program, had significantly reduced the under-five mortality rate to a rate 10 times lower than the overall under-five mortality rate in the Republic of Benin, i.e. 15.5 per 10,000.
It is acknowledged that a comprehensive management of SCD including specific interventions such as neonatal screening, early diagnosis and preventive penicillin therapy, Pneumococcal and Haemophilus influenzae vaccination, malaria prophylaxis in malaria-endemic areas, improved blood safety, hydroxyurea administration, vitamin supplements such as folic acid, early diagnosis of complications and their prompt management may decrease sickle cell disease-related morbidity and mortality, preventing organic damage and therefore improve the quality of life and the life expectancy of SCD patients [Citation3,Citation4,Citation8,Citation9].
In the WHO African Region, it was recommended that by 2020, 50% of states with high prevalence of SCD, including the Democratic Republic of the Congo (DRC), should have developed and implemented well-designed national programs to combat SCD as part of their national strategic health plans. In addition, a comprehensive care policy was recommended including the use of antibiotic prophylaxis and antimalarial drugs, vaccinations, permanent medical follow-up and early detection and management of complications [Citation5]. But this remains a major challenge in many countries [Citation8].
The DRC is the third most affected country in the world by SCD [Citation1]. However, despite its epidemiological importance, the disease is still orphaned and large-scale control actions are scarce. This setting suggests that there may be a poor utilization of standard-of-care practices for SCD patients in the country. Since little is known about this in the DRC, especially in the eastern part of the country, the purpose of this study was to assess current practices regarding the diagnosis of SCD and the features of its management in Kisangani (North-eastern DRC).
Material and methods
Study design and setting
This health facility-based survey was a cross-sectional study carrying out in Kisangani among presumed SCD patients between August 2018 to July 2019, using a paper-based and semi-structured questionnaire developed and pre-tested by a team study from the Faculty of Medicine and Pharmacy of the University of Kisangani.
Kisangani is the capital city of Tshopo province and the third largest urbanized city in the DRC, comprising 1.6 million inhabitants [Citation10], with a neonatal prevalence of SCD of about 1% and 23.3% for the homozygous and heterozygous forms, respectively [Citation11]. A total of six facilities in Kisangani were selected for this study including three public (University hospital of Kisangani, Reference General Hospital of Makiso and Kabondo) and three private (Health Centers of Alabul, Maison médicale and Gracia fondation) health facilities. The choice of these facilities was justified by their high attendance by SCD patients.
Study population and recruitment
All participants were presumed SCD patients enrolled from patients visiting the selected health facilities for any kind of medical care. The inclusion criteria were being registered and followed up as SCD patient in the above health facilities, being present at the time of the survey, and having given free and informed consent to participate in the study. For children under 16 years of age, the consent of parents or guardians was required. The requested minimum number(n) of participants to include was obtained by setting 95% confidence level, 80% statistical power, and considering estimated the prevalence of the sickle cell trait of 23.3% in Kisangani [Citation11], DRC. Thus, at least 117 participants were required to be included in the study.
Data collection
After filling the sociodemographic data (age and sex), the participants underwent a clinical examination which allowed us to collect data on diagnostic methods of the SCD, immunization status (against Pneumococcus, Haemophilus influenza type b [Hib], Salmonella sp), chemoprophylaxis (Penicillin and antimalarial prophylaxis) and specific treatment of SCD (folic acid and hydroxyurea intake, and blood transfusion [history, indication and type of blood transfusion, and origin of transfused blood]). For children under 16 years of age, information were obtained from their parents or guardians. In complement to the clinical examination, we used the medical records of the patients. At the end of the session, capillary whole blood was collected by finger sampling using dried blood spots for laboratory diagnosis to confirm SCD. The samples were sent to the Biochemical Genetics Laboratory of CHU (Centre Hospitalier Universitaire) of Liège (Belgium) where the screening of SCD was carried out by high-performance liquid chromatography coupled to mass spectrometry (TQ5500 – Sciex – Framingham, MA 01701 U.S.A) [Citation12,Citation13]. The laboratory results were collected on standardized sheets.
Ethical statement and statistical analysis
Ethical clearance was obtained from the Ethics Committee of the University of Kisangani (Réf. UNIKIS/CER/007/2018). Furthermore, Freely-given and informed consent to participate in the study was obtained from all participants. For children under 16 years of age, the consent of parents or guardians was required. Data were entered into an Excel file and analyzed on SPSS 20.0 (Chicago, IL). Proportions were calculated for categorical variables; and the results were presented as a 95% confidence interval (CI) using the Wilson score bounds. For the quantitative variables, mean and standard deviation were calculated.
Results
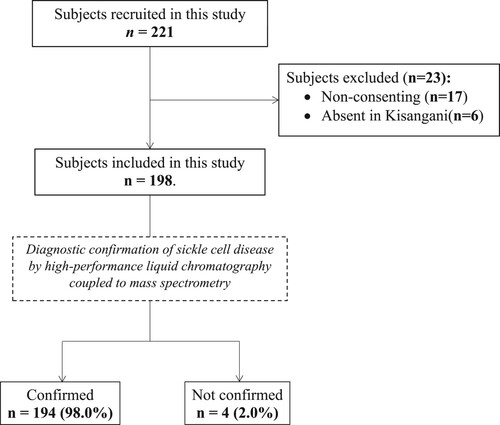
A total of 221 participants were evaluated for eligibility for the study. Among them, 198 participants were finally included in the study whereas 23 were excluded because they did not consent (n = 17) and were absent in Kisangani at the time of the survey (n = 6) (). Data on the sociodemographic characteristics of participants are shown in . In brief, the majority of participants were female and under 16 years of age (mean: 13.74; SD: 9.34; Min: 2; Max: 74).
Table 1. Social demographics and medical story of 198 participants.
As shown in , 194 (98.0%; 95% CI: 94.9–99.2) presumed SCD patients were confirmed as SCD patients with the high-performance liquid chromatography coupled to mass spectrometry (TQ5500 – Sciex – Framingham, MA 01701 U.S.A) whereas 4 (2.0%; 95% CI: 0.8–5.1) presumed SCD patients were not confirmed. When investigating the diagnostic method used in the past for these participants to be considered SCD patients, in general, the Emmel test was predominantly used with 85 (42.9%) participants, while hemoglobin electrophoresis was used in only 12.1% of participants. In 12 (6.1%) participants, the diagnosis was made only on the basis of the clinic and family history of SCD. Of the four participants in whom the diagnosis of SCD was not confirmed, two (50%) were males under 16 years of age, and all were previously diagnosed on the basis of the Emmel test ().
The study showed that 79.3% of participants had history of blood transfusion and 45.8% had previously been transfused with blood units from their parents. In all cases, it was episodic transfusions (). Although irregularly, folic acid was taken by 48.5% of participants and previous intake of hydroxyurea was reported in only 5.1% of participants. In addition, only 13.6% of participants were vaccinated against Pneumococcus and 28.3% against Hib. Penicillin prophylaxis was received by only 1.5% of participants and malaria chemoprophylaxis by 11.6% ().
Table 2. Distribution of 198 participants according to whether they received specific therapy, immunization, and anti-infectious chemoprophylaxis.
Discussion
We herein report on current practices regarding the diagnosis of SCD and the features of its management in Kisangani, DRC. Overall, our findings showed that 98% of the participants were truly SCD patients whereas 2% were not because of the poor performance of the diagnostic tests used. In addition, the vast majority of patients did not receive appropriate healthcare. These findings show that SCD patients in Kisangani are poorly managed by the existing health-care system.
In high-income countries, screening of SCD is done by high-performance liquid chromatography, capillary electrophoresis and sometimes isoelectric focusing which are well-established methods for haemoglobinopathies screening. But, as shown in this study, diagnosis of SCD in the DRC is still largely based on the Emmel test and even only on the basis of clinical examination. Currently, capillary electrophoresis and/or isoelectric focusing is performed in only a very limited number of health facilities in DRC, mainly in Kinshasa and Lubumbashi, which are the two largest cities in the country. Elsewhere, these methods are quasi non-existent. In the context of lack of advanced equipment, local and realistic solutions are to be considered in order to improve the diagnosis of SCD in the DRC. The large-scale use of point-of-care tests such as «HemoType SC®» and «Sickle Scan®» recently introduced in SSA and in the DRC through pilot projects [Citation14], are interesting options. Indeed, these tests based on lateral flow immunoassay devices (Hemotype SC®) or chromatographic immunoassay approach (Sickle Scan®) have demonstrated excellent analytical performance in the screening of sickle cell disease [Citation14,Citation15] and would make it possible to bypass the diagnostic pitfalls associated to the Emmel test.
Our findings showed that folic acid was taken by less than half of the participants, contrasting with the results of Aloni et al. in Kinshasa [Citation16] who observed that 98.0% of the SCD patients were taking folic acid. The fact that Aloni et al conducted their study in a center dedicated to the management of the SCD can account for this result. It has been hypothesized that patients with SCD are at an increased risk for folate deficiency [Citation17,Citation18]. For this reason, SCD patients commonly take folic acid on the premise that it will replace depleted folate stores and reduce the symptoms of anemia. However, this has not yet been formally demonstrated and is still controversial [Citation19]. In our opinion, folic acid supplementation is required in the DRC. Indeed, the precarious nutritional conditions of SCD patients due, among other things, to poverty and the financial burden of the disease on families owing to lack of health insurance system in the country, expose them to various nutritional deficiencies.
Hydroxyurea is the only substantive treatment for SCD. Tshilolo et al. [Citation2], Ofakunrin et al. [Citation20] and Thornburg et al. [Citation21] have recently demonstrated in SSA the safety and benefit of using hydroxyurea in SCD patients indicating the need for wider access to this drug. In this study, only 5.1% of SCD patients have ever been treated with hydroxyurea. Whether this is due to the limited financial accessibility to this molecule, its rarity in our area, the lack of clinical indications, concerns about side effects, the lack or insufficient knowledge of the caregivers about this drug remains to be determined. Studies assessing the barriers to the use of hydroxyurea in SCD patients in the DRC, and more generally in SSA are therefore needed as well as actions related to this drug supply in terms of quantity, quality, availability, and affordability to be considered by the public health authorities. Local production of generic hydroxyurea tablets would be an interesting option as it could make this drug more available and financially affordable for patient. Our result is in accordance with that of Aloni et al in Kinshasa who observed that only 2% of SCD patients were taking hydroxyurea [Citation16], and that of Ofakunrin et al in Nigeria [Citation20] who reported a low use of hydroxyurea among Nigerian SCD patients.
As shown in this study, 45.8% of SCD patients had previously been transfused with blood units from their parents, exposing them to complications. Indeed, it is recognized that increasing hematocrit without decreasing the percentage of circulating sickle cell constitutes a risk factor of stroke and other complications in SCD patients [Citation22]. This may occur when blood units with hemoglobin AS, such as that of the parents of SCD patients, are administered to patients with SCD. In the context of the DRC where the need for blood is always greater than the supply, and where patients often arrive at the emergency services with severe anemia, the question of whether AS donors should be excluded from donating in SCD patients is crucial but difficult to answer. Moreover, Batina-Agasa and colleagues [Citation23] recently demonstrated that the prevalence of hemoglobin AS among blood donors in the DRC was 23.2%.
None of the patients were in a chronic transfusion program. A number of factors can account for this result. The lack of transcranial Doppler equipment to identify children with SCD at risk of stroke, the lack of erythrocytapheresis equipment and the absence of quantitative Hemoglobin S(HbS) and Hemoglobin F(HbF) assays in our area do not make it possible to consider a chronic transfusion program. In addition, the persistence of family and remunerated blood donors is a potential barrier to chronic transfusion in our setting, since a high risk of transfusion-transmitted infections has been reported in this category of donors [Citation24,Citation25]. Moreover, the guidelines recommend to irradiate donations from first or second-degree relatives, even if the patient is immunocompetent, to prevent transfusion-associated graft-vs-host disease (TA-GVHD) [Citation26]. However, data regarding TA-GVHD are not available in DRC.
Encapsulated bacteria especially Streptococcus pneumoniae and Hib are an important cause of morbidity and death in SCD patients mainly in children. Therefore, penicillin prophylaxis and immunization against these two bacteria are measures widely accepted in the management of SCD and their effectiveness has been well demonstrated [Citation9]. In this study, we noticed that only 13.6% of participants were vaccinated against Pneumococcus and 28.3% against Hib. In addition, all participants were vaccinated in childhood according to the immunization schedule in DRC, throughout the Expanded Program on Immunization (EPI) campaigns. These results can mostly be attributed to the fact that it was in 2009 and 2011 respectively that the Hib and Pneumococcal conjugate vaccines (PCV-13) were introduced in the EPI in DRC. Consequently, SCD patients born before 2009 did not benefit from these vaccines. The CDC (Centers for Disease Control and Prevention) recommends vaccination of SCD patients over 5 years of age who have not been immunized or who have been incompletely immunized [Citation27]. Since vaccines against Pneumococcus and Hib are available in the DRC, a government policy that can allow immunization of unvaccinated SCD patients in childhood is required.
Very few SCD patients (1.5%) were receiving penicillin prophylaxis. Elsewhere, Galadanci et al. [Citation28] in Nigeria found that few clinics provided penicillin prophylaxis or pneumococcal vaccination for patients with SCD. Although bacterial resistance to penicillin is increasingly being observed, penicillin prophylaxis is still widely recommended for children with SCD and there is evidence that it significantly reduces the risk of pneumococcal and Hib infection in children with homozygous SCD [Citation5,Citation29,Citation30].
The DRC is the second most malaria-affected country worldwide [Citation31]. As noted in this study, malaria chemoprophylaxis was undertaken by only 11.6% of the participants and this was done irregularly, whereas the WHO recommends this prophylaxis in SCD patients living in malaria-endemic areas [Citation5]. The lack of policy regarding antimalarial chemoprophylaxis for SCD patients in DRC may explain this result. Indeed, in this country, malaria chemoprophylaxis is currently recommended only for pregnant women. A systematic review and network meta-analysis of the safety and efficacy of antimalarial therapy in SCD by Frimpong and colleagues showed that antimalarial prophylaxis reduces the incidence of clinical malaria in children with the disease [Citation32]. Studies on the co-morbidity of SCD and malaria are therefore necessary in the DRC. As a first step, the impact of malaria on the SCD and vice versa should be determined. Then, in a second step, the following questions should be assessed: which drug for which treatment regimen for malaria chemoprophylaxis in SCD patients and for what result? Elsewhere, these questions still arise [Citation32].
In conclusion, this study showed that there is insufficient use of standard-care practices for SCD patients in Kisangani. The Congolese government should regard this disease as a health priority and consider actions to improve its management. The implementation of dedicated and well-equipped SCD health centers and the training of health-care providers will improve the treatment of sickle cell patients.
Acknowledgements
This study was carried out thanks to the PRD-2018 project: ‘DREPAKIS: Contribution à la prise en charge de la drépanocytose dans la ville de Kisangani’, funded by ARES-CCD. Thus, the authors are very grateful to the Belgian ‘Académie de Recherche et d’Enseignement Supérieur’ (ARES). Authors would also like to thank the health facilities, the sickle cell disease patients and their families, Dr Emmanuel Vakanyaki, Dr Apio Nora, Dr Oketa Bala, MrToussaint Agambu and Mr LEDUC Stéphane for their collaboration in the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;38(9861):142–151. DOI:https://doi.org/10.1016/S0140-6736(12)61229-X.
- Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in Sub-Saharan Africa. N Engl J Med. 2019;380(2):121–131. DOI:https://doi.org/10.1056/NEJMoa1813598.
- Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012;59(2):386–390. DOI:https://doi.org/10.1002/pbc.24175.
- Mulumba LL, Wilson L. Sickle cell disease among children in Africa: An integrative literature review and global recommendations. Int J Afr Nurs Sci. 2015;3:56–64. DOI:https://doi.org/10.1016/J.IJANS.2015.08.002.
- WHO, Africa Regional Committee. Sickle-cell anaemia: a strategy for the who African region. Report of the Sixtieth Session Malabo, Equatorial Guinea, 30 August – 3 September 2010AFR/RC60/8 [cited 2020 May 17]. Available from: https://apps.who.int/iris/handle/10665/1682.
- Wailoo K. Sickle cell disease – a history of Progress and Peril. N Engl J Med. 2017;376(9):805–807. DOI:https://doi.org/10.1056/NEJMp1700101.
- Rahimy MC, Gangbo A, Ahouignan G, et al. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol. 2009;62(1):46–48. DOI:https://doi.org/10.1136/jcp.2008.059113.
- De Montalembert M, Tshilolo L. Is therapeutic progress in the management of sickle cell disease applicable in sub-Saharan Africa? Med Trop. 2007;67(6):612–616.
- Allali S, Chalumeau M, Launay O, et al. Conjugate Haemophilus influenzae type b vaccines for sickle cell disease. Cochrane Database Syst Rev. 2018;8(8):CD011199. DOI:https://doi.org/10.1002/14651858.CD011199.
- Tonen-Wolyec S, Batina-Agasa S, Muwonga J, et al. Acceptability, feasibility, and individual preferences of blood-based HIV self-testing in a population-based sample of adolescents in Kisangani, Democratic Republic of the Congo. PLoS One. 2019;14(7):e0218795. DOI:https://doi.org/10.1371/journal.pone.0218795.
- Agasa B, Bosunga K, Opara A, et al. Prevalence of sickle cell disease in a northeastern region of the Democratic Republic of Congo: what impact on transfusion policy? Transfus Med. 2010;20(1):62–65. DOI:https://doi.org/10.1111/j.1365-3148.2009.00943.x.
- Boemer F, Cornet Y, Libioulle C, et al. 3-years experience review of neonatal screening for hemoglobin disorders using tandem mass spectrometry. Clin Chim Acta. 2011;412(15-16):1476–1479. DOI:https://doi.org/10.1016/j.cca.2011.04.031.
- Boemer F, Ketelslegers O, Minon JM, et al. Newborn screening for sickle cell disease using tandem mass spectrometry. Clin Chem. 2008;54(12):2036–2041. DOI:https://doi.org/10.1373/clinchem.2008.106369.
- Kasai E, Boemer F, Djang’eing’a R, et al. Systematic screening of neonatal sickle cell disease with HemoTypeSCTM Kit-test: case study and literature review. Open J Blood Dis. 2020;10:12–21. DOI:https://doi.org/10.4236/ojbd.2020.101002.
- Segbena AY, Guindo A, Buono R, et al. Diagnostic accuracy in field conditions of the sickle SCAN® rapid test for sickle cell disease among children and adults in two West African settings: the DREPATEST study. BMC Hematol. 2018;18:26, DOI:https://doi.org/10.1186/s12878-018-0120-5.
- Aloni MN, Nkee L. Challenge of managing sickle cell disease in a pediatric population living in Kinshasa, democratic Republic of Congo: a sickle cell center experience. Hemoglobin. 2014;38(3):196–200. DOI:https://doi.org/10.3109/03630269.2014.896810.
- Lowenthal EA, Mayo MS, Cornwell PE, et al. Homocysteine elevation in sickle cell disease. J Am Coll Nutr. 2000;19(5):608–612. DOI:https://doi.org/10.1080/07315724.2000.10718958.
- Liu YK. Folic acid deficiency in sickle cell anaemia. Scand J Haematol. 1975;14(1):71–79. DOI:https://doi.org/10.1111/j.1600-0609.1975.tb00295.x.
- Al-Yassin A, Osei A, Rees D. Folic acid supplementation in children with sickle cell disease. Arch Dis Child. 2012;97:A91–A92. DOI:https://doi.org/10.1136/archdischild-2012-301885.219.
- Ofakunrin AOD, Oguche S, Adekola K, et al. Effectiveness and safety of hydroxyurea in the treatment of sickle cell anaemia children in Jos, North Central Nigeria. J Trop Pediatr. 2020;66(3):290–298. DOI:https://doi.org/10.1093/tropej/fmz070.
- Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304–4310. DOI:https://doi.org/10.1182/blood-2012-03-419879.
- Brecher ME. American Association of Blood Banks. Technical manual. 14th ed., Bethesda (MA): Brecher ME; 2002.
- Batina-Agasa S, Kambale-Kombi P, Kabamba P, et al. Sickle cell trait among blood donors in the Democratic Republic of the Congo: which transfusion policy for sickle cell patients? ISBT ScSer. 2020;1–4. DOI:https://doi.org/10.1111/voxs.12580
- Batina A, Kabemba S, Malengela R. Marqueurs infectieux chez les donneurs de sang en République Démocratique du Congo (RDC) [Infectious markers among blood donors in Democratic Republic of Congo (DRC)]. Rev Med Brux. 2007;28(3):145–149.
- Mukendi PC, Katala MK, Ndala DB, et al. Seroprevalence of Hepatitis B among blood donors in Mbuji-Mayi, “Case of Dipumba General Hospital” (DRC). Open Access Library Journal. 2017;2017(04):1–7. DOI:https://doi.org/10.4236/oalib.1103503.
- Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol. 2011;152(1):35–51. DOI:https://doi.org/10.1111/j.1365-2141.2010.08444.x.
- CDC. 2018. Vaccine recommendations for patients with sickle cell disease (includes Hgb SS, Hgb SC, Hgb SB null and Hgb SB minor). [cited 2020 May 30]. Available from: https://missionhealth.org/wp-content/uploads/2018/04/CDC-Vaccine-Recommendations-for-Patients-with-Sickle-Cell-Disease.pdf.
- Galadanci N, Wudil BJ, Balogun TM, et al. Current sickle cell disease management practices in Nigeria. Int Health. 2014;6(1):23–28. DOI:https://doi.org/10.1093/inthealth/iht022.
- Rankine-Mullings AE, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev. 2017;10(10). CD003427. DOI:https://doi.org/10.1002/14651858.CD003427.pub4.
- Yee ME, Bakshi N, Graciaa SH, et al. Incidence of invasive Haemophilus influenzae infections in children with sickle cell disease. Pediatr Blood Cancer. 2019;66(6):e27642. DOI:https://doi.org/10.1002/pbc.27642.
- Taylor SM, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One. 2011;6(1):e16420. DOI:https://doi.org/10.1371/journal.pone.0016420.
- Frimpong A, Thiam LG, Arko-Boham B, et al. Safety and effectiveness of antimalarial therapy in sickle cell disease: a systematic review and network meta-analysis. BMC Infect Dis. 2018;18(1):650, Published 2018 Dec 12. DOI:https://doi.org/10.1186/s12879-018-3556-0.