ABSTRACT
Objectives
The purpose of this study was to evaluate the efficacy and safety of different doses of anthracyclines combined with arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) for induction in newly diagnosed acute promyelocytic leukemia (APL).
Methods
One hundred and forty patients were included between January 2011 and December 2017. Seventy patients received low dose anthracycline, ATO and ATRA for induction chemotherapy; and other seventy patients received standard dose anthracycline, ATO and ATRA for induction chemotherapy.
Results
The outcomes of both groups were similar: low dose group versus standard dose group: early mortality 5.7% vs. 10.0% (P = 0.532), disease-free survival (DFS), probabilities of overall-survival (OS) at 2 years 94.6% vs. 95.1% (P = 0.657), 92.8% vs. 88.2% (P = 0.951), respectively. However, the standard-dose group was associated with a longer duration of neutropenia (p < 0.001) and thrombocytopenia (p < 0.001), more volumes of platelets (p = 0.037) and red blood cell transfusions (p < 0.001), and a higher rate of infections (p = 0.042).
Conclusion
Low-dose group achieves outcomes similar to those of standard dose group for APL patients, but the low-dose group may be even safer than standard-dose group. So the low-dose anthracycline may be a better choice for newly diagnosed APL patients.
1. Introduction
Acute promyelocytic leukemia (APL) is a specific subtype of acute myeloid leukemia (AML) characterized by coagulopathy, high cure rate and sensitivity to anthracyclines, all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) [Citation1]. Over 95% of APL cases are characterized by balanced reciprocal translocation t (15;17), which fuses the PML gene on chromosome 15 to the RAR-α (retinoic acid receptor-α) gene on chromosome 17 [Citation1]. The disease may occur abruptly and is associated with a high risk of early death (ED) mostly due to severe hemorrhages [Citation2]. At present, anthracyclines, ATRA and ATO, are mainly used in induction therapy for APL, which can prolong long-term survival of these patients, significantly reduce recurrence, and control differentiation syndrome (DS) with the addition of chemotherapy drugs [Citation3]. Unfortunately, the combination of these treatment options leads to bone marrow suppression, which increases the risk of infection and bleeding. In addition, the dose of anthracyclines is still controversial, especially in intermediate risk group. Here, we conducted a retrospective multi-center study to evaluate the safety and efficacy of different doses of anthracyclines in the induction treatment of de novo APL.
2. Materials and methods
2.1. Patients and methods
One hundred and sixty-three patients in total with de novo APL underwent induction chemotherapy in the three centers (First Affiliated Hospital of Soochow University, Soochow Guangci Hospital, and Soochow Hopes Hospital of Hematology) between January 2011 and December 2017. A total of 23 cases were excluded as follow: age < 12 years-old (n = 5), with central nervous system (CNS) hemorrhage at disease onset (n = 12), a history of other malignancy (n = 3) and pregnant and/or lactating (n = 3). Therefore, 140 patients (82 males and 58 females), with a median age of 40 (14–68) years old, were included in this study. Seventy patients received low dose anthracycline (IDA ≤ 6 mg/m2/d, d1–3 or d1, 3, 5) combined with ATO and ATRA for induction chemotherapy, while other 70 patients received standard dose anthracycline (IDA 8–12 mg/m2/d, d1–3 or d1, 3, 5) combined with ATO and ATRA for induction chemotherapy. The diagnosis of APL was established based on clinical presentation, morphological criteria of the French-American-British classification systems, immunophenotype, and the presence of t (15;17) and fusion gene of PML-RARα. All patients provided written informed consent for the protocol, which was approved by hospital’s Ethics Committee.
2.2. Therapeutic regimen
Seventy patients received low dose anthracycline chemotherapy and seventy patients received standard dose anthracycline chemotherapy. Our induction therapy was administered as follows: 25 mg/m2 ATRA orally per day and 0.16 mg/kg (maximum 10 mg) ATO intravenously per day until CR. When the patient’s white blood cell count (WBC) exceeded 10 × 109/L, the patient was given anthracycline (IDA ≤ 6 mg/m2/d or 8–12 mg/m2/d, d1–3 or d1, 3, 5). If absolute neutrophil count (ANC) was <1.0×109/L 5 days later after the end of anthracyclines, granulocyte-colony stimulating factor (G-CSF) was injected subcutaneously at 150 μg/day. Red blood cells (RBC) were transfused when hemoglobin (Hb) was <70 g/L. Platelets were transfused when platelet count (PLT) was <50×109/L. To evaluate therapeutic efficacy, a bone marrow puncture was performed either after the induction course, or before the following consolidation therapy. Bone marrow assays included bone marrow morphology, cytogenetic analysis and gene expression levels of PML-RARα fusion gene. After the achievement of CR, to avoid central nervous system (CNS) relapse, all patients received at least once intrathecal injection of three drugs (methotrexate 15 mg + Ara-C 50 mg + dexamethasone 5 mg). Supportive treatment for coagulopathy, oral antimicrobial prophylaxis as well as diagnosis and management of the APL differentiation syndrome were performed according to international standards [Citation3].
2.3. Molecular monitoring
Bone marrow was used to demonstrate the presence of PML-RARα transcripts. Bone marrow samples for morphologic and reverse quantitative transcriptase polymerase chain reaction (qRT-PCR) evaluations were obtained at diagnosis, at the first hematologic complete remission (hCR), the beginning of every cycle of consolidation and intervals of 3 months during maintenance therapy, there after every 3–6 months for at least 2 more years after the end of maintenance. qRT-PCR was performed according to international standards in central laboratories [Citation4].
2.4. Definitions
Low dose anthracycline was defined as the total amount of idarubicin (IDA) ≤ 6 mg/(m2·day) ×3d, on days 1–3, or days 1, 3, 5. Standard dose anthracycline was defined as IDA 8–12 mg/(m2·day) ×3d, on days 1–3, or days 1, 3, 5. If other anthracycline drugs were used, the conversion table of anthracycline dose was consulted [Citation5]. ED was defined as death from any cause either before treatment was initiated or within the first 30 days of induction treatment. The most frequent clinical manifestations of DS were unexplained fever, dyspnea, pleural or pericardial effusion, pulmonary infiltrates, renal failure, unexplained hypotension, weight gain of more than 5 kg. Patients with 4 or more of the above signs or symptoms were classified as having severe DS, while those with 2 or 3 signs or symptoms were considered to have moderate DS [Citation6]. Molecular complete remission (mCR) was defined as the absence of detectable PML-RARα transcripts, and molecular relapse was defined as reversion to PML-RARα positivity, confirmed on serial samples after a previous negative result. Overall survival (OS) was calculated from date of diagnosis to death of any cause or last follow-up. Disease-free survival (DFS) of patients was calculated from date of CR to first event (relapse, or death of any cause).
2.5. Statistical analysis
The chi-square or Fisher exact test was used to compare categorical variables, while the nonparametric test (Mann Whitney U test) was used for continuous ones. The Kaplan–Meier method with the log-rank test was used to calculate the probabilities of OS and DFS for 2 years. The P value was analyzed by bilateral analysis, and P < 0.05 was considered statistically significant. Statistical analysis was performed by SPSS 20.0 windows software (SPSS, Chicago, IL).
3. Results
3.1. Patient demographics
The characteristics of the low-dose group and the standard-dose group are summarized in . In total, 140 consecutive de novo patients from 3 centers with a median age of 40 years were included in this study. Of the patients, 82 (58.6%) were male and 58 (41.4%) were female. As shown in , patients included in the two treatment groups did not differ significantly with regard to age, gender or to the other baseline characteristics.
Table 1. Clinical and laboratory features of acute promyelocytic leukemia patients.
3.2. Early death
Four patients died without hCR within 30 days after diagnosis in the low-dose group, and the causes of death included pneumorrhagia (n = 2), cerebral bleeding (n = 1) and multiple organ failure (n = 1). Seven patients died without hCR within 30 days after diagnosis in the standard-dose group, and the causes of death included pneumorrhagia (n = 4), cerebral bleeding (n = 2) and multiple organ failure (n = 1). The probabilities of ED were similar between the low-dose and the standard-dose group, with 5.7% and 10.0% (P = 0.532).
3.3. Hematologic toxicity
shows the hematologic toxicity and side effects of the 129 survival patients. The median time of grade 4 neutropenia (ANC ≤ 0.5 × 109/L) duration after anthracycline was 0 (range, 0–13) days in the in the low-dose group and 3 (range, 0–23) days in the standard-dose group (p < 0.001); while the median time of grade 3–4 thrombocytopenia (PLT ≤ 50 × 109/L) duration after anthracycline in the low-dose group and the standard-dose group was 12 (range, 0–21) and 15 (range, 3–27) days (p < 0.001). The low-dose group had lower median volumes of platelet transfusions than the standard-dose group, with 6 (range, 0–18) units (U) versus 8 (range, 1–22.5) U (P = 0.037). Moreover, the low-dose group also had notable lower median volumes of red blood cell transfusions, 4 (range, 0–14) U compared with 7 (range, 0–20) U in the standard-dose group (P < 0.001).
Table 2. Hematologic toxicity and side effects of low-dose group and standard-dose group.
During the entire induction chemotherapy, a total of 46 patients both of groups suffered treatment-related severe infections (WHO grade ≥ 3). The low-dose group had a significantly lower rate of infections than the standard-dose group, with 27.3% versus 44.4% (P = 0.042). The most frequent treatment-related severe infection of both groups was respiratory infection.
A complete data set about DS was available in 35 patients (27.1%, 35/129), 16 from the low-dose group and 19 from the standard-dose group. Severe DS was reported in 9 patients (7.0%, 9/129), and moderate DS in 26 patients (20.1%), without differences between groups (P = 0.553).
3.4. Therapeutic effect and follow up
One hundred and twenty-nine patients finished the induction regimen and were evaluated for response. The rate of molecular complete remission (mCR) of the survival patients after induction was similar in the low-dose group than those in the standard-dose group (84.8% vs. 79.4%, P = 0.418).
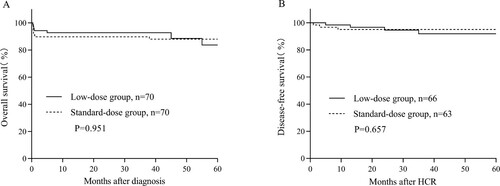
The median follow-up of the 129 eligible patients was 42 (0–97) months. In the low-dose group, 3 of 66 patients (4.5%) died. The causes of death were underlying disease relapse/progressive (n = 2) and infection (n = 1). In the standard-dose group, 1 of 63 patients (1.6%) died. The cause of death was underlying disease relapse/progressive (n = 1). The 2-year OS rate was similar in the low-dose group compared to the standard-dose group (92.8% vs. 88.2%, P = 0.951, A). Similarly, the 2-year DFS did not vary considerably between the low-dose group and the standard group, with 94.6% and 95.1% (P = 0.657, B).
Figure 1. (A) OS and (B) DFS. Patients were grouped according to the different doses of anthracyclines. DFS and OS were calculated using Kaplan–Meier estimators. P-values for curve comparison were calculated using the log-rank test.
As of April 30, 2019, 7 patients had relapsed after a median of 14 months (range 3–41), reaching a relapse rate (RR) of 4.7% at 2 years. In the low-dose group, 3 of 66 patients (4.5%) relapsed: 2 bone marrow relapse, and 1 central nervous system (CNS) relapse. While, in the standard-dose group, 4 of 63 patients (6.3%) relapsed: 1 bone marrow relapse, 1 CNS relapse, and 2 molecular relapses.
4. Discussion
APL was previously known as the most fatal leukemia subtype, but is now considered potentially curable in adults since the introduction of ATRA therapy in the 1980s [Citation7]. The conventional strategy combines cytosine arabinoside (Ara-C), mitoxantrone (MTZ) and anthracycline-based chemotherapy to induce remission, and afterwards consolidation chemotherapy and maintenance therapy. This strategy has yielded CR rates less than 70%, early death rates (EDR) greater than 20%, and long-term cure rates of less than 45% [Citation2,Citation8–10]. However, the addition of ATRA to anthracycline-based chemotherapy has dramatically improved the survival of patients with APL leading to cure in approximately 90% of patients with newly diagnosed APL. It has completely changed the prognosis of APL, turning it into the most curable myeloid leukemia [Citation11]. Currently, many studies have demonstrated that the anthracyclines for patients with de novo APL is safe and effective [Citation12,Citation13]. Therefore, to evaluate the safety and efficacy of different doses of anthracyclines in the induction treatment of newly diagnosed APL, we designed this clinical research.
DS remains as an important adverse event for APL patients during induction therapy. Previous researches had described an incidence of DS ranging from 0.75% to 31% in patients with induction therapy [Citation6,Citation12,Citation14,Citation15]. The GIMEMA group [Citation14], using the ATRA and idarubicin (AIDA regimen), reported the low incidence of DS of 0.75% (6 of 797 patients), but their definition of DS was notably different. DS was defined as ‘definitely present’ in the presence of all the following 5 of the 7 signs and symptoms as originally described by Frankel et al. [Citation16]. Pau Montesinos et al. [Citation6] analyzed patients developing DS in a large series of patients with newly diagnosed APL treated with AIDA regimen who were enrolled in 2 consecutive studies by the PETHEMA Group (LPA96 and LPA99). The classification of the DS in this study was the same with ours, and 183 of 739 patients (24.8%) experienced DS. The incidence of DS was similar to ours. No significant difference of the rate of DS was found between low-dose group and standard-dose group in our study. Our study suggested that reducing the dose of anthracycline may not increase the rate of DS.
Myelotoxicity is a major concern in treatment of leukemia. In three consecutive PETHEMA trials (LPA96 & LPA99 & LPA2005), D Martínez-Cuadrón et al. [Citation17] reported that the mean days of grade 4 neutropenia duration was 21/20/22 days and the mean days of grade 3–4 thrombocytopenia duration was 19/19/20 days, respectively. Other study had described an incidence of grade 3–4 neutropenia (lasting > 15 days) ranging from 35% to 64%, while the incidence of grade 3–4 thrombocytopenia (lasting >15 days) ranging from 38% to 62% [Citation18]. However, in our study, the median time of grade 4 neutropenia duration and the median time of 3–4 thrombocytopenia duration in the low-dose group was shorter compared to those with standard-dose and even substantially shorter than the other group in previous report [Citation17]. In the above report, the durations of neutropenia and thrombocytopenia suppression were defined as the duration of suppression during the entire induction treatment. However, in our study, the median time for neutrophil and platelet recovery were defined as the duration of time after anthracycline discontinuation. Blood routine during the application of anthracyclines was not included in the statistical analysis. The shorter time of hematologic toxicity duration in our study, especially in the low-dose group, may be explained by reducing doses of anthracyclines.
A randomized phase 3 trial comparing ATRA-ATO to the oral ATO realgar-Indigo naturalis formula (RIF)-ATRA protocol and involving several China cooperative groups had shown that the volumes of RBC transfusions were 3 units in the RIF-ATRA group versus 4 in the ATO-ATRA group; while the volumes of platelet transfusions were 3 units in the RIF-ATRA group versus 3 in the ATO-ATRA group [Citation19]. This randomized phase 3 trial only included non-high-risk, and a WHO performance status of 2 or less APL, which may have contributed to the low volumes of RBC and platelet transfusions. Other larger study (MRC AML15 trial) [Citation20] also had shown that the mean volumes of RBC transfusions were 11.4 units in the Spanish arm and 11.1 units in the MRC (Medical Research Council) arm. The mean volumes of platelet transfusions were, respectively, 14.0 units in the MRC arm and 17.4 units in the Spanish arm. The chemotherapeutic drugs in the induction protocol in this clinical trial were daunorubicin, cytarabine combined with etoposide, or idarubicin (IDA 12 mg/m2/day, d1–4). The dose of chemotherapy drugs was higher than that of our study, which may be the cause for the higher blood transfusion volume. In our study, low dose anthracyclines were found to be less hematological toxic. This suggests that low doses of anthracyclines are less hematologic toxic in the induction chemotherapy of de novo APL.
In our study, the low-dose group tended to develop less frequently infections (WHO grade ≥ 3). The rate of infection was substantially lower compared to those with standard-dose group, even less than the German AML-Cooperative Group (AMLCG) and the Spanish PETHEMA (LPA99) group [Citation21]. The general risk of infections during induction therapy was enhanced by the dose intensity of the different doses group, in particular by standard-dose anthracycline (IDA 8–12 mg/(m2·day) ×3d) leading to a longer duration of neutropenia, as to be expected [Citation22]. The results further showed that low-dose anthracycline was safer than the standard-dose anthracycline in the treatment of newly diagnosed APL.
The EDR was typically identified as 5%−10% in most studies of APL [Citation12,Citation23–25]. Our research results showed that the EDR was 7.9%, which was consistent with the most previous studies. A multicenter, non-inferiority, open-label, randomized, controlled phase 3 trial study carried out in China in 105 patients reported the 2-year EFS was 97% (67/69) in the RIF (oral arsenic realgar-Indigo naturalis formula)-ATRA group and 94% (34/36) in the ATO-ATRA group[Citation19]. Another important study by Sanz et al. [Citation22] showed that the 3 year OS was 89% and the 3 year DFS was 94% in the PETHEMA LPA2005 trial. Our study demonstrated that the 2-year DFS in the low-dose group was similar with the standard-dose group as well as the previous studies with patients treated with ATRA-ATO or ATRA-ATO-idarubicin [Citation12,Citation18,Citation25–27]. Moreover, the 2-year probabilities of OS were also similar between the low-dose group and the standard-dose group. Our data was in consistent with most recent studies regimens [Citation12,Citation18,Citation26]. There was no significant difference of the 2-year OS and the 2-year DFS between the low-dose group and standard-dose group, however, the severity of complications including infection and myelosuppression in low-dose group was less than in standard-dose group, in addition, the median blood transfusion volume was decreased. Therefore, low dose anthracycline regimen can decrease the treatment complications without impacting the prognosis of APL patients.
In conclusion, our data demonstrates that the induction therapy regimen of ATRA and ATO plus low dose anthracycline can decrease the treatment complications compared with ATRA and ATO plus standard dose anthracycline regimen in APL patients, and prognosis of APL patients treated with low dose anthracycline were comparable at least with prognosis of standard dose anthracycline. However, definitive conclusions cannot be drawn based on the small number of patients included in our study, so more studies are necessary to confirm our findings.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cull EH, Altman JK. Contemporary treatment of APL. Curr Hematol Malig Repo. 2014;9:193–201.
- Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015;5:e304.
- Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133:1630–1643.
- Lion T, van Dongen JJ, Macintyre E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)-a Europe against cancer program. Leukemia. 2003;17:2474–2486.
- Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28:2–7.
- Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775–783.
- Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572.
- Head D, Kopecky K J, Weick J, et al. Effect of aggressive daunomycin therapy on survival in acute promyelocytic leukemia. Blood. 1995;86:1717–1728.
- Avvisati G, Petti MC, LO Coco F, et al. Induction therapy with idarubicin alone significantly influences event-free survival duration in patients with newly diagnosed hypergranular acute promyelocytic leukemia: final results of the GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up. Blood. 2002;100:3141–3146.
- Mcculloch D, Brown C, Iland H. Retinoic acid and arsenic trioxide in the treatment of acute promyelocytic leukemia: current perspectives. Onco Targets Ther. 2017;10:1585–1601.
- Sanz M A, Iacoboni G, Montesinos P. Acute promyelocytic leukemia: do we have a new front-line standard of treatment? Curr Oncol Rep. 2013;15(5):445–449.
- Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120:1570–1580.
- Liu CC, Hua W, Wang WD, et al. Consolidation therapy of arsenic trioxide alternated with chemotherapy achieves remarkable efficacy in newly diagnosed acute promyelocytic leukemia. Onco Targets Ther. 2015;8:3297–3303.
- Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716–4725.
- Rogers J E, Yang D. Differentiation syndrome in patients with acute promyelocytic leukemia. J Oncol Pharm Pract. 2012;18(1):109–114.
- Frankel SR, Eardley A, Lauwers G, et al. The ‘retinoic acid syndrome’ in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292–296.
- Martínez-Cuadrón D, Montesinos P, Vellenga E, et al. Long-term outcome of older patients with newly diagnosed de novo acute promyelocytic leukemia treated with ATRA plus anthracycline-based therapy. Leukemia. 2018;32(1):21–29.
- Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non–high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35:605–612.
- Zhu H-H, Wu D-P, Du X, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19:871–879.
- Burnett AK, Hills RK, Grimwade D, et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. Leukemia. 2013;27:843–851.
- Eva L, Dennis G, Daniel N, et al. Frontline therapy of acute promyelocytic leukemia: randomized comparison of ATRA and intensified chemotherapy versus ATRA and anthracyclines. Eur J Haematol. 2018;100:154–162.
- Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115:5137–5146.
- Fenaux P, Chevret S, Guerci A, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. Leukemia. 2000;14:1371–1377.
- Hongming Z, Jiong H, Li C, et al. The 12-year follow-up of survival, chronic adverse effects, and retention of arsenic in patients with acute promyelocytic leukemia. Blood. 2016;128:1525–1528.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121.
- Song X, Hu X, Lü S, et al. Incorporation of arsenic trioxide in induction therapy improves survival of patients with newly diagnosed acute promyelocytic leukaemia. Eur J Haematol. 2014;93:54–62.
- Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;4:1237–1243
