ABSTRACT
Objectives
Acute myeloid leukaemia (AML) is a haematopoietic malignancy with a dismal outcome. Consequently, risk stratification based on more effective prognostic biomarkers is crucial to make accurate therapy decisions. T cell receptor gamma alternative reading frame protein (TARP) has been reported in prostate and breast cancers, but its correlation with AML remains unclear.
Methods
Differential expression of TARP mRNA in different AML subtypes was analysed using the UALCAN online platform. Its relationship with baseline clinical attributes, survival and efficacy were analysed based on three GSE1159, GSE425 and GSE6891 microarray datasets downloaded from Gene Expression Omnibus (GEO) and Oncomine databases. Quantitative real-time PCR was performed to determine mRNA levels of TARP in bone marrow mononuclear cells (BMMCs) isolated from AML patients.
Results
TARP was significantly overexpressed in AML patients. In AML, relatively low TARP expression was associated with the CBFβ-MYH11 fusion gene. The proportion of FLT3 mutations was significantly higher in non-adolescent and young adult (non-AYA, >39 years of age) AML patients who had high TARP levels but not in AYA (15–39 years) patients. High expression of TARP was related to poor outcome by univariate analysis but not by multivariate analysis and unsatisfactory therapeutic effects, which could be overcome by haematopoietic stem cell transplantation (HSCT).
Conclusion
Our findings suggest that TARP might be a potential prognostic marker of AML and serve as a promising immunotherapeutic target.
Introduction
Acute myeloid leukaemia (AML), also known as acute nonlymphocytic leukaemia, is a malignancy originating from haematopoietic stem or progenitor cells. AML has a heterogeneous prognosis. According to the French-American-British classification, AML is subdivided into eight subtypes (M0–M7) [Citation1]. Among them, acute promyelocytic leukaemia (APL, M3 subtype) is the most curable AML with a genetic hallmark of chromosomal translocation t (15; 17)(q21; q22), which results in a PML-RARa fusion gene [Citation2]. Except for APL, most adult AML patients have dismal outcomes. Recently, improved outcomes including response to therapy and survival were observed in AML of adolescent and young adult (AYA) patients than in non-AYA cases [Citation3]. However, the underlying mechanisms remain unclear.
Improved understanding of AML at the molecular and genomic levels to identify high-risk patients is crucial for arriving at decisions concerning the best treatment options, for example, whether a patient should be treated using allo-haematopoietic stem cell transplantation (HSCT). Currently, conventional cytogenetics and studies on molecular alterations are recommended for routine testing at baseline for risk stratification; abnormalities such as complex karyotype and FMS-like tyrosine kinase (FLT3) mutation always predict poor prognosis [Citation4]. However, prognostic stratification by commonly used models in clinical settings has been unsuccessful so far [Citation5].
FLT3 is a member of the receptor tyrosine kinase superfamily. FLT3 harbours five domains: an extracellular domain, one transmembrane domain, a cytoplasmic juxtamembrane domain (JMD) and two tyrosine kinase domains (TKD1 and TKD2) [Citation6]. The internal tandem duplication (ITD) alteration of the JMD domain is a gain-of-function mutation in AML that is detected in 25% of de novo AML patients. The mutation induces auto-phosphorylation, which leads to excessive proliferation and survival of AML cells [Citation7]. FLT3-ITD mutations have been reported to be associated with poor outcomes and high rates of relapse [Citation7]. However, the clinical significance of genetic alterations in the TKD domain, including the D835 point mutation and I836 deletion, is not fully understood. A study reported that no difference in overall survival (OS) was observed between AML patients having an FLT3-TKD mutation and those with wild type (wt) FLT3 kinase, and that prognosis of FLT3-TKD positive cases was better than that of FLT3-ITD patients [Citation8]. However, FLT3-TKD abnormalities were observed in no more than 10% of AML cases in Asia and were slightly higher in Europe and the United States [Citation8].
T-cell receptor gamma alternative reading frame protein (TARP) is a protein-encoding gene that is embedded within an intron of the T cell receptor gamma gene on chromosome 7p14.1. Besides T cells, TARP is also expressed as a 7 kD protein in the nuclei of cells such as prostate epithelial cells and breast cancer cells in some non-lymphoid tissues without coexpression with TCRD [Citation9].
Herein, We found that TARP was overexpressed in non-APL AML patients, especially in non-AYA cases. High levels of TARP were associated with FLT3-ITD mutations and poor prognosis. However, the role and underlying mechanisms of this gene in AML pathogenesis and progression remain to be further studied.
Methods
Primary gene expression profiling and relevant clinical data
All primary gene profiling and clinical data were obtained from two free integrated data mining tools: the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) [Citation10] and the Oncomine platform (https://www.oncomine.org) [Citation11]. Original data were from GSE1159 (included samples from 285 AML patients and eight healthy controls) [Citation12], GSE425 (109 AML cases, including 12 APL cases and 97 non-APL cases) [Citation13], and GSE6891 (444 AML cases, including 24 APL cases and 420 non-APL cases) [Citation14] datasets available in the GEO database.
Analysis of TARP mRNA expression in AML patients and normal controls
Expression of TARP mRNA in 285 AML patients and eight normal controls with valid TARP mRNA expression values in GSE1159 were compared using Oncomine (https://www.oncomine.org/).
The UALCAN free online platform (http://ualcan.path.uab.edu/analysis.html) was used to compare TARP mRNA expression levels among different AML subtypes. UALCAN is a comprehensive web-based resource that provides easy access to cancer OMICS data based on The Cancer Genome Atlas and MET500 cohort database [Citation15].
Quantitative real-time polymerase chain reaction (qRT-PCR)
Bone marrow mononuclear cells (BMMCs) were isolated from 29 primary non-M3 AML patients (non-AYA, Supplemental Table 1) who were treated at the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University between January 2019 and November 2020. Informed consents were obtained from all patients in accordance with the declaration of Helsinki. Total RNA was extracted from BMMCs using a commercially available RNAprep pure Kit (TIANGEN Biotech (Beijing) Co, Ltd, Beijing, China). The concentration and purity of the isolated RNA were tested by spectrophotometry using a NanoDrop 2000c apparatus (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from 1 μg of isolated RNA using Reverse Transcriptase Kits (Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. Analysis of TARP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was performed by real-time PCR using 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) under cycling conditions: predenaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s and annealing at 60°C for 30 s. Primers obtaining from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) were: TARP: 5′-TTTGCCATCATCACCTGCTGTCTG-3′ (Forward), 3′-GTATTGTCTGCCACCGTGTTCCTC-5′ (Reverse); and GAPDH: 5′-CTGGGCTACACTGAGCACC-3′ (Forward), 3′-GTAACGGGAGTTGCTGGTGAA-5′ (Reverse).
Analysis of association between TARP mRNA expression and clinical characteristics as well as survival
Analysis of the correlation between TARP expression and baseline clinical attributes, survival and treatment data were based on 29 AML cases at our centre and from AML patients available in the GEO datasets GSE425 and GSE6891 [Citation13,Citation14]. In GSE425, younger patients (<60 years) comprised the HD98A (Panel A) subgroup. They were treated with two cycles of the idarubicin, cytarabine, and etoposide (ICE) regimen as an initial induction regimen, followed by one course of high-dose cytarabine and mitoxantrone (HAM) as consolidation, and later risk-adapted late consolidation therapy according to different cytogenetics (HAM maintenance or autologous/allogeneic HSCT). In the HD98B subgroup (Panel B, >60 years), received two courses of ICE as an induction regimen, one cycle of HAM (with or without high-dose cytarabine and etoposide) as early consolidation and idarubicin and etoposide as late consolidation; autologous or non-myeloablative allogeneic HSCT was considered as alternative experimental consolidations [Citation13].
Statistical analysis
Clinical characteristics were analysed by one-way ANOVA, unpaired t-test, Pearson’s χ2-test and Fisher’s exact test, as appropriate. OS and progression-free survival (PFS) were estimated using Kaplan–Meier analysis. All the variables that influenced OS (p < .1) in the univariate analysis were subjected to a multivariate analysis using the Cox proportional hazards model. All statistical tests were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism v. 8.0 software (GraphPad, La Jolla, CA, USA). p-values < .05 were considered statistically significant.
Results
TARP mRNA was overexpressed in acute myeloid leukaemia
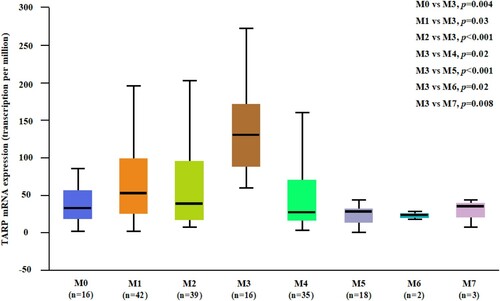
To study the transcriptional level of TARP in AML patients, gene expression profiling data were collected from the Oncomine database. The results showed that TARP was significantly upregulated in non-APL AML patients (p < .0001, Supplemental Figure 1A), and it was significantly higher in patients with APL than in other subgroups ( and Supplemental Figure 1A). cBioPortal (https://www.cbioportal.org/) [Citation16] online analysis did not identify any genetic mutation or fusion involved in TARP (data not shown). However, no prognostic role of TARP expression was observed in patients with APL (data not shown).
Higher TARP expression associated with FLT3 mutation in non-AYA AML
To understand the characteristics correlating to TARP expression in AML patients, we classified all the 97 AML patients (excluding APL cases) from GEO dataset GSE425 (Bullinger leukaemia in the Oncomine database) into groups having relatively higher and lower TARP expression levels depending on whether their TARP expression levels were higher or lower than the average expression level (). Both the subgroups had the same sex and age distributions. The mean white blood cell (WBC) count at diagnosis was 50.07×109/L (range, 2 × 109/L to 157.5 × 109/L) in the low TARP subgroup and 72.0 × 109/L (range, 1.1×109/L to 427 × 109/L) in the high TARP subgroup; however, the difference was not statistically significant (). Preceding malignancy rates in both the groups were similar. However, AML cases harbouring the common fusion genes CBFβ-MYH11, MLL-MLLT3 and RUNX1- RUNXT1 tended to be associated with lower TARP expression (p = .001, ). Further analysis showed that lower TARP expression was related to CBFβ-MYH11, but not the MLL-MLLT3 and RUNX1- RUNXT1 fusion genes (p = .011, 0.123, and 0.524, respectively; data not shown). Relatively higher TARP expression levels correlated with FLT3 mutations in AML, based on analysis of the GSE425 dataset ((A), p < .001), but not in the GSE6891 validation dataset ((D), p = .065). To further understand the association between TARP expression and FLT3 mutated status in different age groups, we conducted subgroup analyses in AYA and non-AYA patients; TARP expression was found to be comparable in both the groups (Supplemental Figure 1B). Results also revealed that TARP expression was unrelated to FLT3 status in AYA AML patients ((B,E), p = .096 and 0.856, respectively). However, in non-AYA patients, high TARP values were positively correlated with FLT3 mutations, especially FLT3-ITD mutations ((C,F), p = .034 and .021, respectively). The same trend was observed in our cohort, but no statistical difference was observed (non-AYA AML patients, (G), p = 0.066). TARP expression did not correlate with FLT3 mutations corresponding to different fusion gene subgroups (RUNX1-RUNXT1, CBFβ-MYH11 and MLL-MLLT3) in all patients as well as in the AYA and non-AYA subgroups (data not shown). High TARP values were related to lower favourable and higher intermediate chromosomal karyotypes based on the GSE425 dataset ((H), p = .0099). However, the same trend was not observed in any of the patients or AYA/non-AYA cases based on the GSE6891 validation dataset ((I), p = .312).
Figure 2. High TARP expression is related to high frequency of Fms-like tyrosine kinase (FLT3) alterations in non-APL patients, especially non-adolescents and young adults (AYA) AML patients. (A–F). Analysis of FLT3 mutations in low and high TARP groups of the total population, AYA subgroup and non-AYA subgroup (on the basis of the datasets GSE425 and GSE6891 downloaded from GEO). (G). Analysis of FLT3-internal tandem duplication (ITD) mutations in low and high relative TARP mRNA groups of non-AYA AML at our institute. (H–I). Composition of chromosomal karyotypes in low and high TARP groups based on analysis of the GSE425 and GSE6891 datasets. wt: wild type; mut: mutation.
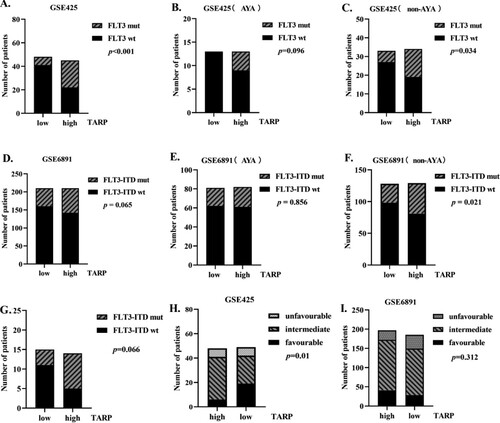
Table 1. Baseline characteristics of non-APL AML in low and high TARP arms of the GSE425 dataset.
Influence of TARP on efficacy and survival in AML
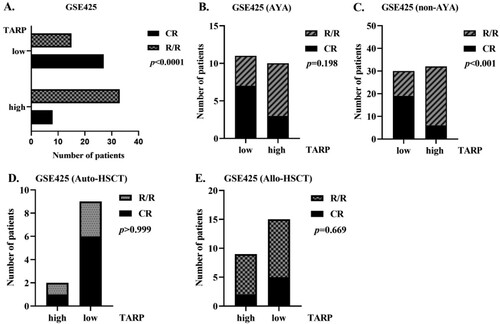
To explore whether an increase in TARP expression affects efficacy, we first compared treatment compositions in the subgroups that showed high and low expression of TARP. The two subgroups shared comparable chemotherapy protocol compositions, while more patients in the low TARP expression arm received transplantation, including auto-HSCT and allo-HSCT (, p = .028). Complete remission (CR) rate was remarkably higher in patients with low TARP. Thus, it seemed that patients who expressed high TARP levels were prone to relapse/refractory (R/R) diseases ( and (A), p < .0001). Further analysis showed that high TARP expression was related to poor therapeutic response in the non-AYA subgroup but not in AYA subgroup ((B,C), p < 0.001 and p = 0.198, respectively). To rule out an imbalance between the HSCT choices, we separately compared CR and R/R rates in allo-HSCT and auto-HSCT patients. No difference was observed between these groups ((E,F), p > 0.999 and p = .669, respectively). Because all the non-AYA patients receiving auto-HSCT had low TARP expression, we did not analyse the role of TARP in its efficacy in auto-HSCT subgroups. However, no differences were observed in therapeutic efficacies between the low and high TARP arms in auto- and allo-HSCT subgroups in AYA patients and those in non-AYA patients (data not shown).
Figure 3. High TARP levels are correlated with poor therapeutic response in non-APL AML subgroup, especially in non-AYA subgroup. (A–C). Therapeutic response of low and high TARP groups of the total population (A), AYA subgroup (B) and non-AYA subgroup (C) (GSE425). (D and E). Therapeutic response of low and high TARP arms in autologous-Hematopoietic stem cell transplantation (HSCT) and allogeneic-HSCT subgroups of the dataset GSE425. CR: complete remission; R/R: Relapsed/Refractory.
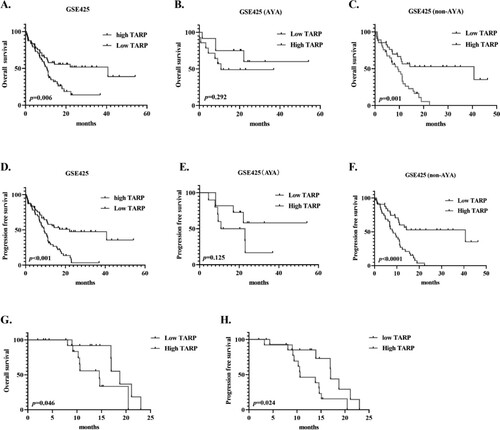
To clarify the effect of TARP expression on survival, Kaplan–Meier univariable analysis revealed that upregulation of TARP correlated with worse survival, with median OS and PFS of 10.53 and 9.367 months, respectively, compared to median OS and PFS of 40.67 and 22.1 months in the low TARP arm, respectively ((A,D), p = .006 and p < .001, respectively). We also investigated the prognostic role of TARP in the AYA and non-AYA subgroups. TARP was a poor predictor of OS and PFS in non-AYA patients, but not in AYA patients ((B,C and E,F)). Our data related to non-AYA cases showed the same trends (G,H).
Figure 4. High TARP levels were associated with poor overall survival (OS) and progression-free survival (PFS) in non-APL AML cases, especially in non-AYA cases. (A–C). OS curves (GSE425); (D–F). PFS curves (GSE425); (G–H). OS and PFS of non-AYA AML patients with low- and high-relative TARP mRNA at our institute.
Univariate analysis indicated that high TARF, FLT3 mutated status, intermediate cytogenetics and poor therapeutic response were inferior prognostic factors in AML (). Considering that imbalanced baseline characteristics were observed between the low and high TARF groups, patients who had undergone treatment (including HSCT) as well as those with preceding malignancies with a p < .1 were included in the Cox proportional hazards models. Unlike the univariable analysis, high expression of TARP did not predict a significant adverse prognosis when considering other factors (, hazard ratio = 1.283, p = .573). Age, preceding malignancy, FLT3 mutated status, HSCT and R/R after induction/consolidation therapies were independent factors in predicting an unfavourable prognosis in AML patients.
Table 2. Univariate and multivariate analyses of risk factors for OS in AML in the GSE425 dataset.
Discussion
AML is a heterogeneous malignancy with a 5-year OS of 20–40% in adults [Citation17]; those who harbour FLT3-ITD aberrations and exhibit complex cytogenetics have even worse survival [Citation1]. Relapse after an initial response due to the persistence of leukaemic stem cells (LSCs) is one of the key factors affecting the outcome of AML [Citation18]. However, LSCs cannot be distinguished from their normal counterparts. Recently, Barbara et al. reported an aberrant higher expression of TARP in AML-LSCs than in normal haematopoietic stem cells (HSCs). As a leukaemic marker, TARP is an immunotherapeutic target in response to TARP-T-cell receptor–engineered cytotoxic T cells [Citation19].
Promotion of proliferation of cancerous cells induced by TARP was first observed in prostate cancer [Citation20]. The role of TARP as an adoptive tumour immunotherapeutic target for cytotoxic T cells and helper T cells in prostate cancer has also been described [Citation20]. TARP expression in LSCs from 13 paediatric and 17 adult AML patients was analysed retrospectively. High TARP expression was associated with FLT3-ITD mutation and central nervous system involvement in paediatric AML patients but not in adult AML patients. In addition, TARP-T cell receptor–engineered cytotoxic T cells exhibited potent leukaemic cell-killing activities in vitro [Citation19]. Unfortunately, no large studies have been designed to delineate the function and prognostic role of TARP in adult AML.
Consistent with a previous report [Citation19], we observed significant overexpression of TARP mRNA in AML, especially in APL. The findings indicated that TARP might be correlated with APL pathogenesis; however, no significance in survival was observed in APL patients. In non-APL AML patients, we observed that low TARP expression was associated with the CBFβ-MYH11 fusion gene. Analysis of the GSE6891 dataset revealed that compared to cases with a normal karyotype, cases with inv16 (which results in the formation of CBFβ-MYH11 fusion gene) had a lower frequency of FLT3 mutation (17.65% vs 50.84%, p = .0003, data not shown). The core-binding factor (CBF) is a heterodimeric DNA-binding transcriptional regulator; CBF-AML comprises approximately 15% of cases of de novo adult AMLs and is considered a superior prognosis subgroup [Citation21]. It was reported that high TARP expression was not restricted to FLT3-ITD in adult AML [Citation19], we observed that aberrant TARP expression was significantly related to FLT3 alteration in non-AYA AML but not in AYA AML on the basis of bioinformatics analysis. Our data revealed the same trend, but no statistical significance was observed ((G), p = .066), possibly due to the small sample size. High TARP values predicted poor prognosis in both therapeutic responses as well as the survival of non-AYA AML patients (although this association was not independent in multivariate analysis). Although AML is a malignancy typically diagnosed in older adults, it still accounts for 33% of adolescent leukaemia. AYA AML was reported to have an improved therapeutic response rate and a trend towards better OS compared to non-AYA AML [Citation22]. Higher frequency of molecular alterations, cytogenetics aspects, such as FLT3 mutations, and unfavourable cytogenetic changes could be a possible explanation for the poor prognosis of non-AYA AML [Citation3]. In consistent with previous studies, multivariate analysis revealed that age, preceding malignancy, FLT3 mutation, unfavourable cytogenetics changes and poor response to treatment were independent inferior prognostic factors related to survival, whereas undergoing HSCT was a favourable factor [Citation1].
To explore the effect of TARP on efficacy, we compared the proportions of CR and R/R in TARP-high and -low subsets. The CR rate was significantly lower in TARP-high patients, whilst no difference was observed in the auto-HSCT and allo-HSCT subgroups. These findings suggest that HSCT could overcome the negative effects caused by the high expression of TARP.
Taken together, these results highlight the probable association of TARP overexpression with AML pathogenesis and unfavourable prognostic factors. Thus, non-AYA AML patients with high TARP expression could be suitable candidates for HSCT and TARP-cytotoxic T-cell therapy.
Supplemental Material
Download MS Word (240.2 KB)Acknowledgements
The authors are grateful to Lars Bullinger and Peter J. M. Valk for kindly providing clinical and prognostic data of MM patients via online datasets. The authors also thank Professor Chunling Wang and Professor Liang Yu for revising the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Stahl M, Tallman MS. Acute promyelocytic leukemia (APL): remaining challenges towards a cure for all. Leuk Lymphoma. 2019;60(13):3107–3115.
- Reville PK, Kadia T. Individualizing treatment for newly diagnosed acute myeloid leukemia. Curr Treat Options Oncol. 2020;21(4):34.
- Kuwatsuka Y, Tomizawa D, Kihara R, et al. Prognostic value of genetic mutations in adolescent and young adults with acute myeloid leukemia. Int J Hematol. 2018;107(2):201–210.
- Long L, Assaraf YG, Lei ZN, et al. Genetic biomarkers of drug resistance: a compass of prognosis and targeted therapy in acute myeloid leukemia. Drug Resist Update. 2020;52:100703.
- Tsapogas P, Mooney C J, Brown G, et al. The cytokine Flt3-ligand in normal and malignant hematopoiesis. Int J Mol Sci. 2017;18(6):1115.
- Abou Dalle I, Ghorab A, Patel K, et al. Impact of numerical variation, allele burden, mutation length and co-occurring mutations on the efficacy of tyrosine kinase inhibitors in newly diagnosed FLT3-mutant acute myeloid leukemia. Blood Cancer J. 2020;10(5):48.
- Kayamori K, Hagihara M, Kanda J, et al. Significance of FLT3-tyrosine kinase domain mutation as a prognostic factor for acute myeloid leukemia. Int J Hematol. 2019;110(5):566–574.
- Weiss AT, von Deetzen MC, Hecht W, et al. Molecular characterization of the feline T-cell receptor gamma alternate reading frame protein (TARP) ortholog. J Vet Sci. 2012;13(4):345–353.
- Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;14(18):93–110.
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6.
- Valk PJ, Verhaak RG, Beijen MA, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350(16):1617–1628.
- Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350(16):1605–1616.
- Verhaak RG, Wouters BJ, Erpelinck CA, et al. Prediction of molecular subtypes in acute myeloid leukemia based on gene expression profiling. Haematologica. 2009;94(1):131–134.
- Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404.
- Pourrajab F, Zare-Khormizi MR, Hekmatimoghaddam S, et al. Molecular targeting and rational chemotherapy in acute myeloid leukemia. J Exp Pharmacol. 2020;12:107–128.
- Valent P, Sadovnik I, Eisenwort G, et al. Immunotherapy-based targeting and elimination of leukemic stem cells in AML and CML. Int J Mol Sci. 2019;20(17):4233.
- Depreter B, Weening KE, Vandepoele K, et al. TARP is an immunotherapeutic target in acute myeloid leukemia expressed in the leukemic stem cell compartment. Haematologica. 2020;105(5):1306–1316.
- Fritzsche FR, Stephan C, Gerhardt J, et al. Diagnostic and prognostic value of T-cell receptor gamma alternative reading frame protein (TARP) expression in prostate cancer. Histol Histopathol. 2010;25(6):733–739.
- Rogers HJ, Wang X, Xie Y, et al. Comparison of therapy-related and de novo core binding factor acute myeloid leukemia: A bone marrow pathology group study. Am J Hematol. 2020;95(7):799–808.
- Pemmaraju N, Kantarjian H, Ravandi F, et al. Patient characteristics and outcomes in adolescents and young adults (AYA) with acute myeloid leukemia (AML). Clin Lymphoma Myeloma Leuk. 2016;16(4):213–222.