ABSTRACT
Objective
To investigate the impact of minimal residual disease (MRD) before allogeneic hematopoietic stem cell transplantation (allo-HSCT) on the outcome of acute leukemia.
Methods
Data from 114 patients who were diagnosed with acute leukemia (AL) and underwent allo-HSCT between Jan 2013 and Dec 2019 were collected and analyzed. The patients were attributed into MRD positive (MRD+) group and MRD negative (MRD−) group.
Results
Among the 114 acute leukemia patients, there were 32 MRD+ patients before transplantation, and 82 MRD− patients. No significant difference was found between the MRD+ group and the MRD− group in the incidence of acute graft-versus-host disease (aGvHD) (p = 0.09). Compared with the MRD+ group, the MRD− group had a higher incidence of chronic graft-versus-host disease (cGvHD) (p = 0.008). There is no significance in relapse between the two groups (p = 0.084), while the incidence of relapse was seemingly higher in the MRD+ group: 36.9% Vs 19.7% . We attributed to the lack of sample size and NRM in MRD+ group was remarkably higher. The MRD+ group had significantly worse one-year overall survival (OS) ( , p = 0.003) and one-year progression-free survival (PFS) (, p = 0.009). In the multivariate analysis, MRD+ was an independent prognostic factor for OS (HR = 1.898; 95%CI 1.042–3.457; p = 0.036).
Conclusion
Pre-transplantation MRD positive status is a risk factor for survival and prognosis after HSCT. Upon this, emphasis should be put on (1) screening more efficient chemo regimen with targeted agents, to help patients reach and keep MRD− status before transplantation; (2) designing better management with different GvHD prophylaxis treatment, timely disease monitoring and preemptive intervention on relapse.
Introduction
Acute leukemia (AL) represents a heterogeneous group of hematological malignancies with varying clinical, morphological, immunological and molecular characteristics [Citation1]. At present, with the widespread use of traditional chemotherapy regimens combined with targeted agents, the prognosis of patients with AL has been improved significantly. But patients with refractory or relapsed AL still have an extremely poor prognosis [Citation2,Citation3]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a crucial treatment option for patients with AL, especially for that primary chemo-refractory disease and early relapse after standard chemotherapy [Citation4,Citation5]. However, even after HSCT relapse is still the major hurdle and leads to poor prognosis.
Minimal residual disease (MRD) refers a state in which traces of leukemia cells could not be detected using morphologic methods when patients in complete remission (CR), while using PCR or flow cytometry leukemia tumor load could still be detected. In recent years, MRD has been widely used in clinical monitoring and an indication of relapse of all kinds of malignant hematological diseases, which has a certain guiding significance for disease risk stratification and chemotherapy scheme selection [Citation6,Citation7]. We retrospectively analyzed the basic clinical characteristics, post-transplantation complications, relapse and survival for 114 patients with AL who underwent allo-HSCT in the department of hematology in Jiangsu Province Hospital, Jiangsu Province P.R. China. The impact of pre-transplantation MRD status on the outcome of allo-HSCT was also addressed.
Materials and methods
Patients
A total of 114 patients with AL who underwent allo-HSCT in the department of hematology in Jiangsu Province Hospital from Jan 2013 to Dec 2019 were enrolled in this study. All patients achieved morphological CR status according to the criteria of NCCN (National comprehensive cancer network) [Citation2] and were arranged to detect MRD status with bone marrow routinely before transplantation. Patients were divided into two groups according to the results of the MRD test before transplantation, including the MRD positive group (MRD+) and MRD the negative group (MRD−). This study was approved by the Medical Ethical Committee of the First Affiliated Hospital of Nanjing Medical University.
Detection of MRD
At diagnosis of AL, leukemia-associated aberrant immunophenotypes (LAIP) were identified by multiparametric flow cytometry (MFC) and all patients had at least one useful phenotype for follow-up throughout leukemia treatment. All patients underwent bone marrow aspiration within one month before transplantation and 2 mL Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulant bone marrow samples were collected for MRD detection by flow cytometry. The following monoclonal antibodies were used to track LAIP by eight-color MFC: CD2, CD3, CD7, CD5, CD13, CD14, CD15, CD19, CD33, CD34, CD10, CD20, CD45, CD61, CD22, CD117 and HLA-DR. Flow cytometry and fluorescent labeled antibodies were produced by BD Biosciences company (Becton Dickinson, Franklin Lakes, NJ, USA). Hemolysin and cleaning solution were purchased from Beckman Company in Germany. CellQuest software (BD Biosciences) was used for data analysis. At least 5 × 104 events were measured with each sample. MRD ≥ 0.01% was defined as positive (MRD+) and MR < 0.01% was considered as MRD negative (MRD−).
Conditioning regimens
All of the patients enrolled were fit for the myeloablative conditioning regimen and the standard Bu/Cy regimen was chosen as busulfan 3.2 mg kg−1·day−1 (from day -7 to -4) combined with cyclophosphamide 60 mg kg−1·day−1 (day -3 to -2). The patient will not receive any chemical toxic therapy and have a rest on day 1. The stem cells were infused intravenously on day 0. The minimum cell counts are defined as 5 × 108/Kg for total nucleated cell (TNC) and 2 × 106/Kg for CD34+ cell.
Evaluation of the engraftment and chimerism
The definition for neutrophil engraftment is neutrophil counts more than 0.5 × 109/L in consecutive 3 days, and the platelet engraftment is considered as platelet counts exceed 20 × 109/L without platelet transfusion in consecutive 7 days. Bone marrow aspiration was routinely performed on day 30, 60, 90, 180, 270 and 360 after the HSCT. Hematopoietic chimerism was identified by sex chromosome determination using the FISH (fluorescence in situ hybridization) method if the donor and recipient were sex-mismatched or short tandem repeats (STR) by PCR (polymerase chain reaction) technique if they were in the same gender group.
Immunosuppression and management
The prophylaxis treatment of graft-versus-host disease (GvHD) for matched sibling donors (MSD) is mainly based on cyclosporine A (minimum concentration ranging from 200–400 ng/mL) combined with short course methotrexate (methotrexate 15 mg/m2 on day 1 and 10 mg/m2 for day 3, 6 and 11). The strategies for haploidentical stem cell transplantation include cyclosporine A, short-term methotrexate, mycophenolate mofetil, basiliximab and anti-thymocyte globulin (ATG, Rabbit Anti-human Thymocyte Globuli, Genzyme Polyclonals S.A.S), in which the doses of cyclosporine and methotrexate are as same as MSD transplantation, and ATG was administrated at 2.5 mg/kg on day -5 to -2, basiliximab 20 mg on day 0, and the oral mycophenolate mofetil (MMF) dose was 500 mg twice a day on day -1 and was tapered off after 28 days if no acute graft-versus-host disease (aGvHD) was observed. We graded each aGvHD patient using the MAGIC consortium [Citation8].
Supportive care
All patients received central venous catheters and were isolated in a laminar airflow room. Empirical antibiotics and anti-fungal agents were administered to the patients when they experienced fever. The cytomegalovirus (CMV) and Epstein–Barr virus (EBV) were measured weekly during the immunosuppressive period and the patients were administered with gancyclovir when CMV was positive. Trimethoprim/sulfamethoxazole was given for the prevention of pneumocystis carinii infection. Irradiated blood components were infused while needed to maintain the hemoglobin above 70 g/L and platelet counts over 20 × 109/L. The subcutaneous G-CSF (5 μg kg−1·day−1) was administered to all recipients from the day 5 after transplantation until neutrophil recovery. All patients were transfused with 10 g of gamma-immunoglobulin each week from transplantation day to 3 months after transplantation.
Statistical analysis
The research data were processed by Statistical Product and Service Solutions (SPSS) (Version 26.0) software package. The measurement data were tested by an independent sample t-test, and the rates were compared by the chi-square test. Kaplan–Meier method was used to draw the survival curve, and Log-Rank was used to test whether there was a statistical difference between the curves. The difference was statistically significant (p < 0.05). Overall survival (OS) is defined as the time from post-transplant to death and progression-free survival (PFS) is considered as the time when the disease progressed for the first time or died of any cause after transplantation.
Results
Patient characteristics
Among the 114 patients with AL, the median age was 39 years old (range from 11y to 60y). The number of ALL and AML patients was 43 (37.7%) and 71 (62.3%) separately. Ninety- seven (85.1%) cases were in the first complete remission (CR1) status and the other 17 (14.9%) cases were in the second complete remission or the third remission (CR≥2) status. A total of 64 patients underwent haploidentical transplantation, and 50 patients experienced MSD transplantation. The comparative results for patient, disease and transplant-related characteristics between the two groups were summarized in .
Table 1. Clinical characteristics of 114 patients before transplantation.
Stem cell engraftment
In the MRD+ group, neutrophil engraftment was successful in all patients, while two patients failed to get platelet engraftment. The median engraft time of neutrophils and platelets were 12 days (9–22 days) and 13 days (10–32 days), respectively. In the MRD− group, there were one patient and 6 patients failed in neutrophil and platelet engraftment, respectively. The median engraft time were 14 days (8–25 days) for neutrophils, and 15 days (5–35 days) for platelets. No significant difference was found in neutrophil engraftment time and platelet engraftment time between the MRD+ group and MRD− group (p > 0.05) ().
Table 2. Comparison of observation indexes after transplantation.
Evaluation of early complications after transplantation
A total of 64 (56.1%) patients developed a GvHD, and 35 (30.7%) patients suffered from chronic graft-versus-host disease (cGvHD). In the MRD+ group, there were 22 (68.8%) cases of aGvHD, including 4 cases of grade III-IV aGvHD. In the MRD− group, there were 42 (51.2%) cases of aGvHD, including 12 cases of grade Ш-Ⅳ aGvHD. The cumulative incidence of grade Ш-Ⅳ acute GvHD was 14.6% in the MRD− group, 12.5% in the MRD+ group (p = 0.78). However, there was a significant difference in the incidence of cGvHD between the two groups (12.5% in MRD+ group Vs 37.8% in MRD− group, p = 0.008) ().
Prognostic impact of MRD
Of the 32 patients in the MRD+ group, 21 patients died and 11 patients survived during the follow-up period. Among all the 21 mortality, 9 patients died of relapse and 12 patients died of non-relapse causes. 27 out of 82 cases in the MRD− group were dead, including 9 patients died of relapse. The one-year OS of the MRD− group and MRD+ group were 70.2% and 46.9%, respectively; and the one-year PFS was 64.3% and 43.8%, respectively. There was a significant difference in OS between the two groups (p = 0.003, (A)). And the same result was found in PFS. (p = 0.009, (B).
Figure 1. Kaplan–Meier curves of (A) overall survival (OS), (B) progression-free survival (PFS), (C) cumulative incidence of relapse between the MRD+ group and MRD− group.

In the MRD+ group, 10 patients relapsed after transplantation, of which 9 patients died after relapse, and one patient survived during the follow-up period. The one-year cumulative relapse rate was 25.2% and the 3-year cumulative relapse rate was 36.9%. In the MRD− group, 14 patients relapsed after transplantation, of which 9 patients died after relapse, and 5 patients survived during the follow-up period. The 1-year cumulative relapse rate was 17.9% and the 3-year cumulative relapse rate was 19.7%. No significant difference was found in the cumulative relapse rate between the two groups (p = 0.084, (C). The cumulative incidence of NRM in the MRD− group did differ from that in the MRD+ group (20.5% in MRD− group Vs 39.7% in MRD+ group, p = 0.046, (A).
Figure 2. Kaplan–Meier curves of (A) non-relapse mortality (NRM), (B) GVHD/Relapse-Free survival (GRFS) between the MRD+ group and MRD− group.
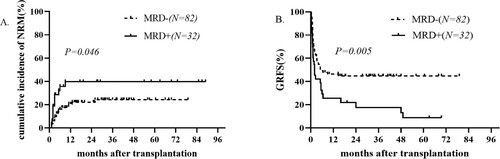
Graft-versus-host disease-free, relapse-free survival (GRFS) in the MRD+ group and MRD− group did differ significantly (46.4% in MRD− group Vs 26.5% in MRD+ group, p = 0.005, (B).
Multivariate analysis of the prognostic impact of MRD
The COX regression model was used to analyze the related factors of OS. Univariate Cox regression analysis revealed that age >50y, MRD+, relapse and Haplo-HSCT were associated with higher mortality. Upon multivariable adjustment, age>50y (HR = 2.254; 95%CI 1.203–4.222; p = 0.011), MRD + (HR=1.898; 95%CI 1.042–3.457; p = 0.036), relapse(HR = 2.447; 95%CI 1.320–4.535; p = 0.004) and Haplo-HSCT(HR = 2.358; 95%CI 1.270–4.376; p = 0.007) were significantly associated with poor survival ().
Table 3. Analysis of risk factors for OS.
Discussion
Acute leukemia is a common kind of hematological malignant tumor. Although low-risk patients can obtain a longer survival time and good prognosis from intensive chemotherapy, the only choice for high-risk patients to prolong survival time is HSCT. Relapse is the major reason that limits the efficacy of HSCT and leads to the failure of transplantation. Residual leukemia cells in patients are the main cause of disease relapse. The OS and PFS for no remission (NR) patients are significantly lower than those for CR patients, and the cumulative relapse rate is also significantly higher than that of remission patients [Citation9,Citation10]. However, even if the patient is in CR status, the MRD may be detected in some patients. MRD can be detected by flow cytometry (FCM), polymerase chain reaction (PCR), real-time quantitative polymerase chain reaction (RQ-PCR), reverse transcriptase polymerase chain reaction (RT–PCR) or next generation sequencing (NGS) [Citation11,Citation12]. The detection of MRD is critical for predicting the outcome of transplantation and for selecting the intensity of further treatment strategy [Citation13–15].
In our study, no significance in the incidence of aGvHD (p = 0.090) between MRD + and MRD− group. But there was a significant difference in the incidence of cGvHD between the two groups (p = 0.008). It might be due to the longer OS in the MRD− group compared with the MRD+ group.The median OS of the MRD+ group was significantly shorter than that of the MRD− group (p = 0.003). The results of the COX multivariate survival analysis showed that the survival risk of MRD− was lower than that of MRD+ (p = 0.032). This is similar to the results of Liu et al., who retrospectively analyzed 79 AL patients with allo-HSCT when they got CR. According to the pre-transplant MRD status, they were divided into 49 MRD+ patients and 30 MRD− patients. There was a significant difference in 3-year OS between MRD positive group and MRD negative group. The results of the univariate analysis showed that the leukemia-free survival time of the MRD positive group was lower than that of the MRD negative group (10.5% in MRD+ group Vs 36.2% in MRD− group) (p < 0.001) [Citation16]. At the same time, other studies also supported our results that the outcome of patients with MRD positive before transplantation is significantly worse than that of patients with MRD negative, mainly characterized by the decrease of PFS and OS [Citation17–19]. The results of Salhotra et al. [Citation20] also showed that the outcome of the MRD positive group was worse than that of the MRD negative group in ALL patients before transplantation. This suggests that we should improve the monitoring of MRD and adjust the treatment plan for patients with ALL as soon as possible. In our study, the PFS and OS of the MRD+ group were also significantly lower than that of the MRD− group, which were the same as the previous study.
In our study, MRD ≥0.01% is considered as MRD positive, while in the J Sanchez-Garcia study [Citation21], MRD was divided into three groups: negative group (<0.01%), low-level group (0.01–0.1%) and high-level group (greater than 0.1%). The results showed that the high-level group had the worst prognosis, while the low-level group had a chance of long-term remission, and the prognosis was better than the high-level group. Federica Lovisa also confirmed the result [Citation22]. Therefore, we speculate that there were some low levels in the MRD positive group in this study, which might prolong the PFS in the MRD+ group.
All of these studies suggested that MRD is indeed an important factor in relapse and survival, the higher level of MRD, might be a sign of worse prognosis. It is necessary to continue chemotherapy for MRD high-level positive patients to make them negative before transplantation, or could we ignore the low level of MRD since it does not affect OS and PFS? For some refractory cases, MRD+ might be the best statue before treatment, they may have no time for further chemotherapy before transplantation. Thus for these high-risk MRD+ patients, we could also design better management with different GvHD prophylaxis treatment, timely disease monitoring and preemptive intervention on relapse. This is still a controversial issue and more objectives are needed to expand this study.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81470329), National Public Health Grand Research Foundation (No.201202017), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (No. JX10231801), Program for Development of Innovative Research Teams in the First Affiliated Hospital of Nanjing Medical University, Project of National Key Clinical Specialty, National Science & Technology Pillar Program (No.2014BAI09B12) and Project Funded by Jiangsu Provincial Special Program of Medical Science (No.BL2014086).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brunning RD. Classification of acute leukemias. Semin Diagn Pathol. 2003;20(3):142–153.
- Brown PA, Shah B, Fathi A, et al. NCCN guidelines insights: acute lymphoblastic leukemia, Version 1.2017. J Natl Compr Canc Netw. 2017;15(9):1091–1102.
- Wang Y, Liu Q-F, Wu D-P, et al. Impact of prophylactic/preemptive donor lymphocyte infusion and intensified conditioning for relapsed/refractory leukemia: a real-world study. Sci China Life Sci. 2020;63(10):1552–1564.
- Su XH, Yao JF, Zhang GX, et al. Allogeneic hematopoietic stem cell transplantation for treatment of refractory and relapsed acute myeloid leukemia: outcomes and prognostic factors. Zhonghua Xue Ye Xue Za Zhi. 2017;38(12):1024–1030.
- Yoo SH, Koh Y, Kim D-Y, et al. Salvage therapy for acute chemorefractory leukemia by allogeneic stem cell transplantation: the Korean experience. Ann Hematol. 2017;96(4):605–615.
- Yalniz FF, Patel KP, Bashir Q, et al. Significance of minimal residual disease monitoring by real-time quantitative polymerase chain reaction in core binding factor acute myeloid leukemia for transplantation outcomes. Cancer. 2020;126(10):2183–2192.
- Juul-Dam KL, Ommen HB, Nyvold CG, et al. Measurable residual disease assessment by qPCR in peripheral blood is an informative tool for disease surveillance in childhood acute myeloid leukaemia. Br J Haematol. 2020;190(2):198–208.
- Harris AC, Young R, Devine S, et al. International, Multicenter Standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10.
- Inagaki J, Fukano R, Noguchi M, et al. Hematopoietic stem cell transplantation following unsuccessful salvage treatment for relapsed acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2015;62(4):674–679.
- Bourlon C, Lacayo-Leñero D, Inclán-Alarcón SI, et al. Hematopoietic stem cell transplantation for adult Philadelphia-negative acute lymphoblastic leukemia in the first complete remission in the era of minimal residual disease. Curr Oncol Rep. 2018;20(4):36.
- Rustad EH, Boyle EM. Monitoring minimal residual disease in the bone marrow using next generation sequencing. Best Pract Res Clin Haematol. 2020;33(1):101149.
- Rastogi P, Sachdeva M. Flow cytometric minimal residual disease analysis in acute leukemia: current status. Indian J Hematol Blood Transfus. 2020;36(1):3–15.
- Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease With clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580.
- Zhao X, Wang Z, Ruan G, et al. Impact of pre-transplantation minimal residual disease determined by multiparameter flow cytometry on the outcome of AML patients with FLT3-ITD after allogeneic stem cell transplantation. Ann Hematol. 2018;97(6):967–975.
- Bader P, Hancock J, Kreyenberg H, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16(9):1668–1672.
- Liu C, Liu L, Chen J-B, et al. [Clinical significance of minimal residual disease monitoring by multi-parameter flow cytometry before allogeneic hematopoietic stem cell transplantation for prognosis of acute leukemia patients]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28(1):262–266.
- Bader P, Salzmann-Manrique E, Balduzzi A, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3(21):3393–3405.
- Gratwohl A, Duarte R, Snowden JA, et al. Pre-transplantation Risks and transplant-techniques in haematopoietic stem cell transplantation for acute leukaemia. EClinicalMedicine. 2019;15:33–41.
- Zhao H, Wei J, Wei G, et al. Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: a multi-center retrospective study. J Hematol Oncol. 2020;13(1):42.
- Salhotra A, Yang D, Mokhtari S, et al. Outcomes of allogeneic hematopoietic cell transplantation after Salvage Therapy with Blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2020;26(6):1084–1090.
- Sanchez-Garcia J, Serrano J, Serrano-Lopez J, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant. 2013;48(3):396–402.
- Lovisa F, Zecca M, Rossi B, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol. 2018;180(5):680–693.
