ABSTRACT
Objective
The aim of this study was to investigate the data of HSCT patients who were admitted to our Hematology ICU due to infections or infectious complications.
Materials and Methods
HSCT patients who were admitted to our Hematology ICU between 01 January 2014 and 01 September 2017 were analyzed retrospectively.
Results
62 HSCT patients were included in this study. The median age was 55.5 years and 58% of the patients were allogeneic HSCT patients. Major underlying hematologic disorders were multiple myeloma (29%) and lymphoma (27.4%). The most common reasons for ICU admission were sepsis/septic shock (61.3%) and acute respiratory failure (54.8%). Overall ICU mortality rate was 45.2%. However, a lot of factors were related with ICU mortality of HSCT patients in univariate analysis, only APACHE II score was found to be an independent risk factor for ICU mortality. While there was infection in 58 patients at ICU admission, new infections developed in 38 patients during ICU stay. The most common new infection was pneumonia/VAP, while the most frequently isolated bacteria were Acinetobacter baumannii. Length of ICU stay, sepsis/septic shock as a reason for ICU admission and the presence of urinary catheter at ICU admission were determined factors for ICU-acquired infections. There was no difference between autologous and allogeneic stem cell transplant patients in terms of ICU morbidities and mortality. However, pneumonia/VAP developed in the ICU was higher in autologous HSCT patients, while bloodstream/catheter-related bloodstream infection was higher in allogeneic HSCT patients.
Conclusion
It was concluded that early or late post-HSCT infections and related complications (sepsis, organ failure, etc.) constituted a major part of the reasons for ICU admission, ICU mortality and ICU morbidities.
Introduction
Hematopoietic stem cell transplantation (HSCT) is currently a treatment modality for patients with certain solid cancers or hematological malignancies, and many congenital or acquired hematopoietic system diseases [Citation1,Citation2]. The success of HSCT has improved dramatically with the advances in transplantation technologies in the last two decades, leading to a growing number of patients having access to HSCT. However, HSCT is associated with multiple infectious and non-infectious complications that often lead to organ failures resulting in admission to the intensive care units (ICUs). Although the rate of admission to the ICU in this patient group varies, the average rate is around 16% (5–55%) [Citation3–6]. However, the mortality rate is high (around 50%) in HSCT patients when ICU admission is required [Citation3–6].
Underlying disorder, stem cell source, conditioning regimen, previous therapies, additional comorbidities, development of HSCT-related complications such as sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) or graft-versus-host disease (GVHD, acute or chronic), and immunosuppressive therapies are the reasons leading to these serious clinical conditions that require ICU support [Citation3–8]. Infectious complications are responsible for the major part of the morbidities and mortality after HSCT. While neutropenia and mucositis are significant risk factors for infection during the pre-engraftment period after transplantation, T-lymphocyte-mediated immunity and opsonization deficiency become more important at early and late post-engraftment periods. Therefore, it is critical to determine the possible infectious agents and antimicrobial susceptibilities during these periods to start appropriate antimicrobial therapies, and to decide the prognosis in this patient group with severely suppressed immune system [Citation9–11].
There are some studies on the indications of ICU admission and prognostic factors after ICU admission in this patient population; however, there is the current literature does not provide profound research on infections, which are the main reason for morbidity and mortality in critically ill HSCT recipients. Therefore, in this study, we aimed to investigate the infections and infectious complications in addition to prognosis of HSCT patients who were hospitalized in our Hematology ICU.
Materials and methods
Setting and patient selection
This retrospective study was conducted at the adult Hematology ICU of Gazi University Hospital, a tertiary care academic center with more than 1000 beds. The four-bed adult Hematology ICU, working on the principle of a closed unit, is managed by a full-time intensive care physician. In this unit, senior internal medicine residents are available 24 h a day, 7 days a week, and physicians from different disciplines can give consultation services at any time when needed. Hematological patients with a potentially favorable long-term prognosis (≥3 months) and all HSCT patients are admitted to this unit in case of life-threatening medical conditions (severe sepsis/septic shock, acute severe respiratory failure, etc.). All adult (≥18 years of age) HSCT recipients admitted to this ICU between 01 January 2014 and 01 September 2017 were included in this study. Patients who did not survive more than 24 h following ICU admission and those who had more than one HSCT procedure were excluded. For patients readmitted to ICU, only the first admission data were included.
Patients data
Demographic and laboratory data were obtained from the electronic hospital database and medical files of the patients. Patients’ age, sex, comorbidities, cause of ICU admission, detailed information about underlying hematological diseases, procedure and complications of HSCT, the current state of immunosuppressive therapy, clinical and microbiological data about previous and ICU-acquired infections, data about supportive therapies, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, Glasgow coma scale (GCS), the requirement of mechanical ventilation support, presence of shock, requirement of hemodialysis (continuous renal replacement therapy or intermittent hemodialysis), biochemical parameters, length of ICU stay, other ICU morbidities and ICU mortality were recorded. Admission GCS, APACHE II and SOFA scores were calculated within 24 h of ICU admission. Infections detected 48 h or later than ICU admission in a different focus or with a different infectious agent from the first focus were considered as ICU-acquired infections. Sepsis and septic shock were defined and diagnosed according to recent guidelines [Citation12]. Diagnosis of infections was based on clinical, laboratory, radiological, and/or microbiological evidence, in accordance with published international consensus guidelines for the diagnosis of infections [Citation13–18]. Acute respiratory distress syndrome (ARDS) was diagnosed according to 2012 Berlin definition [Citation19]. Acute kidney injury was defined according to RIFLE (Risk, Injury, Failure, Lost and End-stage renal disease) criteria [Citation20].
Statistical analysis
Statistical analyses were performed using the IBM SPSS (Statistical Package for Social Sciences) statistical software version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were reported as medians [interquartile ranges]. Frequency and percentage values were used for the presentation of categorical variables. Then, patients were separated into groups as survivors or non-survivors, patients with or without ICU-acquired infections, and allogeneic or autologous HSCT patients for statistical comparison. The Mann–Whitney U test was used to compare the medians of continuous variables, and the χ2 (chi-squared) and Fisher’s exact test were used to compare categorical variables. Logistic regression analysis was used to determine independent risk factors for ICU mortality. After the determination of independent risk factors for mortality, ROC (Receiver Operating Characteristic) curve analyses were performed. P values lower than 0.05 were considered as statistically significant.
This retrospective observational clinical study was approved by Gazi University Local Ethics Committee on 11 September 2017, numbered 407.
Results
HSCT patients admitted to Hematology ICU of Gazi University Hospital between 01 January 2014 and 01 September 2017 were identified. For eight patients with more than one admission to ICU, the first admission data were included. Four patients who died within the first 24 h of ICU admission and six patients who had more than one HSCT procedure were excluded. As a result, a total of 62 patients were included. Thirty-eight (61.3%) patients were male and the median age of the patients was 55.5 [34.25–62] years. The most common hematological diseases presented as indications for HSCT were multiple myeloma in 18 (29%), lymphoma in 17 (27.4%) and acute myeloid leukemia in 14 (22.6%) patients. About 26 (41.9%) patients received autologous stem cell transplantation and 36 (58.1%) patients received allogeneic stem cell transplantation. Peripheral stem cell sources were used in all stem cell transplantations. The conditioning regimens were myeloablative in 25 (40.3%), non-myeloablative in 13 (21%) and reduced intensity in 24 (38.7%) patients. Total body irradiation (TBI) was performed in eight patients in the myeloablative regimen group. The disease stages were progression in 26 (41.9%) patients, remission in 20 (32.3%) and stable disease in 16 (25.8%) at the time of transplantation.
The median interval between HSCT and ICU admission was 189.5 [16.5–826] days. The common causes of ICU admission were sepsis/septic shock (61.3%), acute respiratory failure (54.8%), altered mental status (25.8%), and acute renal failure (19.4%). Complications related to conditioning regimen and HSCT procedure were neutropenia (50%), mucositis (45.2%), GVHD (32.3%) and VOD (3.2%) at the time of ICU admission. Engraftment occurred in 10 HSCT patients during ICU stay. Thirty-four patients (54.8%) received immunosuppressive treatment during ICU admission and the most common drugs were steroid (35.5%) and cyclosporine (33.3%). Also, 10 patients (16.1%) received invasive mechanical ventilation support, 22 patients (35.5%) received non-invasive mechanical ventilation support and 18 patients (29%) received vasopressor support, 32 patients (51.6%) had a central venous catheter, and 41 patients (66.1%) had urinary catheter at the time of ICU admission. Some of the baseline prognostic and organ failure assessment scores (such as APACHE II and SOFA scores, GCS, and RIFLE criteria) are shown in .
Table 1. Severity and organ failure scores, and infection data in HSCT recipients admitted to Hematology ICU.
Based on clinical, laboratory and/or imaging methods, infection was not detected in four patients and there was a suspicion of infection in 19 patients at ICU admission. There was a microbiologically proven infection in 39 patients before or during ICU admission. Antimicrobials (due to prophylactic, empiric, or definite indications) were started before ICU admission in 60 HSCT patients (96.7%). Data on infections is given in .
New infections were detected in 38 patients (61.3%) during ICU stay. The most common infection was nosocomial pneumonia/VAP in 20 patients (32.3%). The microorganisms isolated from the patients’ sputum, endotracheal aspirate (ETA), or bronchoalveolar lavage (BAL) cultures were Acinetobacter baumannii in seven patients, Candida spp. in seven, Klebsiella pneumonia in three, Pseudomonas aeruginosa in three, Escherichia coli in one, and Stenotrophomonas maltophilia in one. CMV replication (detected by Polymerase chain reaction (PCR)-based assays) and galactomannan positivity was detected in the blood and BAL specimens of two patients. Since carbapenemase-producing gram-negative bacteria (Acinetobacter, Pseudomonas and Klebsiella) rates are high in our hospital, polymyxin E (colistin) or polymyxin B was started as antibacterial agents in Acinetobacter, Pseudomonas and Klebsiella infections.
Bloodstream/catheter-related bloodstream infections were documented in 16 patients (25.8%) during ICU stay. Isolated microorganisms were coagulase-negative Staphylococci in eleven patients, Candida spp. in four, Pseudomonas aeruginosa in two, methicillin-resistant Staphylococcus aureus in one, Klebsiella pneumonia in one, Enterococcus spp. in one, Corynebacterium spp. in one, and Stenotrophomonas maltophilia in one. According to our hospital infection protocol for HSCT patients, daptomycin was started in bloodstream/catheter-related bloodstream infections caused by Staphylococci and the catheter was removed if needed.
Urinary tract/catheter-associated urinary tract infections were documented in 10 patients (16.1%) during ICU stay. Isolated microorganisms from urinary cultures were Candida spp. in five patients, Escherichia coli in two, Klebsiella spp. in two, Enterococcus spp. in two, and Streptococcus spp. in one.
Various complications developed in HSCT patients during ICU stay. These complications were the requirement of invasive mechanical ventilation support in 31 patients (50%), ARDS development in 8 (12.9%), septic shock in 36 (58.1%), renal dysfunction in 43 (69.4%) (14 of whom required hemodialysis), and intracranial bleeding in 8 (12.9%). The median length of ICU stay was 5 [3–9] days. ICU mortality rate was 45.2% (28 patients).
HSCT patients who died (28 patients) and survived (34 patients) were compared in terms of some characteristics. Demographic characteristics, primary hematological disease, transplantation type, conditioning regimen, primary disease status at the time of HSCT procedure, and reasons for ICU admission were similar between survivors and non-survivors (p > 0.05). Complications related to conditioning regimens and HSCT procedure such as GVHD, VOD and mucositis were similar between survivors and non-survivors, but the rate of neutropenia at ICU admission was significantly higher in non-survivors (p = 0.041). The number of patients treated with cyclosporine was higher in non-survivors (p = 0.021), while other immunosuppressive treatments were similar between the groups ().
Table 2. Some clinical characteristics of HSCT recipients who survived or died at Hematology ICU.
Eight patients in non-survivors and two patients in survivors were under invasive mechanical ventilation support at ICU admission (p = 0.053). The number of patients admitted to the ICU with vasopressor support and central venous catheter was significantly higher in non-survivors. Urinary catheterization rate was similar between survivors and non-survivors ().
APACHE II and SOFA scores, GCS, RIFLE criteria and some laboratory parameters in survivors and non-survivors at ICU admission are given in .
The frequency of urinary tract/catheter-associated urinary tract infections was significantly higher in non-survivors than survivors (p = 0.008), but the frequencies of other infections were similar between these groups at ICU admission. Gram-positive and fungal infections were both statistically more frequent in non-survivors (p < 0.05). The frequency of gram-negative and CMV infections and antimicrobial treatments were similar between the two groups at ICU admission (p > 0.05).
The number of patients who developed new infections during ICU stay was statistically higher in non-survivors than survivors (p = 0.044). Complications other than infections such as new intubation requirement, acute kidney injury and liver dysfunction were also significantly higher in non-survivors than survivors (p < 0.05) ().
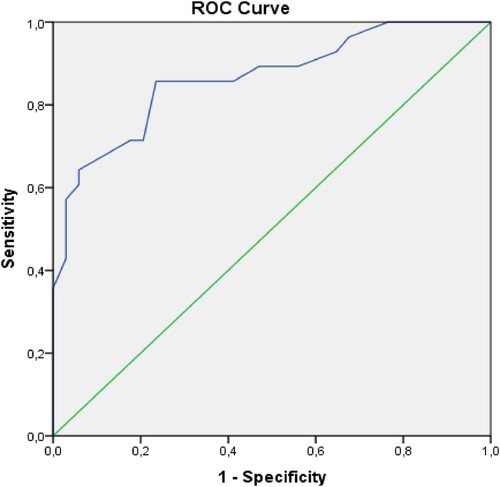
Multivariate analysis was performed on some parameters that were statistically significant in univariate analysis such as the presence of neutropenia at ICU admission, APACHE II and SOFA scores, presence of gram-positive infection and urinary tract/catheter-associated urinary tract infection at ICU admission and new infection development during ICU stay. Only APACHE II score at ICU admission was found as an independent risk factor for ICU mortality (p = 0.017) (). An APACHE II score of greater than 22.5 predicted ICU mortality with 85.7% sensitivity and 76.5% specificity (AUC: 0.864, 0.772–0.957 95%CI, p = 0.0001) ().
Table 3. Independent risk factors for ICU mortality in HSCT patients.
New infections developed in 38 patients (61.3%) during ICU stay. When compared HSCT patients who developed new infections and those who did not, length of ICU stay, sepsis/septic shock as a reason for ICU admission, and the presence of urinary catheter at ICU admission were determined as risk factors for the development of new infections. We were not able to decide precisely on whether prolonged ICU stay was a cause or a consequence of new ICU infections. The transplantation type, the condition of the underlying hematological disease during transplantation, conditioning regimen, the presence of HSCT-related complications such as GVHD, VOD, mucositis, or neutropenia at ICU admission, and immunosuppressive therapies had no effect on the development of new ICU infections. APACHE II and SOFA scores, GCS and RIFLE criteria at ICU admission were similar between groups with and without new ICU infections (p > 0.05). ICU complications other than infections such as new intubation, septic shock and ICU mortality rates were statistically higher in the group with new infections (p < 0.05) ().
Table 4. Clinical characteristics of HSCT recipients with or without new infections during ICU stay.
We thought that it was appropriate to statistically compare autologous and allogeneic stem cell transplant patients, considering that their profiles, treatments, and complications were very different. We also aimed to determine the effects of these two types of stem cell transplantation procedures on infections, ICU mortality, and morbidities (). In our study, autologous stem cell transplant patients were older and mostly multiple myeloma patients. They had more comorbidities, higher creatinine values, and less neutropenia at ICU admission. There was no difference between the two patient groups in terms of morbidities developing in the ICU (kidney injury, septic shock, the requirement of new intubation and invasive mechanical ventilation support, liver dysfunction, etc.). However, pneumonia/VAP developed during ICU stay was higher in autologous HSCT patients, while bloodstream/catheter-related bloodstream infection was higher in allogeneic HSCT patients. There was no difference between the two groups in terms of ICU mortality rates ().
Table 5. Clinical characteristics of allogeneic and autologous HSCT patients at ICU admission and during ICU stay.
Discussion
Every year, about 50,000–60,000 patients undergo HSCT worldwide. Multiple myeloma and lymphoma constitute the most common indications for HSCT, accounting for about half of all cases. Multiple myeloma is the most common indication for autologous transplantation and acute leukemia is the most common for allogeneic transplantation [Citation1,Citation2,Citation21]. We found the most common indications for HSCT as multiple myeloma in 18 (29%) patients and lymphoma in 17 (27.4%). Multiple myeloma was the most common indication (53.8%) for autologous HSCT and acute myeloid leukemia was the most common indication (33.3%) for allogeneic HSCT, consistent with the literature.
Reports suggest that 5–55% of HSCT recipients are admitted to the ICU [Citation22], most commonly due to acute respiratory failure for infectious and non-infectious causes (such as pulmonary edema, diffuse alveolar hemorrhage, etc.) and hemodynamic instability for sepsis and non-sepsis causes (such as hypovolemia, arrhythmia, etc.) [Citation22–24]. In the literature, central nervous manifestations (such as intracranial bleeding, seizure, etc.), hemostatic complications leading to bleeding and thromboembolic events and acute renal failure are also reported to be primary reason for ICU admission [Citation23–25]. We found the most common reason for ICU admission as sepsis/septic shock (61.3%), followed by acute respiratory failure (54.8%). This difference might stem from the various criteria for transferring patients to the ICU in each center. Our criteria mainly include the presence of shock requiring high-dose vasopressor support, respiratory failure requiring invasive mechanical ventilation support, and renal failure requiring continuous renal replacement therapy.
Rates of mortality for HSCT patients admitted to the ICU, particularly allogeneic patients, have decreased from around 80–90% to 20–50% since organ dysfunction, infection, and severe complication outcomes have showed significant improvement [Citation26–30]. Some possible explanations for this improvement include reduced intensity conditioning regimens, more accurate human leukocyte antigen (HLA) typing, more effective antimicrobial prophylaxis, improved patient care, wider use of colony-stimulating factors for neutropenia, more frequent use of autologous transplant, using peripheral blood stem cell for HSCT, earlier use of non-invasive ventilation, lung protective strategies in mechanical ventilation, and proper management of infections, sepsis and organ failures [Citation27–30]. Our ICU mortality rate was 45.2%, consistent with the recent data in the literature.
There are a lot of prognostic factors that may influence the outcome of HSCT recipients admitted to the ICU. Age, functional status and underlying diagnosis in pre-transplant period; disease status at the time of transplantation; conditioning regimen, transplantation type and source of stem cell for transplantation in peri-transplant period; reasons for ICU admission, admission time, type and number of organ failures, and also the severity of critical illness at ICU admission are important factors for prognosis of HSCT recipients in ICU. According to the studies, advanced age, coexisting comorbidities, lower functional status, being allogeneic HSCT recipient, being acute leukemia not in remission during the transplant process, being allogeneic HSCT recipient from unrelated donors, using a stem cell source other than peripheral blood, presence of GVHD, underlying disease relapse, ICU admission during an earlier period following HSCT, presence of pneumonia and gram-negative bacterial infection, respiratory failure requiring invasive mechanical ventilation, ICU admission due to multiorgan failure, sepsis and ARDS, development of ARDS, sepsis and acute kidney injury during ICU stay to determine the prognosis of critically ill HSCT patients [Citation26–34]. Adult ICU prognostic models (such as APACHE, Simplified Acute Physiology Score – SAPS, and Mortality Prediction Model – MPM) have been studied in the critically ill HSCT recipients. Some studies have shown that non-survivors have higher APACHE, SAPS and MPM scores, but in some studies, it has been reported that these models underestimate the mortality rate of the HSCT patient [Citation35–37]. Based on our findings, prognostic factors for critically ill HSCT patients include the presence of neutropenia, vasopressor support and central venous catheter, high organ failure scores, gram-positive and fungal infections, and urinary tract/catheter-associated urinary tract infection at ICU admission and new infections and complications, requiring renal replacement therapy, and liver dysfunction during ICU stay. APACHE II score at ICU admission was the only factor that was independently associated with ICU mortality, indicating a higher risk for scores above 22.5. While the predictive value of these models has been in question, our findings support their reliability. Also, in our cohort, ICU mortality risk was not correlated with demographic characteristics, transplantation type, conditioning regimen, primary disease status at the time of HSCT procedure or reasons for ICU admission.
Accurate estimation of ICU mortality risk is crucial. Studies have focused on disease-specific models since the prognostic models used here are designed for the general public. Groeger et al. developed and validated a model for predicting mortality in cancer patients (253 HSCT recipients included in original study) with 16 predictor variables, obtaining AUC values of 0.812 and 0.802 for development and validation models, respectively. Predictor variables in this model were PaO2/FiO2 ratio, platelet count, respiratory rate, systolic blood pressure, pre-ICU hospital days, intracranial mass effect, allogeneic bone marrow transplantation, recurrent or progressive cancer, albumin less than 2.5 g/dL, bilirubin 2 mg/dL or more, Glasgow Coma Scale less than 6, prothrombin time more than 15 s, blood urea nitrogen more than 50 mg/dL, endotracheal intubation, performance status before hospitalization, and cardiopulmonary resuscitation [Citation38]. Bayraktar et al. developed a model to predict ICU outcomes in allogeneic HSCT patients, called the prognostic index for intensive care after allogeneic hematopoietic stem cell transplantation (PICAT). Their parameters were time from hospital admission to ICU, lactate dehydrogenase, bilirubin, and albumin levels, the reason for ICU admission, prothrombin time-international normalized ratio, conditioning intensity, age, and comorbidity score. PICAT was reportedly better than both APACHE and SOFA in predicting mortality (AUC: 0.80 vs. 0.61 and 0.72) [Citation39]. In our study, the Glasgow Coma Scale was lower, INR longer, total bilirubin higher, neutropenia more frequent and sodium level higher in the patients who died. We thought that the prolongation of INR and high total bilirubin level were due to impaired liver function related to drugs (antimicrobials or immunosuppressive drugs), sepsis, oral intake disorder, total parenteral nutrition (TPN) or GVHD. Interestingly, we found a relationship between serum sodium level and prognosis of HSCT patients. Non-survivors had higher serum sodium levels at ICU admission. Research reports this patient group to be more prone to hyponatremia due to sodium loss from urine (immunosuppressive drugs or inappropriate ADH secretion, etc.) [Citation40,Citation41]. We associated this correlation with the insufficient replacement of fluid loss due to diarrhea or mucositis or with excess saline loading due to septic shock. Another risk factor that we identified was neutropenia. Some studies have marked that neutropenia increased mortality in hematological malignancies and solid cancers due to increased susceptibility to infections, particularly when it was prolonged, although some authors suggest no prognostic significance [Citation3,Citation4,Citation6,Citation11].
Infections remain the most common and significant reason for ICU admission. Although infectious agents (bacterial, fungal, viral, etc.) and foci (pulmonary, bloodstream, urinary, etc.) are different in pre-engraftment, early and late post-engraftment periods, some common reasons such as age, comorbidities, underlying hematological diseases, type of stem cell transplantation (allogeneic or autologous), conditioning regimen and stem cell source, HLA compatibility between the patient and donor, infections and microbial agents before HSCT, neutropenia (especially prolonged), mucosal damage, gastrointestinal tract colonization, and invasive devices used (endotracheal intubation tube, central venous catheter, urinary catheter, etc.) has been held responsible for the development of infections in HSCT patients [Citation11,Citation42]. Our cohort showed numerous infections before and during ICU admission. Yet, infection risk was found to be unrelated to age, primary hematological disease, transplantation type, or conditioning regimen, unlike the literature. Studies report allogeneic HSCT patients to have more infections, which may be associated with longer neutropenia, longer immune reconstruction, and higher length of hospital stay in this group. The lack of such correlation in our research may have stemmed from the low number of patients and late admission to the ICU. In our allogeneic HSCT patients, median admission time to the ICU after HSCT procedure was 220 [66.25–496.75] days, and number of patients admitted to the ICU 100 days or later after HSCT procedure was 24 (66.7%).
During pre-engraftment (within 30 days after HSCT), bacteria are often the leading cause. Although rare, fungal pathogens may be present, mostly Candida spp. In this period, bacterial infections are caused mainly by the endogenous gastrointestinal flora and indwelling vascular catheters [Citation42–48]. Here, the most common infections during pre-engraftment were pneumonia/VAP and bloodstream/catheter-related bloodstream infections, with the most common infection agent being gram-negative bacteria.
During early post-engraftment (30–100 days after HSCT), GVHD-induced infection risk increases, where enteric bacteria may lead to life-threatening infections. In this period, viral and fungal infections become more frequent. Allogeneic patients in particular may be under risk for CMV and molds [Citation11,Citation42,Citation49–51]. In our cohort, pneumonia/VAP and bloodstream/catheter-related bloodstream infections were common in allogeneic patients, although we had a low number of patients admitted to the ICU during early post-engraftment. In this group, the most common infection agent was gram-positive bacteria.
During late post-engraftment (100 days and later after HSCT), infections are often caused by GVHD or opsonization defect and hypogammaglobulinemia. This period may also include encapsulated bacterial infections [Citation42–46]. We observed pneumonia/VAP, bloodstream/catheter-related bloodstream infections, and urinary tract/catheter-associated urinary tract infections in both types of HSCT patients. The infection agents included gram-positive and negative bacteria and fungi.
According to research, risk of developing fungal infections ranges from 6% to 33% in HSCT patients [Citation11,Citation42,Citation50,Citation51]. About 16.1% of our patients had fungal infections at ICU admission, with Candida spp. being the most frequent cause. Our center often uses fluconazole for antifungal prophylaxis in allogeneic patients, although it may lead to resistant Candida strains when used excessively. We also found CMV positivity in 12.9% of our patients, although PCP was not detected. Trimethoprim-sulfamethoxazole prophylaxis has sufficient efficiency for PCP prophylaxis in allogeneic patients.
Sixty-one point three percent (61.3%) of our patients developed new infections during ICU stay. The most common infection was pneumonia/VAP (32.3%), while the most frequently isolated bacteria were Acinetobacter baumannii, Pseudomonas spp., Klebsiella spp., Escherichia coli and Stenotrophomanas spp. Although quinolones are used in antibacterial prophylaxis during HSCT procedure, their excessive use might lead to a resistant type of gram-negative bacteria. Similarly, urinary tract/catheter-associated urinary tract infections were caused mostly by resistant gram-negative bacteria like Klebsiella spp. and Escherichia coli. Besides, bloodstream/catheter-related bloodstream infections were found in 25.8% of our patients, with the most frequent agents being gram-positive bacteria like coagulase-negative Staphylococci (CNS). The literature indicates that skin flora might lead to bloodstream/catheter-related infections in patients undergoing intensive chemotherapy [Citation11,Citation42,Citation47,Citation48]. Pneumonia/VAP during ICU stay was more common in autologous patients, while bloodstream/catheter-related bloodstream infection was more common in allogeneic patients. Developing new infections during ICU stay was found to be associated with the length of ICU stay, presence of sepsis/septic shock, and presence of urinary catheter at admission. Yet, whether prolonged ICU stay was a cause or consequence of new ICU infections remains unclear. We believe that HSCT patients with an impaired immune system would not demonstrate adequate immune response in sepsis and septic shock causing vulnerability to further pathogens. Inserting catheters to these patients may be another reason for their vulnerability.
The retrospective nature of this study, small sample size and analysis of single-center data constitute the limitation of our study. The small sample size and retrospective nature of this study may cause different results and rates from the literature in determining prognostic factors in this cohort. Retrospective nature, small sample size and single-center experience also limit the generalizability of our findings. The study group consisted mostly of patients in late post-engraftment, which may have affected the distribution of infection and microbial agents. This longer period from transplantation to ICU admission creates uncertainties in terms of recurrence or additional treatments. Despite our limitations, this is one of the few studies in Turkey to investigate the ICU course in HSCT patients, potentially shedding light on infection-related complications in this patient group.
In conclusion, this research found that infectious complications after HSCT constituted most reasons for ICU admission, mortality, and morbidities. There still is a need for further multi-center and prospective research to better understand the causes and effects of both early and late infectious complications in HSCT patients.
Acknowledgments
No financial support or grant was received for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Holowiecki J. Indications for hematopoietic stem cell transplantation. Pol Arch Med Wewn. 2008; 118(11): 658–663. Doi: https://doi.org/10.20452/pamw.516.
- Balassa K, Danby R, Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med (Lond). 2019; 80(1): 33–39. Doi: https://doi.org/10.12968/hmed.2019.80.1.33.
- Afessa B, Tefferi A, Hoagland HC, Letendre L, Peters SG. Outcome of recipients of bone marrow transplants who require intensive-care unit support. Mayo Clin Proc. 1992; 67(2): 117–122. Doi: https://doi.org/10.1016/s0025-6196(12)61310-x.
- Galindo-Becerra S, Labastida-Mercado N, Rosales-Padrón J, García-Chavez J, Soto-Vega E, Rivadeneyra-Espinoza L, et al. Outcome of recipients of hematopoietic stem cell transplants who require intensive care unit support: a single institution experience. Acta Haematol. 2015; 134(2): 119–124. Doi: https://doi.org/10.1159/000381301.
- Denardo SJ, Oye RK, Bellamy PE. Efficacy of intensive care for bone marrow transplant patients with respiratory failure. Crit Care Med. 1989; 17(1): 4–6. Doi: https://doi.org/10.1097/00003246-198901000-00002.
- Jackson SR, Tweeddale MG, Barnett MJ, Spinelli JJ, Sutherland HJ, Reece DE, et al. Admission of bone marrow transplant recipients to the intensive care unit: outcome, survival and prognostic factors. Bone Marrow Transplant. 1998; 21(7): 697–704. Doi: https://doi.org/10.1038/sj.bmt.1701158.
- Matulis M, High KP. Immune reconstitution after hematopoietic stem-cell transplantation and its influence on respiratory infections. Semin Respir Infect. 2002; 17(2): 130–139. Doi: https://doi.org/10.1053/srin.2002.33441.
- Boyacı N, Aygencel G, Turkoglu M, Yegin ZA, Acar K, Sucak GT. The intensive care management process in patients with hematopoetic stem cell transplantation and factors affecting their prognosis. Hematology. 2014; 19: 338–345. Doi: https://doi.org/10.1179/1607845413Y.0000000130.
- Barnes RA, Stallard N. Severe infections after bone marrow transplantations. Curr Opin Crit Care. 2001; 7(5): 362–366. Doi: https://doi.org/10.1097/00075198-200110000-00008.
- Ninin E, Milpied N, Moreau P, André-Richet B, Morineau N, Mahéet B, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001; 33(1): 41–47. Doi: https://doi.org/10.1086/320871.
- Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010; 24(2): 257–272. Doi: https://doi.org/10.1016/j.idc.2010.01.010.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315(8): 801–810. Doi: https://doi.org/10.1001/jama.2016.0287.
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults With hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63(5): e61–111. Doi: https://doi.org/10.1093/cid/ciw353.
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. .Clin Infect Dis. 2011; 52(9): e162-93. doi: https://doi.org/10.1093/cid/cir257.
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee . Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010; 31(4): 319-26. doi: https://doi.org/10.1086/651091.
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, et al . Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48 (5): 503–535. Doi: https://doi.org/10.1086/596757.
- Maertens J, Marchetti O, Herbrecht R, Cornely OA, Flückiger U, Frêre P, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 update. Bone Marrow Transplant. 2011; 46: 709–718. Doi: https://doi.org/10.1038/bmt.2010.175.
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002; 34(8): 1094–1097. Doi: https://doi.org/10.1086/339329.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012; 307(23): 2526–2533. Doi: https://doi.org/10.1001/jama.2012.5669.
- Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria. J Intensive Care Med. 2007; 22(4): 187–193. Doi: https://doi.org/10.1177/0885066607299510
- Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for blood and Marrow transplant activity survey report. Bone Marrow Transplant. 2017; 52(6): 811–881. Doi: https://doi.org/10.1038/bmt.2017.34.
- Afessa B, Azoulay E. Critical care of the hematopoietic stem cell transplant recipient. Crit Care Clin. 2010; 26 (1): 133–150. Doi: https://doi.org/10.1016/j.ccc.2009.09.001
- Agarwal S, O’Donoghue S, Gowardman J, Kennedy G, Bandeshe H, Boots R . Intensive care unit experience of haemopoietic stem cell transplant patients. Intern Med J. 2012; 42: 748–754. Doi: https://doi.org/10.1111/j.1445-5994.2011.02533.x.
- Saillard C, Blaise D, Mokart D. Critically ill allogeneic hematopoietic stem cell transplantation patients in the intensive care unit: reappraisal of actual prognosis. Bone Marrow Transplant. 2016; 51: 1050–1061. Doi: https://doi.org/10.1038/bmt.2016.72.
- Scales DC, Thiruchelvam D, Kiss A, Sibbald WJ, Redelmeier DA. Intensive care outcomes in bone marrow transplant recipients: a population-based cohort analysis. Crit Care. 2008; 12(3): R77. Doi: https://doi.org/10.1186/cc6923
- Paz HL, Crilley P, Weinar M, Brodsky I. Outcome of patients requiring medical ICU admission following bone marrow transplantation. Chest. 1993; 104(2): 527–531. Doi: https://doi.org/10.1378/chest.104.2.527
- Soubani AO, Kseibi E, Bander JJ, Klein JL, Khanchandani G, Ahmed HP, et al. Outcome and prognostic factors of hematopoietic stem cell transplantation recipients admitted to a medical ICU. Chest. 2004; 126(5): 1604–1611. Doi: https://doi.org/10.1378/chest.126.5.1604
- Lueck C, Stadler M, Koenecke C, Hoeper MM , Dammann E, Schneider A, et al. Improved short- and long-term outcome of allogeneic stem cell recipients admitted to the intensive care unit: a retrospective longitudinal analysis of 942 patients. Intensive Care Med. 2018; 44(9): 1483–1492. Doi: https://doi.org/10.1007/s00134-018-5347-x.
- Azoulay E, Pene F, Darmon M, Lengliné E, Benoit D, Soares M, et al. Managing critically Ill Hematology patients: time to think differently. Blood Rev. 2015; 29: 359–367. Doi: https://doi.org/10.1016/j.blre.2015.04.002
- Michel CS, Teschner D, Schmidtmann I, Theobald M, Hauptrock B, Wagner-Drouet EM, et al. Prognostic factors and outcome of adult allogeneic hematopoietic stem cell transplantation patients admitted to intensive care unit during transplant hospitalization. Sci Rep. 2019; 9(1): 19911. Doi: https://doi.org/10.1038/s41598-019-56322-0.
- Lindgaard SC, Nielsen J, Lindmark A, Sengeløv H. Prognosis of allogeneic Haematopoietic stem cell recipients admitted to the intensive care unit: a retrospective, single-centre study. Acta Haematol. 2016; 135(2): 72–78. Doi: https://doi.org/10.1159/000440937.
- Trinkaus MA, Lapinsky SE, Crump M, Keating A, Reece DE, Chen C,et al. Predictors of mortality in patients undergoing autologous hematopoietic cell transplantation admitted to the intensive care unit. Bone Marrow Transplant. 2009; 43: 411–415. Doi: https://doi.org/10.1038/bmt.2008.336.
- Benz R, Schanz U, Maggiorini M, Seebach JD, Stussi G, et al. Risk factors for ICU admission and ICU survival after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014; 49: 62–65. Doi: https://doi.org/10.1038/bmt.2013.141.
- Mokart D, Granata A, Crocchiolo R, Sannini A, Chow-Chine L, Brun JP, et al. Allogeneic hematopoietic stem cell transplantation after reduced intensity conditioning regimen: outcomes of patients admitted to intensive care unit. J Crit Care. 2015; 30: 1107–1113. Doi: https://doi.org/10.1016/j.jcrc.2015.06.020.
- Neumann F, Lobitz O, Fenk R, Bruns I, Köstering M, Steiner S, et al. The sepsis-related organ failure assessment (SOFA) score is predictive for survival of patients admitted to the intensive care unit following allogeneic blood stem cell transplantation. Ann Hematol. 2008; 87: 299–304. Doi: https://doi.org/10.1007/s00277-008-0440-9.
- Afessa B, Tefferi A, Dunn WF, Litzow MR, Peters SG. Intensive care unit support and acute physiology and chronic health evaluation III performance in hematopoietic stem cell transplant recipients. Crit Care Med. 2003; 31: 1715–1721. Doi: https://doi.org/10.1097/01.CCM.0000065761.51367.2D.
- Gilli K, Remberger M, Hjelmqvist H, Ringden O, Mattsson J. Sequential organ failure assessment predicts the outcome of SCT recipients admitted to intensive care unit. Bone Marrow Transplant. 2010; 45: 682–688. Doi: https://doi.org/10.1038/bmt.2009.220.
- Groeger JS, Lemeshow S, Price K, Nierman DM, White P Jr, Klar J, et al. Multicenter outcome study of cancer patients admitted to the intensive care unit: a probability of mortality model. J Clin Oncol. 1998; 16: 761–770. Doi: https://doi.org/10.1200/JCO.1998.16.2.761.
- Bayraktar UD, Milton DR, Shpall EJ, Rondon G, Price KJ, Champlin RE, et al. Prognostic index for critically Ill allogeneic transplantation patients. Biol Blood Marrow Transplant. 2017; 23: 991–996. Doi: https://doi.org/10.1016/j.bbmt.2017.03.003.
- Kobayashi R, Iguchi A, Nakajima M, Sato T, Yoshida M, Kaneda M, et al. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion complicating stem cell transplantation. Bone Marrow Transplant. 2004; 34(11): 975–979. Doi: https://doi.org/10.1038/sj.bmt.1704688.
- Yuda S, Mori T, Kato J, Koda Y, Kohashi S, Kikuchi T, et al. Sodium-losing nephropathy caused by tacrolimus after allogeneic hematopoietic stem cell transplantation. Rinsho Ketsueki. 2013; 54(12): 2187–2191. Doi: https://doi.org/10.11406/rinketsu.54.2187.
- Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016; 22: 505–514. Doi: https://doi.org/10.1016/j.jiac.2016.05.006.
- Schuster MG, Cleveland AA, Dubberke ER, Kauffman CA, Avery RK, Husain S, et al. Infections in hematopoietic cell transplant recipients: results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect Dis. 2017;4(2):ofx050. Doi: https://doi.org/10.1093/ofid/ofx050.
- César-Arce A, Volkow-Fernández P, Valero-Saldaña LM , Acosta-Maldonado B , Vilar-Compte D, Cornejo-Juárez P Infectious complications and multidrug-resistant bacteria in patients with hematopoietic stem cell transplantation in the first 12 months after transplant. Transplant Proc. 2017; 49(6): 1444–1448. Doi: https://doi.org/10.1016/j.transproceed.2017.03.081.
- Altuntaş F, Yıldız O, Eser B, Alp E, Sarı İ, Çetin M, et al. Microbiologically documented infections following peripheral blood stem cell transplantation: single center experience. Turk J Haematol. 2005; 22(3): 133–145. PMID: 27264835.
- Oliveira AL, de Souza M, Carvalho-Dias VMH, Ruiz MA, Silla L, Tanaka PY, et al. Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007; 39: 775–781. Doi: https://doi.org/10.1038/sj.bmt.1705677.
- Chen CY, Tsay W, Tang JL, Tien HF, Chen YC, Chang SC, et al.; Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiol Infect. 2010; 138(7): 1044–1051. Doi: https://doi.org/10.1017/S0950268809991208.
- Ali N, Adil SN, Shaikh MU. Bloodstream and central line isolates from hematopoietic stem cell transplant recipients: data from a developing country. Transpl Infect Dis. 2014; 16(1): 98–105. Doi: https://doi.org/10.1111/tid.12176.
- Fuji S, Einsele H, Kapp M. Cytomegalovirus disease in hematopoietic stem cell transplant patients: current and future therapeutic options. Curr Opin Infect Dis. 2017; 30(4): 372–376. Doi: https://doi.org/10.1097/QCO.0000000000000375.
- Bays DJ, Thompson GR 3rd . Fungal infections of the stem cell transplant recipient and hematologic malignancy patients. Infect Dis Clin N Am. 2019; 33: 545–566. Doi: https://doi.org/10.1016/j.idc.2019.02.006.
- Omer AK, Ziakas PD, Anagnostou T, Coughlin E, Kourkoumpetis T, McAfee SL, et al. Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant. 2013; 19(8): 1190–1196. Doi: https://doi.org/10.1016/j.bbmt.2013.05.018.