ABSTRACT
Objectives
To determine the rate of RhD-alloimmunization in injured RhD-negative patients in the age range of childbearing potential who were transfused with at least one unit of RhD-positive red blood cells (RBC) or low titer group O whole blood (LTOWB).
Methods
Injured RhD-negative patients between the ages of 13–50 at an American Level 1 trauma center who were transfused with at least one unit of RBCs or LTOWB during their resuscitation and who had an antibody detection test performed at least 14 days afterwards were included.
Results
Over a 20-year period, 96 study-eligible patients were identified, of which 90/96 (93.8%) were male. The median age of these 96 patients was 33 (5th–95th percentiles: 19–49) years. The majority of these patients (71/96, 74.0%) had an injury severity score (ISS) greater than 15. Overall, 41/96 (42.7%; 95% CI: 32.7%–53.2%) of these patients became alloimmunized after receipt of a median of 3 (5th–95th percentiles: 1–35) units of RhD-positive RBCs and/or LTOWB. There was no association between receipt of leukoreduced RBCs or receipt of LTOWB and the RhD-alloimmunization rate.
Discussion
The rate of RhD-alloimmunization in this study was at the higher end of rates that have been reported. None of the previous studies focused exclusively on trauma patients in the childbearing age range.
Conclusion
The 42.7% rate of RhD-alloimmunization in a predominantly male trauma population could probably be extrapolated to women in the same age range when estimating their risk of RhD-alloimmunization following RhD-positive transfusion.
Introduction
There is emerging evidence from both the military and civilian literature showing that the early transfusion of blood products during a trauma resuscitation improves survival [Citation1,Citation2]. In particular, the combination of red blood cells (RBC) and plasma was shown to be the most beneficial compared to either component alone or crystalloids in a secondary analysis of a randomized trial of injured civilians [Citation3]. In light of this evidence, emergency medical services (EMS) such as ground ambulances and rescue helicopters that currently do not administer blood products while the patient is being transported might start considering whether to provide them. Similarly, hospitals that receive injured patients might attempt to provide transfusions early in the resuscitation by having blood products available in the emergency department (ED), if they do not do so already. In both the EMS and ED situations, the patient's ABO and RhD-type is unlikely to be known at the time that the transfusions are administered; in these cases, group O RBCs or low titer group O whole blood (LTOWB) are selected as this group will be compatible with the recipient's naturally occurring anti-A and/or -B. Ideally, RhD-negative RBCs or LTOWB would be provided to females of childbearing potential (FCP), commonly defined as between ages 13–50, if her RhD-type is negative or unknown because if she becomes sensitized to the RhD antigen, future pregnancies could be affected by hemolytic disease of the fetus and newborn (HDFN).
Unfortunately, RhD-negative RBCs or LTOWB are a scarce resource. In a multinational study of blood centers, only 10% of the total number of RBCs distributed to hospitals were O RhD-negative [Citation4]. Similarly, at a large American blood collector, only 3% of all donors are eligible to donate RhD-negative LTOWB (M. Yazer, personal communication, January 2021). Thus, there might be some reluctance to supply EMS bases with these products for fear of wastage, and group O RBCs are often in short supply at hospitals. In these cases, RhD-positive RBCs and LTOWB might be selected for emergency transfusion.
While RhD-alloimmunization is of minimal clinical significance for males and females who are beyond childbearing potential, the concern about alloimmunization and HDFN in FCPs depends in part on the rate of RhD-alloimmunization. This alloimmunization rate has been evaluated in several previous studies of trauma, surgery, and/or general hospitalized patients and has been found to range between approximately 11%–50% () [Citation5–15]. However, there is significant heterogeneity in the design of these retrospective studies, including the nature of the patients studied and the method by which the alloimmunization rate was calculated. In particular, none of the previous studies have focused on injured patients in the childbearing years and instead studied patients who were, on the whole, over 50 years old, and who were not necessarily injured. The intention of the current study was to analyze the RhD-alloimmunization rate in injured patients who were between 13 and 50 years old so as to be able to understand the risk involved with transfusing FCPs of unknown or negative RhD-type with RhD-positive RBCs or LTOWB during their trauma resuscitation vis-à-vis future HDFN risk.
Table 1. Summary of studies reporting RhD-alloimmunization rate on trauma and/or general hospitalized patients.
Methods
This is a secondary analysis of a study that investigated the rate of RhD-alloimmunization per transfused unit of RhD-positive RBCs or LTOWB amongst 335 hospitalized patients [Citation16]. This secondary analysis focused specifically on the RhD-alloimmunization rate in those patients who were admitted to one hospital with trauma and who were in the childbearing age years.
Patients for were identified this study as previously described [Citation16]. Briefly, the trauma patient database at one American Level 1 trauma center that contains information on all injured patients treated at this hospital was merged with the electronic database of transfusion recipients obtained from the hospital's transfusion service for the calendar years 2000–2019. This combined database of injured patients and transfusion recipients was then searched to identify injured RhD-negative trauma patients between 13 and 50 years of age who received at least one RhD-positive RBC or LTOWB unit during their admission. Both females and males were included in this analysis because it was assumed that too few RhD-negative FCPs would have received RhD-positive RBC or LTOWB units to perform a meaningful analysis alone.
Recording the results of antibody detection tests (commonly called ‘antibody screens’) that were performed on study-eligible patients was performed until 1 December 2020, although the last possible date for transfusing RhD-positive RBC or LTOWB units for inclusion in this study was 31 December 2019. This was done to allow for at least one year of passive serological surveillance following the transfusion of RhD-positive products in 2019.
To be included in the analysis, injured RhD-negative recipients of at least one RhD-positive RBC or LTOWB unit during their trauma admission had to have had a follow up antibody detection test performed at least 14 days after the index RhD-positive transfusion, i.e. the first RhD-positive transfusion administered during their trauma admission. Exclusion criteria included having a history of anti-RhD or an active anti-RhD at the time that the RhD-positive RBC or LTOWB units were administered, or having received Rh immunoglobulin (RhIg) following the RhD-positive transfusion. Additionally, to minimize the inclusion of RhD antibodies that were the result of a secondary immune (anamnestic) response, any patient whose anti-RhD was detected within 14 days of the index RhD-positive transfusion was excluded. RhD-negative patients who received RhD-positive platelets without any RhD-positive RBC or LTOWB units were also excluded due to the low rate of RhD-alloimmunization following RhD-positive platelet transfusion [Citation17].
The RhD-alloimmunization rate was calculated as the number of patients who became alloimmunized during the study period divided by the total number of patients who met inclusion criteria for the study. The total number of transfused RhD-positive RBC or LTOWB units during the study period was determined for patients who did not become RhD-alloimmunized, while the total number of transfused RhD-positive RBC or LTOWB units prior to the detection of anti-RhD was calculated for the RhD-alloimmunized patients. Data-driven strata for the total number of units of RhD-positive products were identified in order to determine the effect of the number of units transfused on the RhD-alloimmunization rate.
In addition, the length of serological follow up, defined as the number of days between the index RhD-positive RBC or LTOWB transfusion and the last documented antibody detection test in which anti-RhD was not detected, was calculated for those who did not become alloimmunized to the RhD antigen during the study period. For those who became RhD-alloimmunized, the length of serological follow up was defined as the number of days between the first antibody detection test performed following the index RhD-positive RBC or LTOWB transfusion and the date on which the anti-RhD was detected.
A Chi-squared test or Fisher's exact test, where appropriate, was used to compare the differences between categorical/dichotomous variables. A median test was performed to determine the likelihood that two or more independent samples came from populations with the same median. Royston's trend test for proportions was performed to determine if there was a significant trend in the RhD-alloimmunization rate across the strata of the number of RhD-positive units transfused. Data analysis was performed in Stata version 16 (Statacorp, TX). P-values were not adjusted for multiple comparisons. This protocol was approved by the University of Pittsburgh's Institutional Review Board.
Results
A total of 96 trauma patients met the inclusion criteria. The median (5th–95th percentile) age of all of the included patients was 33 (19–49) years, and 90/96 (93.8%) were male. The majority of these patients (71/96, 74.0%) had an injury severity score (ISS) greater than 15, and 23/96 (24.0%) had a penetrating injury. The median (5th–95th percentile) trauma and injury severity score (TRISS) probability of survival was 96.2% (10.2%–99.4%). The median (5th–95th percentile) length of stay was 22 (5–53) days and only three patients (3.1%) died during their admission. The median (5th–95th percentile) number of RhD-positive RBC or LTOWB units transfused to this cohort was 3 (1–35) units. The majority (85/96, 88.5%) of patients received their first RhD-positive RBC or LTOWB unit within the first calendar day of admission and most (79/96, 82.3%) patients received all of their RhD-positive RBC or LTOWB units within three days of the first transfused RhD-positive unit.
The demographic and clinical features, as well as the median length of serologic follow-up and the median number of negative antibody screens in the non-alloimmunized and RhD-alloimmunized patients are shown in . Overall, there were 41/96 [42.7% (95% exact binomial confidence interval, CI: 32.7%–53.2%)] recipients who became alloimmunized to the RhD antigen. The RhD-alloimmunization rate did not increase significantly as the number of transfused RhD-positive RBC or LTOWB units increased (p = 0.137 for linear trend, p = 0.415 for departure from linear trend. ).
Figure 1. RhD-alloimmunization rate stratified by the total number of transfused RhD-positive RBC and LTOWB units. For non-alloimmunized patients, the total number of RhD-positive RBC or LTOWB units transfused during the entire study period was calculated, while for RhD-alloimmunized patients, the number of RhD-positive RBC or LTOWB units transfused prior to the detection of anti-RhD was calculated. The number of patients (n) in each group is shown within each bar. The average RhD-alloimmunization rate for the entire study cohort of 42.7% (95% exact binomial confidence interval, CI: 32.7%–53.2%) is indicated by the horizontal dashed line. There was no significant difference in the RhD-alloimmunization between any of the groups that received two or more units and the group that received only one unit.
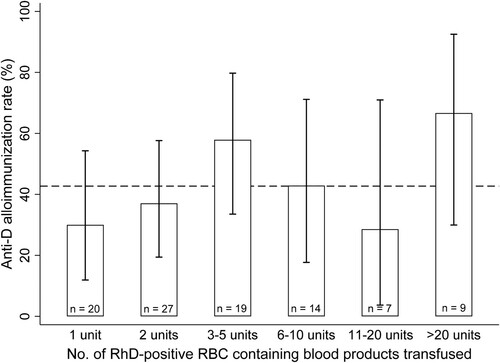
Table 2. Demographics of the RhD-negative recipients of RhD-positive RBC or LTOWB units included in this study, stratified by those who became alloimmunized to the RhD antigen.
There were 76/96 (79.2%) patients who did not receive any leukoreduced RBC or LTOWB units, while 20/96 (20.8%) patients received at least one leukoreduced RBC or LTOWB unit; 9/20 patients (45.0%) received solely leukoreduced products. The median (5th–95th percentiles) number of leukoreduced RBC or LTOWB units transfused to these 20 recipients was 2 (1–34). The RhD-alloimmunization rate was not significantly different between the 76 patients who did not receive any leukoreduced products and the 20 patients who received at least one leukoreduced product (29/76, 38.2% versus 12/20, 60.0%; p = 0.079).
Discussion
This study of injured predominantly male patients in the age range of the childbearing years revealed an RhD-alloimmunization rate of 42.7%. This rate is important to consider when estimating the risk of HDFN complicating future pregnancies should an RhD-negative female of childbearing age receive RhD-positive RBCs or LTOWB during trauma resuscitation.
This RhD-alloimmunization rate is at the higher-end of the rates that had been previously published (). Perhaps this is because this study focused exclusively on younger-aged patients who would have been expected to have been healthy and immunocompetent before their injury; in fact, the previous study that had demonstrated a 50% RhD-alloimmunization rate also focused exclusively on trauma patients, although the mean age of the patients in that study of 18 patients was not reported [Citation12]. One study of hospitalized patients reported an RhD-alloimmunization rate of 26% [Citation13]; an alternative interpretation of the risk in this study is a rate of 40% (), which is very similar to the 42.7% rate found in the current study. Conversely, a recent study of injured patients on whom the hospital's massive transfusion protocol had been activated revealed a 7.8% RhD-alloimmunization rate. The median age of the patients in this study was similar to that in the current study, and both studies focused on trauma patients. However, it is not clear why the rates of RhD-alloimmunization are so widely divergent even when the included patients were of similar age and diagnosis, however, it is possible that differences in study design contributed. For example, several of the previous studies did not report requiring the RhD-negative recipients of RhD-positive RBCs to have had an antibody detection test performed at a designated interval after the transfusion of the index RhD-positive RBCs as an inclusion criterion. Thus, in those studies, it is unclear how closely the patients were followed serologically and if some RhD-alloimmunizations were missed. It is also important to note that a relatively small number of patients were included in many of those studies, with some studies reporting on results from only 8 or 9 transfused RhD-negative patients. These limitations make it difficult to combine the alloimmunization rates from the previous studies into an overall rate.
It is also important to keep the 42.7% alloimmunization rate in the context of all of the events that have to occur for a transfused RhD-negative FCP to have a pregnancy that is affected by severe HDFN. The overall rate of fetal demise occurring in an RhD-negative FCP who received an RhD-positive transfusion and survived her trauma, became alloimmunized, and then carried an RhD-positive fetus was calculated to be 0.3% [Citation18], largely because of the excellent HDFN diagnosis and treatment modalities available in some academic centers [Citation19]. The rate of 0.3% was calculated using an RhD-alloimmunization rate of 21%, which was derived by combining the results of three of the larger studies on RhD-alloimmunization. If the 42.7% rate from this study was substituted into the calculation, the new overall rate of fetal demise would rise to 0.7%. Considering other factors in this calculation such as the maternal age at the time of RhD-alloimmunization and the number of pregnancies that she is likely to have after she recovers from her trauma increases the risk of HDFN [Citation20], and a higher RhD-alloimmunization rate would further raise the predicted rates of HFDN. Still, these calculated HDFN rates have to be balanced against the life-saving benefits of the early administration of blood products, especially in the pre-hospital phase of the resuscitation [Citation1–3], where RhD-positive products would most likely be administered.
This study has several limitations. As this was a retrospective study, it was not possible to serially follow the patients with antibody detection tests. Thus, the exact timing of the alloimmunization cannot be determined. As having an antibody detection test performed 14 days after the index RhD-positive transfusion was an inclusion criterion, alloimmunizations amongst transfused patients who did not have a test performed by that time, perhaps because of death or hospital discharge, would not have been detected thereby underestimating the true RhD-alloimmunization rate. In contrast, patients who received RhD-positive units and never became alloimmunized to the RhD antigen would also have been excluded from this study if they did not have a follow-up antibody detection test at least 14 days after their transfusion. Thus, the observed RhD-alloimmunization rate in this study may in fact represent an overestimate of the true rate. Similarly, alloimmunizations that occurred after the last antibody detection test was performed in the non-responders would also not have been detected, although the median length of serological follow-up in this group of patients was 51 days, which should have been sufficient to have detected a new anti-RhD. The alloimmunization rate in this study of injured patients between 13 and 50 years of age was 42.7%, which was higher than the 30.6% rate that was observed in the primary analysis where all hospitalized RhD-negative patients in the same age range who received at least one unit of RhD-positive RBCs or LTOWB were studied [Citation16]. Although there was not a statistically significant difference in the RhD-alloimmunization rate between the patients who received one RhD-positive unit and those who received multiple units (), the number of patients in each RBC quanta was relatively small. Therefore, the conclusion that the RhD-alloimmunization risk does not increase with exposure to RhD-positive units in trauma patients between 13 and 50 years of age should be interpreted with caution and should ideally be analyzed in a larger study. Lastly, there were only 6/96 (6.3%) females in this secondary analysis; while this low rate was expected based on the trauma-focused nature of this study, any gender-specific differences in RhD-alloimmunization would not have been detected. Therefore, it is not certain that the 42.7% RhD-alloimmunization risk applies to transfused FCPs in trauma.
That only 6/96 (6.3%) of the trauma patients between the ages of 13–50 in this study were female suggests that reserving a certain quantity of RhD-negative RBCs for use when these patients experience trauma or massive bleeding in general should not put a large burden on the RhD-negative inventory at the treating hospital. However, because of the low number of FCPs that experience trauma, reserving RhD-negative RBCs that will not be frequently used effectively removes those scarce RBCs from the hospital blood bank's general inventory and if they are used for other patients, the risk is run of not having RhD-negative RBCs for FCPs of unknown RhD-type who are bleeding thereby potentially compelling the use of RhD-positive RBCs. This is particularly true if RBCs or LTOWB are to be provided in the pre-hospital phase of the resuscitation. Thus, while RhD-negative RBCs or LTOWB should be provided to FCPs of unknown RhD-type who are experiencing a massive bleed when possible, understanding the RhD-alloimmunization risk is important when deciding when to administer RhD-positive RBCs or LTOWB if RhD-negative units are not available or are in short supply.
This study revealed a 42.7% RhD-alloimmunization risk for RhD-negative predominantly male patients in the childbearing age range who were transfused with RhD-positive RBCs or LTOWB during trauma resuscitation. This patient age-specific alloimmunization rate should be considered when developing a program for transfusing injured FCPs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–326.
- Shackelford SA, Del Junco DJ, Powell-Dunford N, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. Jama. 2017;318:1581–1591.
- Guyette FX, Sperry JL, Peitzman AB, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann Surg. 2021;273:358–364.
- Yazer MH, Jackson B, Beckman N, et al. Changes in blood center red blood cell distributions in the era of patient blood management: the trends for collection (TFC) study. Transfusion. 2016;56:1965–1973.
- Frohn C, Dumbgen L, Brand JM, et al. Probability of anti-D development in D- patients receiving D+ RBCs. Transfusion. 2003;43:893–898.
- Dutton RP, Shih D, Edelman BB, et al. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59:1445–1449.
- Yazer MH, Triulzi DJ. Detection of anti-D in D- recipients transfused with D+ red blood cells. Transfusion. 2007;47:2197–2201.
- Gonzalez-Porras JR, Graciani IF, Perez-Simon JA, et al. Prospective evaluation of a transfusion policy of D+ red blood cells into D- patients. Transfusion. 2008;48:1318–1324.
- Miraflor E, Yeung L, Strumwasser A, et al. Emergency uncrossmatched transfusion effect on blood type alloantibodies. J Trauma Acute Care Surg. 2012;72:48–53. discussion -3.
- Tchakarov A, Hobbs R, Bai Y. Transfusion of D+ red blood cells to D- individuals in trauma situations. Immunohematology. 2014;30:149–152.
- Meyer E, Uhl L. A case for stocking O D+ red blood cells in emergency room trauma bays. Transfusion. 2015;55:791–795.
- Flommersfeld S, Mand C, Kuhne CA, et al. Unmatched type O RhD+ red blood cells in multiple injured patients. Transfus Med Hemother. 2018;45:158–161.
- Selleng K, Jenichen G, Denker K, et al. Emergency transfusion of patients with unknown blood type with blood group O rhesus D positive red blood cell concentrates: a prospective, single-centre, observational study. Lancet Haematol. 2017;4:e218–ee24.
- Williams LA, 3rd, Sikora J, Aldrees R, et al. Anti-Rh alloimmunization after trauma resuscitation. Transfus Apher Sci. 2019;58:102652.
- Raval JS, Madden K, Neal MD, et al. Anti-D alloimmunization in Rh(D) negative adults with severe traumatic injury. Transfusion. in press.
- Yazer MH, Triulzi DJ, Sperry JL, et al. Rate of RhD-alloimmunization after the transfusion of multiple RhD-positive primary red blood cell containing products. Transfusion. in press.
- Cid J, Lozano M, Ziman A, et al. Low frequency of anti-D alloimmunization following D+ platelet transfusion: the anti-D alloimmunization after D-incompatible platelet transfusions (ADAPT) study. Br J Haematol. 2015;168:598–603.
- Yazer MH, Delaney M, Doughty H, et al. It is time to reconsider the risks of transfusing RhD negative females of childbearing potential with RhD positive red blood cells in bleeding emergencies. Transfusion. 2019;59:3794–3799.
- Zwiers C, Oepkes D, Lopriore E, et al. The near disappearance of fetal hydrops in relation to current state-of-the-art management of red cell alloimmunization. Prenat Diagn. 2018;38:943–950.
- Seheult JN, Stram M, Pearce T, et al. The risk to future pregnancies of transfusing Rh(D)-negative females of childbearing potential with Rh(D)-positive red blood cells during trauma resuscitation is dependent on their age at transfusion. Vox Sang. in press.
