ABSTRACT
Objectives
Optimal selection of pretransplant conditioning is crucially vital for improving survival and quality-of-life of patients who receive allogeneic hematopoietic cell transplantation (allo-HCT), particularly in those with high-risk diseases. In this study, we evaluated the efficacy and safety of recently-developed reduced-toxicity myeloablative regimen that combines fludarabine, intravenous busulfan, and melphalan (FBM).
Methods
We conducted a single-center retrospective analysis of 39 patients (23 with myeloid neoplasms and 16 with lymphoid neoplasms), with a median age of 50 (range, 17–68) years, who underwent their first allo-HCT using the FBM regimen. Graft types were bone marrow in 11, peripheral blood in 11, and cord blood in 17 patients. Cyclosporine- or tacrolimus-based graft-versus-host disease (GVHD) prophylaxis was administered. The primary end point of the study was the overall survival rate at 2-year after transplantation.
Results
After a median follow-up of 910 days for the surviving patients, 2-year overall survival was 62% for the entire cohort; 73% in the low-to-intermediate-risk group and 44% in the high-to-very high-risk group classified by the refined CIBMTR Disease Risk Index. Cumulative incidences of engraftment, grade II-IV acute GVHD, chronic GVHD, relapse, and non-relapse mortality were 95%, 56%, 56%, 31%, and 17%, respectively.
Conclusion
These results suggest that our FBM regimen can be applied to allo-HCT using various graft types and yields acceptable outcomes with relatively low non-relapse mortality in both myeloid and lymphoid neoplasms. Also, we observed a promising survival in the group of patients with high-risk diseases, warranting more accumulation of patients and longer follow-up.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is an established curative treatment modality to overcome hematologic diseases resistant to conventional therapy. Historically, myeloablative preparatory conditioning with high-dose cyclophosphamide (CY) plus lethal doses of total-body irradiation (TBI) or myeloablative doses of oral busulfan (BU) had been considered essential to overcome histocompatibility barrier as well as to achieve better control of the disease, which strictly restricted the candidates of allo-HCT. However, over these two decades, clinical development and increasing use of reduced-intensity or reduced-toxicity conditioning broadly expand the opportunity to receive transplant into the elderly population and patients with some organ complications.
Currently, various reduced-toxicity conditioning regimens, mostly that combines fludarabine (FLU) plus alkylating agents with or without low-dose TBI, have been developed and utilized with comparable outcomes to allo-HCT using classical myeloablative conditioning [Citation1–3]. Importantly, in two large retrospective studies of allo-HCT for acute myeloid leukemia (AML) in remission by the European Society for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Transplant Research (CIBMTR), ablative TBI-based transplantation was shown to have similar or inferior outcomes with higher risks of developing severe acute GVHD as compared with transplants using intravenous BU plus high-dose CY [Citation4,Citation5]. Additionally, in a prospective study that compared myeloablative TBI-based regimens with ablative BU-based regimens including FLU plus BU (FLU-BU) and BU plus CY (BU-CY) in myeloid neoplasms, 2-year overall survival was proven to be significantly better in the BU-based arm [Citation6]. Retrospective comparisons between classical CY plus TBI (CY-TBI) regimens with intravenous BU-based or melphalan (MEL)-based non-TBI regimens in precursor and mature lymphoid neoplasms also have shown similar post-transplant survival rates between TBI-containing and non-TBI regimens [Citation3,Citation7–9]. Collectively, these previous pivotal studies suggest that the use of ablative dose of TBI is not an essential component of conditioning for allo-HCT in the modern era at least in patients with standard-risk hematopoietic neoplasms.
On the other hand, in patients with chemotherapy-resistant high-risk diseases, problems remain with reduced-intensity or reduced-toxicity regimens because of concerns over the greater risk of relapse after transplantation [Citation10,Citation11]. There have been several reports that compare myeloablative FLU-BU, one of most widely-used reduced-toxicity regimens, with traditional BU-CY or CY-TBI in cohorts including patients carrying high-risk diseases [Citation12–14]. Although these studies yielded conflicting results, FLU-BU has been associated with similar or worse disease-free survival in the high-risk groups. Another problem with ablative FLU-BU is that it does not support reliable engraftment of cord blood graft [Citation15]. To more utilize the advantages of FLU-BU combination as conditioning for broader types of transplantation, non-TBI reduced-toxicity regimens that incorporate MEL in addition to FLU-BU are recently being developed and have shown acceptable outcomes [Citation16–19]. Here, we report the results of allo-HCT for hematopoietic neoplasms conditioned with FLU-BU plus MEL at a dose of 80 or 140 mg/m2 for a better understanding of its efficacy and safety, particularly in patients with high-risk diseases.
Patients and methods
Study design and patients
This is a single-center retrospective study that included a cohort of consecutive patients who received allo-HCT using a myeloablative triplet of FLU-BU-MEL (FBM) regimen as pretransplant conditioning between August 2013 and July 2019 at our center. Patients with a history of prior autologous HCT were included, but those with the previous allo-HCT were excluded from the cohort.
End points and definitions
The primary end point of the study was an overall survival (OS) at 2-year after transplantation. Secondary end points included 1-year OS, the time to engraftment, incidences of grade II-IV and grade III-IV acute GVHD, chronic GVHD of any severity, relapse, non-relapse mortality (NRM), and regimen-related toxicity. All time to events were calculated as days from the date of transplantation to the first occurrence of defined events, and surviving patients were censored at the last follow-up visit. For survival, the event was defined as death from any cause. Engraftment was defined when an absolute neutrophil count in peripheral blood exceeded 0.5 × 109/L for 3 consecutive days with evidence of more than 90% of donor-type hematopoiesis by the standardized quantitative chimerism analysis of whole bone marrow nucleated cells. Acute GVHD was assessed by the standard criteria irrespective of confirmation of engraftment [Citation20], and chronic GVHD was evaluated according to the National Institute of Health (NIH) criteria [Citation21]. The date of relapse was defined as the first day when progression or recurrence of original diseases was identified by either of molecular, cytogenetic, morphologic, or radiographic examinations. Regimen-related toxicity was defined as grade 3 or 4 events according to NIH Common Terminology Criteria for Adverse Events version 5.0 occurring within 28 days after transplant. Diagnosis of sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) was made according to the revised criteria proposed by EBMT [Citation22]. Transplant-associated thrombotic microangiopathy (TMA) was diagnosed based on consensus criteria of the EBMT International Working Group [Citation23]. For the subgroup analysis of overall survival, the standard-risk and high-risk groups were defined using the refined CIBMTR Disease Risk Index (CIBMTR-DRI) as follows [Citation24]. Patients with low- and intermediate-risk diseases were classified as the standard-risk group and those with high- and very high-risk diseases were included in the high-risk group.
Transplantation protocols
The conditioning regimen comprised of 30 mg/m2/day of FLU from days −6 to −3, 3.2 mg/kg/day of intravenous BU from days −6 to −3 (BU4) and 80 mg/m2/day (MEL80) or 140 mg/m2/day (MEL140) of MEL on day −2 administered with oral cryotherapy. Three patients who underwent cord blood transplantation until March 2015 additionally received TBI 2 Gy with an intent to facilitate engraftment. However, this policy was amended by the experience of early toxic death in a patient with highly refractory AML who received FLU-BU4-MEL140 plus TBI 2 Gy regimen after the repetitive course of remission-induction therapy. Therefore, the subsequent 36 patients received FBM without TBI. In principle, patients over 55 years of age or with a history of heavy treatment such as high-dose chemotherapy supported by autologous HCT had received a combination of FLU-BU4 plus MEL80. All other patients were conditioned with FLU-BU4 plus MEL140.
The sources of stem cells were related or unrelated bone marrow (BM), related or unrelated mobilized peripheral blood (PB), and unrelated single-unit cord blood (CB). All patients received GVHD prophylaxis by continuous intravenous administration of cyclosporine (CSP) or tacrolimus (TAC) starting on day −1, both combined with methotrexate (MTX), except for one patient who received TAC and mycophenolate mofetil. MTX was administered by rapid drip infusion at doses of 10 mg/m2 on day 1 and 7 mg/m2 on days 3, 6. In peripheral blood progenitor cell and HLA-allele-mismatched marrow transplants, MTX was also given on day 11 unless severe adverse events that precluded the use of MTX had occurred. In principle, we used a combination of CSP plus MTX for patients who received HLA-matched sibling donor or unrelated cord transplants and those who had the high-risk diseases. TAC plus MTX was preferentially used for patients with the standard-risk diseases who received peripheral blood or unrelated graft transplants. As additional GVHD prophylaxis, 1.25 mg/kg/day of rabbit anti-thymocyte globulin (thymoglobuline) on day −3 and day −2 was administered in one patient who received unrelated peripheral blood graft. During and after transplant, all patients received antiviral prophylaxis with acyclovir and antifungal prophylaxis with either fluconazole, voriconazole, or echinocandins. If patients had no suspected bacterial infections, prophylactic antimicrobial was not routinely administered [Citation25]. Granulocyte colony-stimulating factor was regularly used in cord blood transplants from day 7 until when neutrophil engraftment was achieved or occasionally in cases when serious infections developed before engraftment. After the confirmation of durable engraftment, pharmacologic prophylaxis against Pneumocystis jirovecii pneumonia was given to the patients until at least 6 months after the withdrawal of immunosuppressing agents.
Statistical analysis
Background patient and transplant characteristics were summarized as descriptive statistics. Comparisons between the patients who received FLU-BU4-MEL140 and FLU-BU4-MEL80 groups were done by the Wilcoxon rank-sum test for median age and by the Fisher’s exact test for the other categorical variables. The probability of overall survival was calculated using the Kaplan-Meier method. The incidence rates of neutrophil engraftment, acute and chronic GVHD, non-relapse mortality (NRM), and relapse were estimated by the cumulative incidence curves that accommodate the competing events as follows: graft rejection and death without GVHD for acute and chronic GVHD, disease-associated death for non-relapse mortality. Univariable comparison between the groups was performed using the log-rank test for overall survival and using Gray’s test for engraftment, GVHD, relapse, and NRM. Pretransplant variables that might affect overall survival were evaluated by univariable and multivariate Cox proportional hazards models. Variables included in the analyses were as follows: age (younger than 50 years vs 50 years or more), sex, disease category (myeloid vs lymphoid), disease risk (standard-risk vs high-risk), graft type (marrow vs peripheral blood or cord blood), doses of MEL (80 mg/m2 vs 140 mg/m2), and GVHD prophylaxis (CSP-based vs TAC-based). Multivariate models were constructed using a forward stepwise selection of variables associated with a P-value of less than 0.20 in univariable analyses. All tests were two-sided and P-values of less than 0.05 was considered to suggest statistical significance without adjustment for multiple comparisons. All the statistical analysis was performed by Stata (version 14.2; Stata Corporation).
Results
Patient characteristics
A total of 39 patients (male 25, female 14) with a median age of 50 (range, 17–68) years at the time of transplantation were included in the study. The median follow-up period for surviving patients as of 31 December 2019 was 910 days (range, 196–2326 days). Demographic and transplant characteristics of these patients were shown in . As compared with the MEL140 group, the MEL80 group had significantly higher ages. Primary diagnoses were myeloid neoplasms in 23 patients (AML in 14, myelodysplastic syndrome in 2, myeloproliferative neoplasms and myelodysplastic/ myeloproliferative neoplasms in 5, therapy-related myeloid neoplasms in 2), and lymphoid neoplasms in 16 patients (ALL in 10, mature B cell neoplasms in 2, and mature T/NK cell neoplasms in 4). According to the refined CIBMTR-DRI [Citation14], 23 patients had low-risk (N = 3) and intermediate-risk (N = 20) diseases (collectively classified as the standard-risk group), while 16 patients had high-risk (N = 14) or very high-risk diseases (N = 2) (collectively allocated as the high-risk group). At the time of transplantation, 7 (61%) of 13 patients in the MEL80 group and 9 (35%) of 26 patients with the MEL140 group had active diseases. The proportions of the high-risk group patients among myeloid and lymphoid neoplasms were 52% and 25%, respectively. It is important to note here that 14 (88%) of 16 patients in the high-risk group had an active disease at transplant. The graft sources were bone marrow (N = 11; related in 2, unrelated in 9), granulocyte colony-stimulating factor-mobilized peripheral blood (N = 11; related in 10, unrelated in 1), and unrelated cord blood (N = 17). For GVHD prophylaxis, 28 patients received CSP plus MTX, while 11 patients received TAC-based regimens.
Table 1 Patient and transplant characteristics.
Overall survival
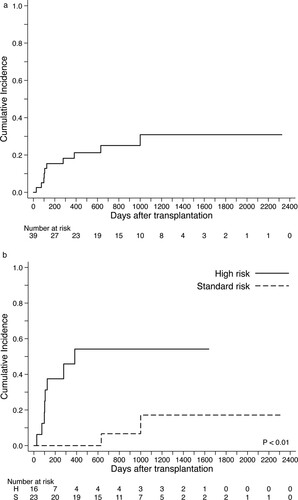
The respective OS estimates at 1-year and 2-years after transplantation were 76% (95% confidence interval [CI], 59–87) and 62% (95% CI, 42–76) for the entire cohort (A); 91% (95% CI, 69–98) and 73% (95% CI, 46–88) among the standard-risk group compared with 52% (95% CI, 24–74) and 44% (95%CI, 17–67) among the high-risk group (P = 0.036 by log-rank test) (B). When we compared patients who received FLU-BU-MEL140 and those received FLU-BU-MEL80 in an unadjusted manner, the MEL140 group had a better survival rate (1-yr OS 68% vs 80%, P = 0.045) (C). At the time of last follow-up, 19 (73%) of 26 patients in the MEL140 group and 6 (46%) of 13 patients in the MEL80 group were alive in CR. In univariable proportional hazard analysis, higher patient age (50 yrs or higher) (the hazard ratio [HR] for mortality 9.15; 95% CI, 1.98–42.2: P = 0.005) and the presence of high-risk disease (HR 3.13; 95% CI, 1.02–9.65: P = 0.046) were significantly associated with worse OS (). However, the only factor significantly associated with OS was the age at transplant (HR 6.60; 95% CI, 1.31–33.2: P = 0.022) by multivariate analysis.
Figure 1. Overall survival after allogeneic hematopoietic cell transplants using FBM regimen. (A) Survival rate for the entire cohort. (B) Survival rates stratified by the disease risk groups.
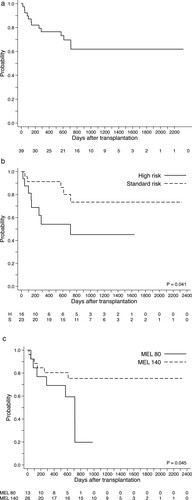
Table 2 Univariable and multivariate analyses for pretransplant variables potentially associated overall mortality.
Engraftment
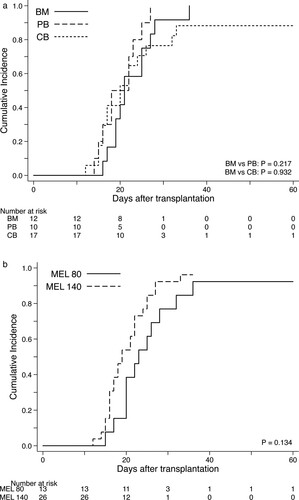
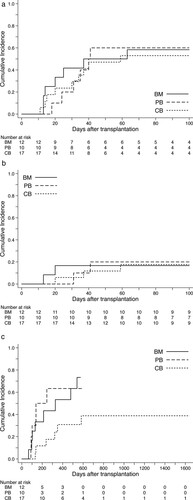
The probability of neutrophil engraftment of 39 patients was 95% (95% CI 81–99). BM and PB recipients showed an engraftment rate of 100% (95% CI, 72–100 for BM; 95% CI, 47–100 for PB), whereas patients who received CB transplant experienced a lower rate of engraftment (88%; 95% CI, 61–97), although the median days to engraftment were not statistically associated with types of graft source (). Primary graft failure occurred in one CB recipient who had subsequently undergone the immediate salvage CB transplantation and was successfully rescued by engraftment of the third graft. In addition, one patient who received BM from an HLA allele-fully matched unrelated donor developed secondary graft failure. These 2 patients encountered with graft failure were conditioned with the FLU-BU4-MEL80 regimen.
GVHD
The cumulative incidences of gradeⅡ-Ⅳand grade III-IV acute GVHD at day 100 for the whole cohort were 56% (95% CI, 40–70) and 18% (95% CI 8–31), respectively ((A,B)). By univarible comparison, types of graft source had no significant impact on the incidence of acute GVHD. Most cases of acute GVHD were manageable and responding to the standard dosage of corticosteroids; however, 5 patients were resistant to primary therapy and required treatment by anti-thymogobulin or mesenchymal stem cell infusion. The estimated incidence of chronic GVHD diagnosed by the NIH criteria was 56% (95% CI, 38–71) for the entire cohort and it appeared to be higher in PB and BM recipients compared with those who received CB transplants (C).
Figure 3. Cumulative incidence of acute and chronic GVHD according to types of graft source. (A) Incidence of grade II to IV acute GVHD within 100 days after transplantation. (B) Incidence of grade III or IV acute GVHD within 100 days after transplantation. (C) Incidence of chronic GVHD of any severity by NIH criteria.
Regimen-related toxicity
Grades 3–4 organ toxicities evaluated by NIH CTCAE criteria were observed in 31 (79%) patients within 28 days after transplant (), most of which were gastrointestinal toxicities such as oral mucositis and manageable with opioids and topical supportive therapy. Vascular toxicity, including SOS/VOD (N = 3) and TMA (N = 2), was observed in 5 patients (13%). While most of them were recovered with supportive therapy or with the use of recombinant thrombomodulin [Citation26], 2 patients with severe SOS/VOD succumbed to multi-organ failure (MOF) leading to death. Two more cases of late-onset SOS/VOD occurred after day 28 and one was associated with mortality. The incidence of neurological, pulmonary, or renal complications was low. No cardiac toxicity was experienced. In terms of infectious complications, 29 (74%) patients developed cytomegalovirus antigenemia or DNA-emia, and 2 of them had gastroenteritis. Documented viral hemorrhagic cystitis occurred in 8 (21%) patients and all of them were resolved by supportive therapy. Detected viruses were BK virus in 6, adenovirus in 2, and JC virus in 3 including 2 cases of overlapping infections.
Table 3 Incidence of grade 3–4 regimen toxicities, VOD/SOS and TMA within 28 days after transplant.
Relapse and non-relapse mortality
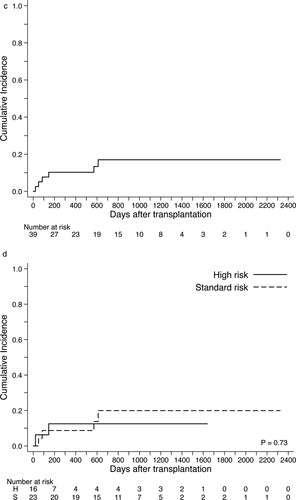
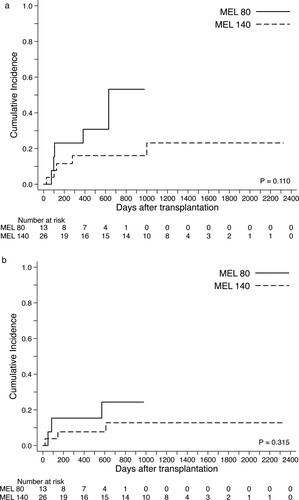
During the observed period, cumulative relapse incidence was 31% (95% CI 15–48), and death associated with disease progression was occurred in 6 patients (A). Of 9 patients who experienced relapse, 2 patients (1 in the standard-risk group and 1 in the high-risk group) were still alive in complete remission after second allo-HCT (N = 1) or re-induction chemotherapy (N = 1). Relapse incidence of the high-risk group was 54% (95% CI, 26–76) and significantly higher than that of the standard-risk group (17%; 95% CI, 3–43) (B). Cumulative incidence of NRM was 17% (95% CI, 6–31) without significant differences between the standard-risk (20%; 95% CI, 9–40) and high-risk groups (13%; 95% CI, 2–33) ((C,D)). When we compared the MEL140 group and the MEL80 group, no significant statistical differences were observed in relapse incidence and NRM ((A,B)). The causes of death without relapse were toxic MOF associated with SOS/VOD (N = 2; day 21, day 85), idiopathic pneumonia syndrome (N = 1; day 50), uncontrolled acute GVHD followed by intracranial hemorrhage (N = 1; day 146), late-onset MOF (N = 1; day 571), and accidental death (N = 1, day 611).
Figure 4. Cumulative relapse incidence and non-relapse mortality after allogeneic hematopoietic cell transplants using FBM regimen. (A) Incidence of disease progression and relapse for the entire cohort. (B) Incidence of disease progression and relapse stratified by the disease risk groups. (C) Non-relapse mortality for the entire cohort. (D) Non-relapse mortality stratified by the disease risk groups.
Discussion
In this study, we retrospectively analyzed a consecutive series of patients with hematopoietic neoplasms who underwent allo-HCT conditioned with the combination of myeloablative FLU-BU-MEL at our center. We found that transplant outcomes using the FBM regimen were comparable to those of conventional myeloablative transplants with relatively lower NRM both in the standard-risk and the high-risk groups. Both MEL140 and MEL80 regimens conferred durable engraftment of all types of stem cell source, although further improvement is required to prevent graft dysfunction when applied to single-unit cord blood transplants. As the primary end point, 2-yr OS rate of 62% for the entire cohort is acceptable. It appears to be particularly encouraging in the high-risk group with a survival rate plateau at 44%, given that 14 (88%) of 16 patients in this group had a chemoresistant active disease at the time of transplant, although early relapse remained a significant hurdle. Although the MEL80 group appeared to have a lower survival rate compared with the MEL140 group, this was not statistically significant in multivariate analysis. We assume that this observation might reflect the higher proportion of patients with active disease in the MEL80 group. The strength of our study is that we adopted the revised CIBMTR-DRI to define the risk of primary disease in our cohort. Although there have been different ways of classifying the standard- and high-risk diseases to predict transplant outcomes, they are often neither exchangeable nor reproducible across the studies. CIBMTR-DRI is an objective risk stratification system that was validated by more than 13000 adult patients. It was proven to be a robust tool to stratify the groups with better and worse post-transplant prognosis irrespective of conditioning intensity, patient age group, and type of graft source [Citation24]. With regard to regimen-related toxicities with the FBM regimen, mild-to-moderate grades of gastrointestinal complications were frequent but serious adverse events leading to early mortality were rare. However, it should be noted here that 3 patients with highly refractory diseases were suffered from early toxic death due to SOS/VOD and idiopathic pneumonia syndrome within 100 days after transplant, alerting the use of FBM in cases with high tumor burden.
The FBM regimen was originally developed as a dose-intensified myeloablative regimen for allo-HCT in pediatric patients with juvenile chronic myelomonocytic leukemia, notably with a high survival rate in this dismal disease [Citation27,Citation28]. However, this regimen consisted of FLU and myeloablative BU plus high doses of MEL at 180–210 mg/m2 is considered prohibitive in a majority of population who requires allo-HCT for hematopoietic neoplasms. More recently, the dose-reduced combinations of this triplet regimen that can be applied to adult recipients were developed by some groups and have shown encouraging results [Citation16,Citation18,Citation19]. Although FLU-BU is widely used as the standard reduced-toxicity myeloablative conditioning, especially in allo-HCT for myeloid neoplasms, several studies have reported that the use of MEL-based regimen as compared with FLU-BU conferred a trend toward better survival of patients with high-risk diseases [Citation29–33]. Also, in a large retrospective study of autologous transplantation in high-risk AML, better survival after BU-MEL combination was reported as compared with the use of standard BU-CY regimen [Citation34]. Another caveat of FLU-BU regimen is that it does not support the engraftment of unrelated cord blood units [Citation15]. Because MEL has dose-dependent myeloablative and immunoablative effects, it is reasonable to combine MEL with FLU-BU as conditioning for allo-HCT to achieve better disease control and deeper host-versus-graft immunosuppression. In line with this assumption, FBM was successfully used in HLA-haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide [Citation35].
Additional advantage of FBM regimen might be its potential activity against high-risk lymphoid neoplasms. As conditioning for autologous HCT, myeloablative intravenous BU plus an intermediate dose (140 mg/m2) of MEL has been shown to be more effective than a single infusion of MEL 200 mg/ m2 against plasma cell myeloma as well as against relapsed lymphomas [Citation36,Citation37]. Although previous experiences of oral BU-CY regimen in allo-HCT for high-risk lymphoid diseases were not satisfactory, intravenous BU plus MEL140 combination was proven to be an effective regimen in transplants in adults ALL [Citation38]. Because new therapeutic modalities such as chimeric antigen receptor-transduced T cells are very rapidly emerging for refractory lymphoid neoplasms [Citation39], it is essential for buiding a safe bridge to allo-HCT through the development of less toxic transplant protocol. Notably, a recent retrospective analysis including more than 1000 patients with 18–60 years old who received transplants for ALL revealed similar survival rates between the intravenous BU-based non-TBI regimen group and the TBI-based regimen group [Citation8]. In this study, 71% of patients had received FLU-BU or BU plus clofarabine and NRM was lower in the BU-based group at the cost of higher incidence of relapse. Therefore, incorporation of MEL to FLU-BU might be beneficial for reducing post-transplant relapse risk in this group of patients without increasing excessive toxicity.
The present study has inherent limitations due to its retrospective nature, the small number of patients, and heterogeneity of the analyzed cohort. Therefore, these results should be confirmed by larger studies, ideally in a prospective fashion, to exclude possible biases. However, our observations in this study provided further evidence for the usefulness of the FBM regimen in a real-world practice of allo-HCT for hematopoietic neoplasms using various graft sources.
Ethical approval
This study was approved by the ethical committee of researches involving human participants at Hiroshima University (the approval number E-1563) and conducted under the ethical principles of the Declaration of Helsinki and its later amendments. All the patients provided written informed consent to the protocol of transplantation, including conditioning regimen, type of stem cell source, and GVHD prophylaxis.
Author’s contributions
TI designed the study and TE, TK, and TI drafted the original manuscript; TE, TK, HN, KF, KT, TMiyama, TMino, TY, TMorioka, YH, MNishizawa and NF collected data and revised the original draft; TE, TK, and TI analyzed data; MNoma and TF collected data regarding graft sources; TE, TK and TI edited the manuscript for submission.
Acknowledgments
We thank all of the staff in our department and members of Hiroshima University Hospital who dedicatedly provided the best care for patients in this study and their donors, with a special gratitude to compassionate hematopoietic cell transplant coordinators: Yuka Asano, Akihiro Osawa, and Kaho Ogawa. We also thank Sachiko Fukumoto, Nanae Nakaju, Sajeda Chowdhury, Emi Nakai, Sanae Furuya, and Masako Ninomiya for their excellent technical and secretarial assistance.
Disclosure statement
The Next Generation Development of Genome and Cellular Therapy Program at RIRBM is cooperatively funded by Repertoire Genesis Inc. and Hiroshima University. TI has received speaker honoraria from Bristol-Myers Squibb, Celgene, Janssen Pharmaceutical K.K., and Kyowa Kirin Co. and research funding from Astellas Pharma, Chugai Pharmaceutical Co., CSL Behring, Eisai Co., FUJIFILM Wako Chemicals., Kyowa Kirin Co., Ono Pharmaceutical Co., Pfizer, Nippon Shinyaku Co., MSD, Otsuka Pharmaceutical Co., Repertoire Genesis Inc., Sumitomo Dainippon Pharma Co., Taiho Pharmaceutical Co., Takara Bio Inc., Takeda Pharmaceutical Co., Zenyaku Kogyo Co. No other authors have relevant conflict-of-interest to declare.
Additional information
Funding
References
- Gyurkocza B, Sadmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344–353. doi:https://doi.org/10.1183/blood-2014-02-514778.
- Jain T, Alahdab F, Firwana B, et al. Choosing a reduced-intensity conditioning regimen for allogeneic stem cell transplantation, fludarabine/busulfan versus fludarabine melphalan: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2019;25(4):728–733. doi:https://doi.org/10.1016/j.bbmt.2018.11.016.
- Abdul Wahid SF, Ismail NA, Mohd-Idris MR, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells Dev. 2014;23(21):2535–2552. doi:https://doi.org/10.1089/scd.2014.0123.
- Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen–a report from the acute Leukemia working party of the European Group for blood and marrow transplantation. J Clin Oncol. 2013;31(28):3549–3556. doi:https://doi.org/10.1200/JCO.2013.48.8114.
- Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122(24):3863–3870. doi:https://doi.org/10.1182/blood-2013-07-514448.
- Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122(24):3871–3878. doi:https://doi.org/10.1182/blood-2013-08-519009.
- Mitsuhashi K, Kako S, Shigematsu A, et al. Comparison of cyclophosphamide combined with total body irradiation, oral busulfan, or intravenous busulfan for allogeneic hematopoietic cell transplantation in adults with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2016;22(12):2194–2200. doi:https://doi.org/10.1016/j.bbmt.2016.09.007.
- Kebriaei P, Anasetti C, Zhang MJ, et al. Intravenous busulfan compared with total body irradiation pretransplant conditioning for adults with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2018;24(4):726–733. doi:https://doi.org/10.1016/j.bbmt.2017.11.025.
- Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12(12):1326–1334. doi:https://doi.org/10.1016/j.bbmt.2006.08.035.
- Shimoni A, Nagler A. Optimizing the conditioning regimen for allogeneic stem-cell transplantation in acute myeloid leukemia; dose intensity is still in need. Best Pract Res Clin Haematol. 2011;24(3):369–379. doi:https://doi.org/10.1016/j.beha.2011.05.002.
- Veltri L, Rezvani K, Oran B, et al. Allotransplants for patients 65 years or older with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2019;25(3):505–514. doi:https://doi.org/10.1016/j.bbmt.2018.09.032.
- Gooptu M, Kim HT, Ho VT, et al. A comparison of the myeloablative conditioning regimen fludarabine/busulfan with cyclophosphamide/total body irradiation for allogeneic stem cell transplantation in the modern era: a cohort analysis. Biol Blood Marrow Transplant. 2018;24(8):1733–1740. doi:https://doi.org/10.1016/j.bbmt.2018.03.011.
- Fedele R, Messina G, Martinello T, et al. Tolerability and efficacy of busulfan and fludarabine as allogeneic pretransplant conditioning therapy in acute myeloid leukemia: comparison with busulfan and cyclophosphamide regimen. Clin Lymphoma Myeloma Leukemia. 2014;14(6):493–500. doi:https://doi.org/10.1016/j.clml.2014.04.006.
- Harris AC, Boelens JJ, Ahn KW, et al. Comparison of pediatric allogeneic transplant outcomes using myeloablative busulfan with cyclophosphamide or fludarabine. Blood Adv. 2018;2(11):1198–1206. doi:https://doi.org/10.1182/bloodadvances.2018016956.
- Horowitz ME, Morris A, Gasparetto C, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant. 2008;14(5):591–594. doi:https://doi.org/10.1016/j.bbmt.2008.02.0116.
- Yamamoto H, Uchida N, Yuasa M, et al. A novel reduced-toxicity myeloablative conditioning regimen using full-dose busulfan, fludarabine, and melphalan for single cord blood transplantation provides durable engraftment and remission in nonremission myeloid malignancies. Biol Blood Marrow Transplant. 2016;22(10):1844–1850. doi:https://doi.org/10.1016/j.bbmt.2016.06.017.
- Katsanis E, Sapp LN, Pelayo-Katsanis L, et al. Alternative donor hematopoietic cell transplantation conditioned with myeloablative busulfan, fludarabine, and melphalan is well tolerated and effective against high-risk myeloid malignancies. J Pediatr Hematol Oncol. 2016;38(8):e315–e318. doi:https://doi.org/10.1097/MPH.0000000000000621.
- Ueda T, Maeda T, Kusakabe S, et al. Addition of melphalan to fludarabine/busulfan (FLU/BU4/MEL) provides survival benefit for patients with myeloid malignancy following allogeneic bone-marrow transplantation/peripheral blood stem-cell transplantation. Int J Hematol. 2019;109(2):197–205. doi:https://doi.org/10.1007/s12185-018-2562-8.
- Ueda T, Jo T, Okada K, et al. Curative potential of fludarabine, melphalan, and non-myeloablative dosage of busulfan in elderly patients with myeloid malignancy. Int J Hematol. 2020;111(2):247–255. doi:https://doi.org/10.1007/s12185-019-02763-2.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi:https://doi.org/10.1016/j.bbmt.2014.12.001.
- Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for blood and marrow transplantation. Bone Marrow Transplant. 2016;51(7):906–912. doi:https://doi.org/10.1038/bmt.2016.130.
- Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95–100.
- Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi:https://doi.org/10.1182/blood-2014-01-552984.
- Kanda J, Ichinohe T, Saito T, et al. Impact of discontinuing fluoroquinolone prophylaxis on early mortality after allogeneic marrow or peripheral blood SCT with myeloablative conditioning. Bone Marrow Transplant. 2010;45(8):1369–1371.
- Yakushijin K, Ikezoe T, Ohwada C, et al. Clinical effects of recombinant thrombomodulin and defibrotide on sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(5):674–680. doi:https://doi.org/10.1038/s41409-018-0304-4.
- Yabe M, Sako M, Yabe H, et al. A conditioning regimen of busulfan, fludarabine, and melphalan for allogeneic stem cell transplantation in children with juvenile myelomonocytic leukemia. Pediatr Transplant. 2008;12(8):862–867. doi:https://doi.org/10.1111/j.1399-3046.2008.00931.x.
- Yabe M, Ohtsuka Y, Watanabe K, et al. Transplantation for juvenile myelomonocytic leukemia: a retrospective study of 30 children treated with a regimen of busulfan, fludarabine, and melphalan. Int J Hematol. 2015;101(2):184–190. doi:https://doi.org/10.1007/s12185-014-1715-7.
- Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/ melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21(10):2109–2116.
- Damlaj M, Alkhateeb HB, Hefazi M, et al. Fludarabine-busulfan reduced-intensity conditioning in comparison with fludarabine-melphalan is associated with increased relapse risk in spite of pharmacokinetic dosing. Biol Blood Marrow Transplant. 2016;22(8):1431–1439. doi:https://doi.org/10.1016/j.bbmt.2016.04.026.
- Kawamura K, Kako S, Mizuta S, et al. Comparison of conditioning with fludarabine/busulfan and fludarabine/melphalan in allogeneic transplantation recipients 50 years or older. Biol Blood Marrow Transplant. 2017;23(12):2079–2087. doi:https://doi.org/10.1016/j.bbmt.2017.09.003.
- Jain T, Kunze KL, Temkit M, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2019;54(2):204–211. doi:https://doi.org/10.1038/s41409-018-0226-1.
- Oshrine B, Adams L, Nguyen ATH, et al. Comparison of melphalan- and busulfan-based myeloablative conditioning in children undergoing allogeneic transplantation for acute myeloid leukemia or myelodysplasia. Pediatr Transplant. 2020;24:e13672. doi:https://doi.org/10.1111/petr.13672.
- Gorin NC, Labopin M, Blaise D, et al. Optimizing the pretransplant regimen for autologous stem cell transplantation in acute myelogenous leukemia: better outcomes with busulfan and melphalan compared with busulfan and cyclophosphamide in high risk patients autografted in first complete remission: A study from the acute leukemia working party of the EBMT. Am J Hematol. 2018;93(7):859–866. doi:https://doi.org/10.1002/ajh.25105.
- Jaiswal SR, Chakrabarti A, Chatterjee S, et al. Haploidentical peripheral blood stem cell transplantation with post-transplantation cyclophosphamide in children with advanced acute leukemia with fludarabine-, busulfan-, and melphalan-based conditioning. Biol Blood Marrow Transplant. 2016;22(3):499–504. doi:https://doi.org/10.1016/j.bbmt.2015.11.010.
- Cornillon J, Daguenet E, Bay JO, et al. BAM conditioning before autologous transplantation for lymphoma: a study on behalf of the Francophone society of bone marrow transplantation and cellular therapy (SFGM-TC). Ann Hematol. 2019;98(8):1973–1980. doi:https://doi.org/10.1007/s00277-019-03704-z.
- Bashir Q, Thall PF, Milton DR, et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Haematol. 2019;6(5):e266–e275. doi:https://doi.org/10.1016/S2352-3026(19)30023-7.
- Kebriaei P, Madden T, Wang X, et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant. 2013;48(1):26–31. doi:https://doi.org/10.1038/bmt.2012.114.
- MacKay M, Afshinnekoo E, Rub J, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol. 2020;38(2):233–244. doi. https://doi.org/10.1038/s41587-019-0329-2.