ABSTRACT
Objective
As COVID-19 is a new emerging disease, the hematological/immunological changes that develop in the infected patients remain unknown. This study aims to systematically review the hematologic autoimmune complications in these patients.
Method
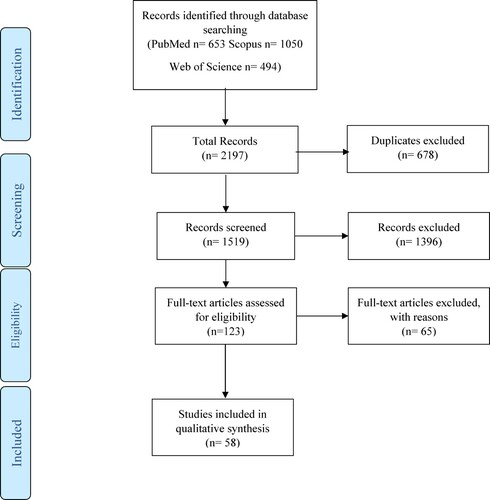
Data from three online databases including Medline (via PubMed), Scopus and Web of Science were searched on 19 December 2020, and after excluding duplicate, irrelevant and inappropriate records, eligible documents were identified. Afterwards, information such as patients’ history, presentations, paraclinical data, treatment course and outcome were extracted from the records.
Results
A total of 58 documents were considered to be eligible for data extraction which described 94 patients with COVID-19 who developed hematologic autoimmune disorder in their course of infection. Of these patients with COVID-19, the most common hematologic autoimmune disorder was immune thrombocytopenic purpura (55 cases) followed by autoimmune hemolytic anemia (22 cases). Other hematologic autoimmune disorders include antiphospholipid syndrome, thrombotic thrombocytopenic purpura, Evans syndrome and autoimmune neutropenia.
Conclusion
The current study would help us to always consider an autoimmune etiology for cases with abnormal hematologic finding which further lead to an appropriate treatment of the patients, especially when the symptoms present in about 1–2 weeks after the first manifestation of the infection symptoms. Maybe, at least in this pandemic, it should be recommended to evaluate patients with unexpected and unexplained decrease in their hemoglobulin or platelet count for COVID-19. Another challenging issue is the treatment options. Given the multiorgan involvement and multifaceted nature of the infection, an individualized approach should be taken for each patient.
Introduction
Late in 2019, a novel viral strain from Coronaviridae family was isolated in the throat culture of a series of patients complained of influenza-like manifestations. This newly emerged strain was subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Citation1]. Due to its high primary attack rate, extensive containment measures including universal lockdowns and social distancing were imposed to impede the spread [Citation2]. However, despite these preventive measures, the virus is rapidly spreading through the world turning it from a public health emergency of international concern into a pandemic, declared by World Health Organization (WHO) on 11 March 2020 [Citation3].
While SARS-CoV-2 mainly targets the respiratory tract, with respiratory failure and acute respiratory distress syndrome as the leading cause of mortality among the afflicted patients, extra-respiratory systems’ manifestations are also frequently observed [Citation4]. The typical clinical manifestations of coronavirus disease 2019 (COVID-19) include fever, dry cough, fatigue, sore throat, malaise and myalgia. Furthermore, there are other less common symptoms such as headache, dizziness, diarrhea and nausea and vomiting [Citation4,Citation5].
Recently, an increasing number of studies have demonstrated that SARS-CoV-2 infection could result in alteration in immune system functions [Citation6]. These alterations could range from an inappropriate immune response and abnormal cytokine/chemokine production to immune system hyperactivation and dramatic increase in immune-inflammatory parameters. These vigorous immune responses could lead to autoimmunity and cytokine storm [Citation7]. Besides, researches have reported several cases of patients with COVID-19, among whom autoimmune events were developed such as vasculitis, Guillain–Barré syndrome, etc. [Citation8]. These findings indicate that SARS-cov-2 infection may be associated with the induction of autoimmune disorders. Among these autoimmune manifestations, several hematologic autoimmune disorders have been also reported and showed that could complicate the management and be in association with outcome in patients with COVID-19 [Citation9–12]; however, they are less paid attention to. As COVID-19 is a new emerging disease, little is known about these immunological changes that occur in the afflicted patient. Given this, we aimed to systematically review hematologic autoimmune disorders reported in patients with SARS-CoV-2 to shed light on this era for better management of patients.
Method
Search sources and strategies
In the current study, a systematic review for relevant records about hematologic autoimmune events developing in the course of SARS-CoV-2 infection was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation13]. Data were gathered from three different online databases including Medline (via PubMed), Scopus and Web of Science. The search has been conducted by the third author in 16 August 2020 and updated in 19 December. In order not to miss any data, a broad search strategy was developed with a combination of key terms of both coronavirus and hematologic autoimmune disorders. The keywords used for coronavirus were COVID-19, coronavirus, SARS-CoV-2 and 2019-nCoV. Furthermore, the following keywords were considered for hematologic autoimmune disorders: autoimmune, autoimmune anemia, autoimmune hemolytic anemia, hemolytic anemia, cold agglutinin, AIHA, pernicious anemia, immune thrombocytopenia, immune thrombocytopenic purpura, idiopathic thrombocytopenic purpura, ITP, thrombotic thrombocytopenic purpura, TTP, Evans syndrome, autoimmune cytopenia, autoimmune neutropenia, antiphospholipid syndrome, anticardiolipin, Beta-2-glycoprotein, lupus anticoagulant and APS.
Study selection
Six hundred and fifty-three articles in PubMed, 1050 articles in Scopus articles and 494 articles in Web of Sciences were found following the search and then their citations were exported into endnote X9(Clarivate Analytics, USA). At first, duplicate records (678 documents) were removed from the library and afterwards, first screening was conducted by the first and second authors; in this step, titles and abstracts of the records were evaluated in order to remove those which are clearly irrelevant; however, those records which were unclear whether they are eligible for final qualitative analysis or not were kept in the study for subsequent screening (); in the second round of the screening process, full texts of the records were evaluated for relevancy. Records from papers with any design or methodology which did not describe the cases who developed hematologic autoimmune disorders including their presentations, their paraclinical workups, their treatment course, etc. were excluded in the second screening round. In this study, not only case reports and case series but also letters or commentaries that included information of SARS-CoV-2-infected patients with hematologic autoimmune disorders were considered eligible. Besides, not only documents of the patients with new onset of hematologic autoimmune disorders, but also documents that reported a relapse or flare of a chronic hematologic autoimmune disorder were included in the qualitative synthesis. There were no restrictions based on the country of origin or the language used in the articles. Besides, both published papers and those that are ahead of print were considered for data extraction.
Data extraction
An Excel spreadsheet was created to extract the following data: study title, first author, access date, type of study, history of the patient, the clinical symptoms and signs the patient presented with, patient’s paraclinical information, the hematologic complication observed in the patient, the treatment course including for both hematologic disorder and SARS-CoV-2 infection and the patient’s outcome. Among patients’ paraclinical workups, only those helpful for diagnosis of the hematologic disorder the patients afflicted to were gathered from the selected records.
Results
A total of 94 patients with SARS-CoV-2 infection who developed hematologic autoimmune disorders in their course of infection were included in the final analysis. Male and female patients constituted an almost equal proportion of the study cases, 47 and 44 cases, respectively. The gender status of three cases of a study was not identified in the document [Citation14]. The study cases had a wide age range from 2 years to 94 years with a median age of 60 and the mean age of 56 ± 18.5. The elder population constitute the majority of cases with 48.9% (46 cases). There were only three pediatric cases and these three patients were all afflicted to idiopathic thrombocytopenic purpura (ITP). A majority of cases had comorbidities (69 percent); however, 14 out of 29 remaining cases were described in a study in which the underlying disorders of the patients were not reported [Citation15]. The most common presentation in these cases was fever reported in 69 (73%) of them, followed by coughing in 56 (59%) and dyspnea in 40 patients (42%).
Among 94 patients, the most common hematologic autoimmune disorder was ITP in 55 cases (58%) followed by autoimmune hemolytic anemia (AIHA) in 22 cases (23%). Other hematologic autoimmune disorders observed in the literature include antiphospholipid syndrome (APLS) in 10 individuals, thrombotic thrombocytopenic purpura (TTP) in 3 individuals, Evans syndrome in 3 individuals and autoimmune neutropenia in one individual (). The laboratory workups which led to these diagnoses for each case is documented in Supplementary material 1.
Table 1. Autoimmune hematologic complications of SARS-CoV-2 infections.
Overall, the time of onset of the hematologic autoimmune presentations from the SARS-CoV-2 infection presentations and not from the time of COVID-19 diagnosis is demonstrated in . The mean time for all categories of hematologic autoimmune disorders was 11.8 ± 7.1 days (95% confidence interval of 10.4–13.3); the mean time in ITP, AIHA, APLS, TTP and Evans syndrome were 13.3 ± 7.3, 8.9 ± 5.02, 14 ± 7.5, 4.6 ± 1.5 and 5.5 ± 2.1, respectively.
Among these patients, 88 patients were alive and in the recovery process and their hematological indices related to the autoimmune disorders improved. Among the deceased cases, three were with AIHA, two were with ITP and one was afflicted to APLS. All three patients with AIHA deceased early, two due to the hemodynamic collapse as a result of the hemolysis process and one due to delayed detection of intracerebral hemorrhage. Among the two deceased patients with ITP, one died of an intracerebral hemorrhage within first 24 h and the other responded significantly to the treatment but passed away due to his poor condition and not due to the autoimmune sequel. For the deceased patient with APLS, the medical team decided to discontinue the plasmapheresis due to their concern of possible removal of antibodies needed for the adaptive response to infection. There were no statistically significant association between the presence of age, gender, having comorbidity and timing of hematological presentation and outcome (recovered or deceased) of the study cases (all p-values were above .05). Therefore, it could be concluded that among those who the autoimmune disorder-oriented treatment was administered (93 out of 94 patients), the remission rate was 95.6% (89 out of 93 patients).
Among the patients with ITP, 15 patients received intravenous immunoglobulin (IVIG), 13 patients received steroids, 13 patients received a combination of IVIG and steroid, 6 patients received a combination of IVIG and thrombopoietin-receptor agonist (TPO-RA) and 4 patients received only supportive care including platelet cell transfusion and treatment of the underlying disorder which is the SARS-CoV-2 infection. Among the patients who started their treatment with IVIG, two patients failed to respond to IVIG (86% remission rate) and further steroid was added to their treatment regimen and then their platelet level was raised. Similarly, among those with steroid, response was not observed in two patients (84% remission rate) and IVIG was initiated for them. The majority of patients who developed AIHA were treated with supportive care (59%) and for others steroid or combination of steroid with IVIG or immunosuppressor medications was administered.
Discussion
Through our study, it was revealed that AIHA, ITP, TTP, APS, autoimmune neutropenia and Evans syndrome are among the observed hematologic autoimmune sequels in the patients infected with SARS-CoV-2 infection. However, the exact pathogenesis from which these autoimmune sequels are developing remains to be fully determined. Although one could label these autoimmune disorders as incidental findings in the patients with SARS-CoV-2 infection, there are a number of evidences that are in favor of the existence of a causative relationship. From a pathogenesis point of view, secondary to viral infection, immunological tolerance could be disturbed through a variety of mechanisms that include molecular mimicry, bystander activation, epitope spreading and autoreactive effector cells immortalization [Citation8]. Several viral infections have been identified so far to be associated with hematologic autoimmune disorders such as human immunodeficiency virus, hepatitis C virus and Epstein–Barr virus [Citation68]. Therefore, CoV infection could be attributed to trigger a cascade of both the innate and adaptive immune arms activation resulting in autoimmunity. Consistently, previous studies on other coronaviruses, SARS-CoV-2’s cousins with a high rate of similarity in their genomic sequence and receptor-binding domains, revealed that this viral family could also lead to autoimmune disorders including hematologic ones [Citation69–71]. Furthermore, the temporal sequence of autoimmune events described in suggests that COVID-19 could play a causal role.
The mean days between the presentations of the hematologic autoimmune disorders and the presentations of SARS-CoV-2 infection were 11.8 ± 7.1 days for the included cases. Consideration of this point could help in early susception of an autoimmune underlying basis in the case of deterioration of the clinical condition or when there is an abnormal hematologic index even in the COVID-19 setting (for example, in these patients, thrombocytopenia is frequently seen but very low level of platelet which led to bleeding diathesis or bleeding complications is rare [Citation72]), especially when the event occurs in 10–13 days after the first manifestations of the infection.
A wide range of hematologic abnormalities have been observed in the patient with SARS-CoV-2 infection [Citation73]. Among these hematologic abnormalities, a hypercoagulable state associated with thromboembolic complications and poor prognosis develops in a majority of patients [Citation74]. However, in about one-third of patients with SARS-CoV-2 infection, mild to severe degree of thrombocytopenia is found [Citation75]. Several possible mechanisms for thrombocytopenia in the course of CoV infection have been proposed including platelet production insufficiency rooted from classic cytokine storm in infectious diseases or direct attack on hematopoietic stem cells, increased peripheral platelet destruction and declined circulating platelet secondary to lung injury [Citation76–78].
In most patients experiencing thrombocytopenia in the course of infection, there are no definitive data revealing increased risk of bleeding and the platelet count did not decrease to a level at which bleeding occurs [Citation76]. A small subgroup of this thrombocytopenia is found to be immune-mediated in which the immune response had been triggered by SARS-CoV-2 infection [Citation79]. An immune etiology should be specially considered in the setting of acute and profound decrease in the platelet count in which no other causes are found, especially those that may induce abrupt feature of thrombocytopenia. Consistently, in the ITP cases reported in Supplementary material 1, first, a series of paraclinical workups, such as viral markers, peripheral blood smear and/or bone marrow study, were done for patients with abnormally low platelet count to excluded classic causes of thrombocytopenia and then, the diagnosis of ITP was made in the case with the absence of any demonstrable primary disease [Citation80]. For example, as a noticeable percent of patients with COVID-19 are indicated to receive anticoagulation, heparin-induced thrombocytopenia should always be kept in mind. It is important to take the patients’ setting into the consideration of diagnosis; in pregnant women, HELLP syndrome (Hemolysis, Elevated Liver function tests, Low Platelet count) and gestational thrombocytopenia should be suspected and evaluated. Furthermore, meticulously and detailed history for potential risk factors for the emergence of ITP could help in diagnosis. A majority of cases reported to have ITP, had a risk factor including a previous ITP episode, use of immunomodulating medications, chemotherapy, etc.
Given the multiorgan involvement and multifaceted nature of SARS-CoV-2 infection and the complexity of its management, development of newly diagnosed or recurrent ITP may raise many therapeutic challenges. Besides inherent hypercoagulable state seen in most cases with COVID-19, ITP, itself and its treatment-related factors such as thrombopoietin-receptor agonists are also reported to be associated with a mild elevation in thrombotic risk [Citation77]. Management of these coexisting conditions warrants an individualized approach to achieve a precise balance between the risk of severe bleeding and of thromboembolic events. Furthermore, there is still lack of data whether the available treatment options could interfere with the adaptive immune response against the pathogen that has already assaulted the body.
The treatment regimens for ITP target mainly whether the immune-mediated peripheral platelet destruction process or the thrombopoiesis process occurred in the bone marrow. Routinely, in most patients with ITP, the first-line treatment consists of IVIG or steroid or a combination of both [Citation81]. Likewise, in the study cases described in , most cases received the first-line therapy and their platelet level increased in response to the treatment. About 90% of patients for whom first-line therapy was administrated (40 out of 44 patients), the response was observed. Steroid treatment is the most widely used standard first-line therapy for the management of new-onset or relapsed ITP. Although steroid administration theoretically poses a higher risk of the development of viral infection and may lead to suppression of immune system [Citation82], in the cases with ITP who received steroid, no worsening of COVID-19 course or symptoms were reported. However, further evidence with more robust methodology and design is required to fill the gap. Another concern associated with glucocorticoid use is that as seen in , most patients with ITP had an underlying disease which warranted great caution for the use of glucocorticoids including hypertension, hyperglycemia and osteoporosis, etc [Citation83]. In these cases, it should be considered to use the minimum necessary dose and duration. Another first-line therapy for ITP is IVIG, which is more suggested for cases with very low levels of platelet and at risk for severe bleeding; many patients respond to IVIG within the first 24 h of its administration and typically the increase is observed in the platelet count in 2–4 days in those who are going to respond to IVIG while the improvement of platelet count in the course of glucocorticoids takes several days to weeks [Citation84]. Although, first-line treatment failure was not common in the reported cases of COVID-19 with ITP (six patients who later responded to the second therapeutic regimens), it should be considered to strictly monitor the hematological indices to avoid foreseeable complications. A practical guideline by Pavord et al. [Citation77] for the management of ITP recommends to use steroids as the first-line option with minimum dosing and duration and suggest to use IVIG in two settings: as a first-line option in those with risk for severe bleeding and as an alternative in those who failed to respond to steroids.
Studies have shown that there is a high risk of coagulopathy and thrombosis formation in patients with COVID -19 [Citation74,Citation85]. Although the exact mechanisms involved remain unclear, it could be assumed that expression of procoagulant and antifibrinolytic factors is attributed mostly to systemic inflammatory state, endothelial activation and hypoxia resulting in altered hemostatic balance [Citation74]. However, an additional factor is detected in bloodstream circulation of patients with SARS-CoV-2 infection with a higher rate of activation of blood coagulation and thrombosis. This additional factor is the presence of antiphospholipid antibodies indicating an APS-like condition including lupus anticoagulant, anticardiolipin and anti-β2-glycoprotein antibodies. In a study on 150 patients with COVID-19 hospitalized in the intensive care unit, 64 thrombotic events were observed that most of the patients with these events had positive antiphospholipid antibodies [Citation85]. Zhang et al. [Citation62] described three critically ill patients diagnosed with COVID-19 who developed thromboembolic events. These patients were detected to be positive for Anticardiolipin and anti-β2-glycoprotein antibodies which is indicative of a notion of APS-like phenotype in these patients.
Another hematologic autoimmune disorder seen in patients with COVID-19 is AIHA. This disorder should mainly be suspected in patients with severe anemia and those with an abrupt decrease in their hemoglobin concentration in the setting that no other attributable cause be identified. As the mainstay of treatment for AIHA, especially cold agglutinin syndrome, is the detection of the underlying cause and its specific treatment, it is important to diagnose SARS-CoV-2 infection in the asymptomatic patients and those with mild symptoms. These findings may suggest to gather samples for SARS-CoV-2 RT–PCR in all new-onset hemolytic anemia even with minor or without respiratory symptoms. In most patients with AIHA of this systematic review, the hemoglobulin level stabilized in response to supportive therapy including packed cell transfusion and the use of steroid or immunomodulators should be preserved for those with treatment failure and those warrant a rapid correction of hemodynamic status [Citation86].
Conclusion
Even with confirmation of the diagnosis of hematologic autoimmune disorder in the patients, a causative relationship between SARS-CoV-2 infection and these autoimmune events still requires further studies. These systematic reviews help us gain a more comprehensive understanding of the risk of development of hematologic autoimmune disorders in infected patients; besides, it helps us always consider an autoimmune etiology for cases with abnormal hematologic finding which further led to an appropriate treatment of the patients, especially when the symptoms present in about 1–2 weeks after the first manifestation of the infection symptoms. With this in mind, the doctor will constantly monitor the patient and consider the likelihood of such events occurring. This will result in the prompt physician’s clinical suspicion when the early manifestations of the autoimmune disorders of the patient appear. Maybe, at least in this SARS-CoV-2 pandemic, it should be recommended to evaluate patients with an unexpected and unexplained decrease in their hemoglobulin or platelet count for SARS-CoV-2 infection. Another challenging issue in this field is treatment regimens for ITP. Based on the literature, it is suggested that, in overall, steroids be used as the first line of therapy if there are no contraindications; however, given the multiorgan involvement and multifaceted nature of SARS-CoV-2 infection, an individualized approach should be taken for each patient.
Supplemental Material
Download MS Word (105.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mohammadi M, Meskini M, do Nascimento Pinto AL. 2019 novel coronavirus (COVID-19) overview. Z Gesundh Wiss. 2020: 1–9. doi:https://doi.org/10.1007/s10389-020-01258-3.
- Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–673. doi:https://doi.org/10.1016/j.jiph.2020.03.019.
- Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID-19). StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2020, StatPearls Publishing LLC; 2020.
- Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020:145561320920762. doi:https://doi.org/10.1177/0145561320920762.
- Taherifard E, Taherifard E. Neurological complications of COVID-19: a systematic review. Neurol Res. 2020: 1–8. doi:https://doi.org/10.1080/01616412.2020.1796405.
- Kadkhoda K. COVID-19: an Immunopathological view. mSphere. 2020;5(2). doi:https://doi.org/10.1128/mSphere.00344-20.
- Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. doi:https://doi.org/10.1016/j.autrev.2020.102597.
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020. doi:https://doi.org/10.1016/j.jaut.2020.102506.
- Zulfiqar AA, Lorenzo-Villalba N, Hassler P, et al. Immune thrombocytopenic purpura in a patient with covid-19. N Engl J Med. 2020;382(18):e43. doi:https://doi.org/10.1056/NEJMc2010472.
- Hindilerden F, Yonal-Hindilerden I, Akar E, et al. Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res. 2020;195:136–138. doi:https://doi.org/10.1016/j.thromres.2020.07.005.
- Hindilerden F, Yonal-Hindilerden I, Akar E, et al. Severe autoimmune hemolytic anemia in COVID-19 infection, safely treated with steroids. Mediterr J Hematol Infect Dis. 2020;12(1):e2020053. doi:https://doi.org/10.4084/mjhid.2020.053.
- Huscenot T, Galland J, Ouvrat M, et al. SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann Hematol. 2020;99(8):1943–1944. doi:https://doi.org/10.1007/s00277-020-04129-9.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi:https://doi.org/10.1186/2046-4053-4-1.
- de la Cruz-Benito B, Rivas-Pollmar MI, Álvarez Román MT, et al. Paradoxical effect of SARS-CoV-2 infection in patients with immune thrombocytopenia. Br J Haematol. 2020. doi:https://doi.org/10.1111/bjh.17077.
- Mahévas M, Moulis G, Andres E, et al. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190(4):e224–e2e9. doi:https://doi.org/10.1111/bjh.17024.
- Zagorski E, Pawar T, Rahimian S, et al. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19). Br J Haematol. 2020. doi:https://doi.org/10.1111/bjh.16892.
- Patil NR, Herc ES, Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. 2020. doi:https://doi.org/10.1016/j.hemonc.2020.06.005.
- Capes A, Bailly S, Hantson P, et al. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99(7):1679–1680. doi:https://doi.org/10.1007/s00277-020-04137-9.
- Moonla C, Watanaboonyongcharoen P, Suwanpimolkul G, et al. Cold agglutinin disease following SARS-CoV-2 and Mycoplasma pneumoniae co-infections. Clin Case Rep. 2020. doi:https://doi.org/10.1002/ccr3.3152.
- Maslov DV, Simenson V, Jain S, et al. COVID-19 and cold agglutinin hemolytic anemia. TH Open. 2020;4(3):e175–e177. doi:https://doi.org/10.1055/s-0040-1715791.
- Jensen CE, Wilson S, Thombare A, et al. Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clin Infect Prac. 2020;7:100041. doi:https://doi.org/10.1016/j.clinpr.2020.100041.
- Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190(1):29–31. doi:https://doi.org/10.1111/bjh.16794.
- Lopez C, Kim J, Pandey A, et al. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190(1):31–32. doi:https://doi.org/10.1111/bjh.16786.
- Jacobs J, Eichbaum Q. COVID-19 associated with severe autoimmune hemolytic anemia. Transfusion. 2020. doi:https://doi.org/10.1111/trf.16226.
- Vega Hernández P, Borges Rivas Y, Ortega Sánchez E, et al. Autoimmune hemolytic anemia in a pediatric patient with severe acute respiratory syndrome coronavirus 2 infection. Pediatr Infect Dis J. 2020;39(9):e288. doi:https://doi.org/10.1097/inf.0000000000002809.
- Jawed M, Hart E, Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep. 2020;13(12):e238118. doi:https://doi.org/10.1136/bcr-2020-238118.
- D'Aloisio R, Nasillo V, Gironi M, et al. Bilateral macular hemorrhage in a patient with COVID-19. Am J Ophthalmology Case Rep. 2020;20:100958. doi:https://doi.org/10.1016/j.ajoc.2020.100958.
- Sereno M, Gutiérrez-Gutiérrez G, Sandoval C, et al. A favorable outcome of pneumonia COVID 19 in an advanced lung cancer patient with severe neutropenia: is immunosuppression a risk factor for SARS-COV2 infection? Lung Cancer (Amsterdam, Netherlands). 2020;145:213–215. doi:https://doi.org/10.1016/j.lungcan.2020.04.029.
- Wahlster L, Weichert-Leahey N, Trissal M, et al. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer. 2020: e28382. doi:https://doi.org/10.1002/pbc.28382.
- Li M, Nguyen CB, Yeung Z, et al. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190(2):e59–e61. doi:https://doi.org/10.1111/bjh.16846.
- Vadlamudi G, Hong L, Keerthy M. Evans syndrome associated with pregnancy and COVID-19 infection. Case Rep Obstet Gynecol. 2020;2020:8862545. doi:https://doi.org/10.1155/2020/8862545.
- Revuz S, Vernier N, Saadi L, et al. Immune thrombocytopenic purpura in patients with COVID-19. Eur J Case Rep Intern Med. 2020;7(7):001751. doi:https://doi.org/10.12890/2020_001751.
- Bennett J, Brown C, Rouse M, et al. Immune thrombocytopenia purpura secondary to COVID-19. Cureus. 2020;12(7):e9083. doi:https://doi.org/10.7759/cureus.9083.
- Bomhof G, Mutsaers P, Leebeek FWG, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61–e64. doi:https://doi.org/10.1111/bjh.16850.
- Hindilerden F, Yonal-Hindilerden I, Sevtap S, et al. Immune thrombocytopenia in a very elderly patient with covid-19. Front Med (Lausanne). 2020;7:404. doi:https://doi.org/10.3389/fmed.2020.00404.
- Lorenzo-Villalba N, Zulfiqar AA, Auburtin M, et al. Thrombocytopenia in the course of COVID-19 infection. Eur J Case Rep Intern Med. 2020;7(6):001702. doi:https://doi.org/10.12890/2020_001702.
- Deruelle E, Ben Hadj Salem O, Sep Hieng S, et al. Immune thrombocytopenia in a patient with COVID-19. Int J Hematol. 2020. doi:https://doi.org/10.1007/s12185-020-02943-5.
- Lévesque V, Millaire É, Corsilli D, et al. Severe immune thrombocytopenic purpura in critical COVID-19. Int J Hematol. 2020. doi:https://doi.org/10.1007/s12185-020-02931-9.
- Merli M, Ageno W, Sessa F, et al. Recurrence of immune thrombocytopenia at the time of SARS-CoV-2 infection. Ann Hematol. 2020;99(8):1951–1952. doi:https://doi.org/10.1007/s00277-020-04130-2.
- Yang Y, Zhao J, Wu J, et al. A rare case of immune thrombocytopenic purpura, secondary to COVID-19. J Med Virol. 2020. doi:https://doi.org/10.1002/jmv.26051.
- Martincic Z, Skopec B, Rener K, et al. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int J Infect Dis. 2020;99:269–271. doi:https://doi.org/10.1016/j.ijid.2020.08.002.
- Humbert S, Razanamahery J, Payet-Revest C, et al. COVID-19 as a cause of immune thrombocytopenia. Med Mal Infect. 2020;50(5):459–460. doi:https://doi.org/10.1016/j.medmal.2020.05.003.
- Pedroso A, Frade L, Trevas S, et al. Immune thrombocytopenic purpura – different presentations in Two COVID-19 patients. Cureus. 2020;12(10):e11202. doi:https://doi.org/10.7759/cureus.11202.
- Nesr G, Garnett C, Bailey C, et al. Immune thrombocytopenia flare with mild COVID-19 infection in pregnancy: a case report. Br J Haematol. 2020;190(3):e146–e148. doi:https://doi.org/10.1111/bjh.16928.
- Metallidis S, Gioula G, Papaioannou M, et al. Immune thrombocytopenia in a patient recovering from COVID-19. HemaSphere. 2020;4(5):e419. doi:https://doi.org/10.1097/hs9.0000000000000419.
- Tsao HS, Chason HM, Fearon DM. Immune thrombocytopenia (ITP) in a pediatric patient positive for SARS-CoV-2. Pediatrics. 2020;146(2). doi:https://doi.org/10.1542/peds.2020-1419.
- Tang MW, Nur E, Biemond BJ. Immune thrombocytopenia due to COVID-19 during pregnancy. Am J Hematol. 2020;95(8):E191–E192. doi:https://doi.org/10.1002/ajh.25877.
- Murt A, Eskazan AE, Yılmaz U, et al. COVID-19 presenting with immune thrombocytopenia: a case report and review of the literature. J Med Virol. doi:https://doi.org/10.1002/jmv.26138.
- Levraut M, Ottavi M, Lechtman S, et al. Immune thrombocytopenic purpura after COVID-19 infection. Int J Lab Hematol. 2020. doi:https://doi.org/10.1111/ijlh.13346.
- Lobos P, Lobos C, Aravena P. Immune thrombocytopenic purpura associated with coronavirus disease 2019 infection in an asymptomatic young healthy patient. JAAD Case Rep. 2020;6(11):1129–1131. doi:https://doi.org/10.1016/j.jdcr.2020.08.037.
- Kewan T, Almhana F, Schwartzman L, et al. COVID-19 patient with immune thrombocytopenic purpura. Int J Lab Hematol. 2020;42(6):e260–e262. doi:https://doi.org/10.1111/ijlh.13303.
- Hayden A, Vyas-Lahar A, Rella V, et al. Severe refractory thrombocytopenia in a woman positive for coronavirus disease 2019 with lupus and antiphospholipid syndrome. Lupus. 2020;29(11):1472–1474. doi:https://doi.org/10.1177/0961203320940389.
- Molinaro E, Novara E, Bonometti R, et al. Isolated immune thrombocytopenic purpura in a young adult covid-19 patient. Eur Rev Med Pharmacol Sci. 2020;24(20):10850–10852. doi:https://doi.org/10.26355/eurrev_202010_23447.
- Pascolini S, Granito A, Muratori L, et al. Coronavirus disease associated immune thrombocytopenia: causation or correlation? J Microbiol Immunol Infect. 2020. doi:https://doi.org/10.1016/j.jmii.2020.08.006.
- Soares A, Loggetto SR, Manga FCM, et al. Outcome of SARS-CoV-2 and immune thrombocytopenia in a pediatric patient. Hematol Transfus Cell Ther. 2020. doi:https://doi.org/10.1016/j.htct.2020.09.145.
- Clerici B, Birocchi S, Bertinato E, et al. A case of newly diagnosed immune thrombocytopenia in the COVID-19 era. Intern Emerg Med. 2020: 1–5. doi:https://doi.org/10.1007/s11739-020-02553-3.
- Kondo Y, Kaneko Y, Oshige T, et al. Exacerbation of immune thrombocytopaenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann Rheum Dis. 2020. doi:https://doi.org/10.1136/annrheumdis-2020-218157.
- Hu Z, Chen W, Liang W, et al. Severe exacerbation of immune thrombocytopenia and COVID-19: the favorable response to corticosteroid-based therapy – a case report. Ann Hematol. 2020:1–3. doi:https://doi.org/10.1007/s00277-020-04070-x.
- Albiol N, Awol R, Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann Hematol. 2020;99(7):1673–1674. doi:https://doi.org/10.1007/s00277-020-04097-0.
- Capecchi M, Mocellin C, Abbruzzese C, et al. Dramatic presentation of acquired TTP associated with COVID-19. Haematologica. 2020. doi:https://doi.org/10.3324/haematol.2020.262345.
- Sung J, Anjum S. Coronavirus disease 2019 (COVID-19) infection associated with antiphospholipid antibodies and four-extremity deep vein thrombosis in a previously healthy female. Cureus. 2020;12(6):e8408. doi:https://doi.org/10.7759/cureus.8408.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi:https://doi.org/10.1056/NEJMc2007575.
- Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:https://doi.org/10.1016/j.thromres.2020.04.014.
- Hossri S, Shadi M, Hamarsha Z, et al. Clinically significant anticardiolipin antibodies associated with COVID-19. J Crit Care. 2020;59:32–34. doi:https://doi.org/10.1016/j.jcrc.2020.05.017.
- Maria ATJ, Diaz-Cau I, Benejean JM, et al. Flare of antiphospholipid syndrome in the course of COVID-19. TH Open. 2020;4(3):e207–e210. doi:https://doi.org/10.1055/s-0040-1716735.
- Mantovani Cardoso E, Hundal J, Feterman D, et al. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020;39(9):2811–2815. doi:https://doi.org/10.1007/s10067-020-05310-1.
- Frankel M, Feldman I, Levine M, et al. Bilateral adrenal hemorrhage in coronavirus disease 2019 patient: a case report. J Clin Endocrinol Metab. 2020;105(12). doi:https://doi.org/10.1210/clinem/dgaa487.
- Smatti MK, Cyprian FS, Nasrallah GK, et al. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi:https://doi.org/10.3390/v11080762.
- Magdi M, Rahil A. Severe immune thrombocytopenia complicated by intracerebral haemorrhage associated with coronavirus infection: a case report and literature review. Eur J Case Rep Intern Med. 2019;6(7):001155. doi:https://doi.org/10.12890/2019_001155.
- Lo AW, Tang NL, To KF. How the SARS coronavirus causes disease: host or organism? J Pathol. 2006;208(2):142–151. doi:https://doi.org/10.1002/path.1897.
- Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East Respiratory Syndrome. J Clin Neurol (Seoul, Korea). 2017;13(3):227–233. doi:https://doi.org/10.3988/jcn.2017.13.3.227.
- Li Q, Cao Y, Chen L, et al. Hematological features of persons with COVID-19. Leukemia. 2020;34(8):2163–2172. doi:https://doi.org/10.1038/s41375-020-0910-1.
- Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99(7):1421–1428. doi:https://doi.org/10.1007/s00277-020-04103-5.
- Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi:https://doi.org/10.1016/j.thromres.2020.06.029.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:https://doi.org/10.1056/NEJMoa2002032.
- Zhang Y, Zeng X, Jiao Y, et al. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb Res. 2020;193:110–115. doi:https://doi.org/10.1016/j.thromres.2020.06.008.
- Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br J Haematol. 2020;189(6):1038–1043. doi:https://doi.org/10.1111/bjh.16775.
- Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2020. doi:https://doi.org/10.1159/000512007.
- Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. 2020:1–11. doi:https://doi.org/10.1007/s42399-020-00521-8.
- Andrès E, Fothergill H, Mecili M. Life-threatening autoimmune hematological disorders. In: Khamashta MA, Ramos-Casals M, editors. Autoimmune diseases: acute and complex situations. London: Springer London; 2011. p. 259–273.
- Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. P & T. 2017;42(12):756–763.
- Wei Y, Ji X-b, Wang Y-w, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302.
- Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. doi:https://doi.org/10.1186/1710-1492-9-30.
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi:https://doi.org/10.1182/blood-2009-06-225565.
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi:https://doi.org/10.1007/s00134-020-06062-x.
- King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol. 2005;42(3):131–136. doi:https://doi.org/10.1053/j.seminhematol.2005.04.003.