ABSTRACT
Background: Mixed chimerism (MC) frequently occurs in patients with severe aplastic anemia (SAA) after allogeneic hematological stem cell transplantation (allo-HSCT).
Methods
A retrospective study on 287 patients with SAA who underwent allo-HSCT between October 2012 and January 2020 was conducted to explore the outcomes, risk factors and treatment options for MC. Among 287 AA patients who excluded Fanconi anemia (FA), Congenital dyskeratosis (DKC), Paroxysmal nocturnal hemoglobinuria (PNH), etc.112 underwent matched sibling donor (MSD)-HSCT, 91 matched unrelated donor-HSCT and 84 haploidentical-HSCT. Patients were divided into the following 4 groups: group 1: Donor chimerism (DC); group 2: MC without cytopenia; group 3: MC with cytopenia; group 4: secondary graft failure (SGF).
Results
Compared with the other three groups, SGF predicted a poor prognosis of SAA (P< 0.001). In addition, SGF was associated with the early (within 3 months after transplantation) presence of MC and the high levels of MC. Uni- and multivariate logistic regression analysis showed that donor/recipient sex-mismatching and CTX + ATG regimen were high-risk factors for MC. Of note, in MC patients with cytopenia (group 3), the effective response rate reached 55% (6/11) following enhanced immunosuppression combined with cellular therapy, while only one of the four was effective who received enhanced immunosuppression alone.
Conclusion
SGF was associated with poor prognosis, early presence of MC and increased levels of recipient chimerism. The donor/recipient sex-mismatching and CTX + ATG regimen based MSD-HSCT were risk factors for MC. Cellular therapy could improve the effective response rate of patients with progressive MC.
Introduction
Aplastic anemia (AA) is a life-threatening hematological disease caused by multiple pathogenic mechanisms. Several complications, such as infection, hemorrhage and severe anemia, are common factors leading to mortality, particularly in patients with severe and very severe aplastic anemia (SAA and VSAA, respectively). Immunosuppressive therapy (IST) and allogeneic hematological stem cell transplantation (allo-HSCT) are the principal interventions for SAA at present [Citation1]. According to the treatment guidelines, matched sibling donor (MSD)-HSCT is recommended for patients with SAA aged <50 years [Citation2]. However, >2/3 of patients lacked a matched sibling. In recent years, considerable improvements have been made in matched unrelated donor (MUD)-HSCT and haploidentical (Haplo)-HSCT, primarily due to optimal conditioning regimens and advances in medications and supportive care, which enable patients with SAA and VSAA to undergo salvage treatment in emergency situations[Citation1,Citation3–8].
It is well known that stable long-term donor cell engraftment is crucial for successful allo-HSCT. However, mixed chimerism (MC) and graft failure (GF) seriously affect the implantation stability of donor cells, and they have a higher frequency in AA than hematological malignancies [Citation9,Citation10]. MC and GF may be caused by the use of non myeloablative conditioning regimen in AA transplantation [Citation10]. In addition, various studies have reported MC in both primary and secondary GF (SGF) [Citation9,Citation11–14]. Donor-recipient chimerism is a dynamic process early after HSCT. The status of MC may be transient mixed chimerism (TMC), which can evolve, towards graft failure or to completely donor chimerism (DC) [Citation9,Citation11,Citation15–17]. However, some patients develop stable MC, which has mainly been reported in patients with nonmalignant hematological diseases include AA, keeping with long-term normal complete blood cell count [Citation18]. The risk factors, clinical outcomes and treatment for AA patients with MC or SGF remain to be elucidated. The influence of MC on the outcome of SAA transplantation is still controversial up to now, and there is no data indicating the correlation between MC and SGF in SAA patients after transplantation. Therefore, this paper takes this as the starting point for demonstration.
The clinical information of 287 patients with SAA and VSAA who received allo-HSCT in the Guangzhou First People’s Hospital (Guangzhou, China) was explored in the present study, to investigate the risk factors, clinical outcomes and therapeutic interventions for MC and SGF.
Materials and methods
Patients
The clinical information of 287 patients with SAA and VSAA who received allo-HSCT from the Guangzhou First People’s Hospital between October 2012 and January 2020 were obtained. AA was diagnosed by peripheral blood (PB) count, bone marrow (BM) biopsy, chromosomal fragility and cytogenetics [Citation19]. The exclusion criteria were as follows: (a) Congenital BM failure such as Fanconi anemia, congenital dyskeratosis; (b) paroxysmal nocturnal hemoglobinuria; (c) early transplantation-related mortality before myeloid engraftment; (d) primary GF or accidental death following transplantation; (e) incomplete follow-up information. In patients who underwent MUD-HSCT, mobilized peripheral blood stem cells (PBSCs) were the sole stem cell source, whereas patients who underwent MSD-HSCT and Haplo-HSCT received both BM and PBSCs. The date of the last follow-up for all surviving patients was 31 Jan 2020, and the median follow-up time for surviving patients was 32 months (range, 3–86 months). The clinical characteristics of AA patients are listed in , and the conditioning regimens and the efficacy evaluation criteria of patients with MC are showed in Supplementary material S1. The present study was approved by the Ethics Committee of Guangzhou First People’s Hospital. All participants provided written informed consent.
Table 1. The clinical characteristics of SAA patients (n=287).
Detection of chimerism in AA patients after allo-HSCT
Chimerism evaluation was performed monthly in the first 3 months, every 3 months between 3 months and 1 year, and every 6 months after 1 year from allo-HSCT using bone marrow or peripheral blood mononuclear cell. If a decrease in the PB cell count was detected during the follow-up, chimerism evaluation was then performed at any time. When the sex was different between the patient and the donor, fluorescence in situ hybridization and microsatellite DNA fingerprinting (also known as short tandem repeat) were used to detect chimerism. When the sex was the same, only microsatellite DNA fingerprinting was used for detection. DC was defined as >95% of donor cells, MC as 5-95% of recipient cells, and GF was defined as <5% of donor cells detected at any time following HSCT. The level of MC was classified based on the percentage of residual host cells (RHCs) in the PBs (level 1, <10% RHCs; level 2, 10-25% RHCs; level 3, >25% RHCs). Cytopenia was defined as fluctuating blood count and no recovery following treatment (specifically, neutrophil count <0.5 × 109, granulocyte stimulating factor treatment ineffective for 2 weeks, platelet count <20 × 109, infusion and other treatments ineffective for 2 weeks, hemoglobin concentration <60 g/l and no increase in the infusion and other treatments for 2 weeks). Finally, according to the percentage of donor-recipient chimerism and the presence or absence of cytopenia, patients with AA were divided into the following four groups: Group 1, DC; group 2, MC without cytopenia; group 3, MC with cytopenia; group 4, SGF.
The treatment of MC patients
When MC occurred following allo-HSCT in patients with SAA, no clinical intervention was performed in addition to close monitoring of immunosuppressant concentration if the complete blood cell count continued to be normal of the patients. The serum concentrations of cyclosporine A (CSA) or tacrolimus were monitored and maintained at 150-250 ng/mL and 6-12 μg/mL, respectively. In addition, glucocorticoids or mycophenolic acid were added no more than 3 months to strengthen the immune suppression when MC consistently existed. When the donor cells percentage progressively declined in a short period of time and was accompanied by cytopenia(group3), mesenchymal stem cell (MSC) or umbilical cord blood stem cell (UCB) infusion was performed. Furthermore, donor stem cell infusion (DSI) was used in MC patients who could obtain hematopoietic stem cells from donor PBs, with an infusion quantity of mononuclear cells (MNCs) of 2-4 × 108 cells/kg. Following DSI, patients with MC were closely observed for at least 2 months. If the patient continued to exhibit cytopenia, repeat DSI or a second transplantation was performed.
Statistical analysis
All statistical analyses were performed using SPSS software (version 23.0, IBM Corp.) and R (version 3.6.1, https://www.rprpject.org/). The “survival” and “survminer” package was used to plot Kaplan-Meier curves, and the difference was compared by log-rank test. The forward stepwise method in uni- and multivariate logistic regression analyses was used to select risk factors. The comparison between two qualitative data was performed by chi-square test. Restricted Mean Survival Time (RMST) was determined by “survRM2” package. A two-sided P <0.05 was considered to indicate a statistically significant difference.
Results
Outcome based on time of onset and level of MC
A total of 58/287 (20.2%) AA patients developed MC following allo-HSCT (). As showed in (A), among the 26 patients who presented with MC within 3 months from HSCT, 8(30.8%) patients rejected the graft, while 11patients (42.3%) progressed to DC and 7(26.9%) patients exhibited stable MC. Among the 14 patients who developed MC within 3-6 months from HSCT, 7 patients progressed to DC and 1 patient to GF. Among the 10 patients who developed MC between 6 months and 1 year from HSCT, 1 patient (10%) progressed to DC, 1 patient (10%) progressed to GF and 8 patients (80%) exhibited stable MC. Among the 8 patients who developed MC 1 year from HSCT, 3 patients (37.5%) progressed to DC and 5 patients (62.5%) exhibited stable MC, and 0 rejected the graft. These results indicated that there were differences in the development of MC among the different time points following HSCT, and that SGF was associated with the early presence of MC.
Figure 1. Frequency and outcome of mixed chimerism (MC) based on (A) time of onset and (B) initial MC levels. Level 1, <10%residual host cells (RHCs); level 2, 10-25% RHCs; level 3, >25% RHCs. Dynamic changes in MC were monitored. The left panel of (B) shows the initial MC levels and the right panel the final MC level. In (B), the left side of picture B is the initial chimerism when MC appeared, we tracked the dynamic changes of chimerism of these patients. The right side of the picture shows the final outcome of chimerism.
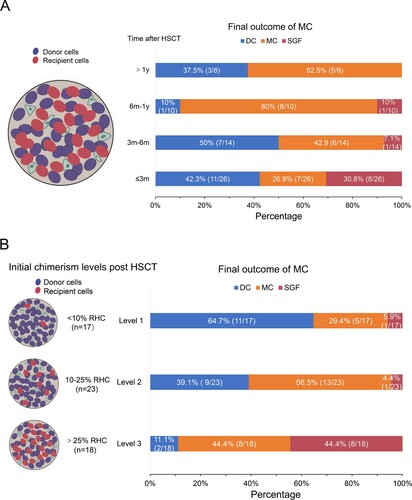
Among the 58, 17 patients had level 1, 23 patients had level 2 and 18 level 3 MC (B). Most (11/17, 64.7%) patients with level 1 MC progressed to DC, 5/17 (29.4%) patients exhibited persistent MC and 1/17 (5.9%) patients progressed to GF. In addition, 13/23 (56.5%) patients with level 2 exhibited persistent MC, while 1 patient rejected the graft. Graft rejection occurred in 8/18 (44.4%) patients with level 3 MC. These findings suggested that SGF was associated with the high levels MC.
Prognostic analysis of patients with MC
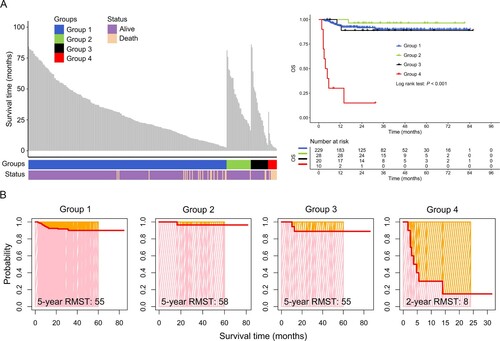
As shown in the left panel of (A), compared with groups 1, 2 and 3, group 4 patients (AA with SGF) had a shorter overall survival (OS) time. Furthermore, the survival curve suggested that these patients had a significantly poorer OS compared with patients from the other groups (right panel of A; P<0.001). In addition, the RMST of patients in group 4 was significantly shorter than that in groups 1, 2 and 3, and the 2-year RMST was only 8 months (B). These results showed that SGF can predict poor clinical outcome in patients with AA.
Figure 2. Prognostic analysis of aplastic anemia (AA)patients with different chimerism statuses following allogeneic hematological stem cell transplantation (allo-HSCT). (A) Distribution of survival time (left panel) and curves (right panel) of AA patients. (B) restricted mean survival time (RMST) of patients with severe aplastic anemia (SAA) with different chimerism statuses following allo-HSCT. Group 1, donor chimerism (DC); group 2, mixed chimerism (MC) without cytopenia; group 3, MC with cytopenia; group 4, secondary graft failure (SGF). OS, overall survival.
Risk factors for MC
The risk factors for MC without transfusion dependence was investigated by comparing group 2 with group 1. Uni- and multivariate logistic regression analyses found that donor/recipient sex-mismatching was an independent risk factor for MC without transfusion dependence (OR = 3.86, 95% CI 1.227, 12.144, P = 0.021). However, compared with the CTX + ATG regimen, the FCA and BUCY + ATG regimens were independent protective factors (OR<1). In addition, PBSCs were another independent protective factor compared with BM + PBSCs (OR = 0.177, 95% CI 0.049, 0.643, P = 0.009) (). Similarly, risk factors for MC with transfusion dependence were explored by comparing group 3 with group 1. Of note, it was found by uni- and multivariate logistic regression analysis that donor/recipient sex-mismatching was also an independent risk factor for MC with transfusion dependence (OR = 4.111, 95% CI 1.142, 14.793, P = 0.030). Furthermore, the FCA and BUCY + ATG regimens were also independent protective factors (OR <1) (). However, no independent risk factors for group 4 patients were identified (Table S1). These results suggested that donor/recipient sex-mismatching and the CTX + ATG regimen were risk factors for MC. Further investigation found that the percentage of MC was 53.6% (15/28) in patients who had been treated with CTX + ATG, while in patients with donor/recipient sex-mismatching it was 25.3% (41/162) (). In addition, univariate logistic regression analysis suggested that human leukocyte antigen (HLA) matching and MSD-HSCT may be risk factors for MC, and that percentages of MC are 22.7% (46/203) and 33% (37/112), respectively (). In the present study, the clinical course of MC was investigated, with a particular focus on the use of post-transplant immunosuppressants. A total of 16/58 patients (27.6%) with MC exhibited a low immunosuppressant concentration for >2 weeks in the 2 months prior to MC onset. This may indicate that a decrease in IS may be a high-risk factor for MC.
Table 2. Risk factors for MC patients without transfusion dependence.
Table 3. Risk factors for MC patients with transfusion dependence.
Therapeutic effect of MC patients with cytopenia or SGF
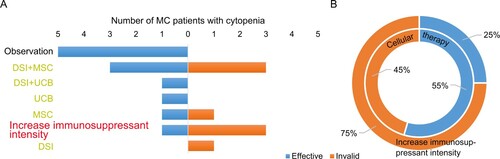
The effective response rate in group 3 following the use of DSI, MSC or UCB combined with enhanced immunosuppression for the treatment of progressive MC reached 55% (6/11), while only one of the four was effective who received enhanced immunosuppression alone (Table S2 and ). These results indicated that cellular therapy was a good choice for MC with cytopenia. The therapeutic effect of MC patients with SGF was further investigated, and it was found that 0/10 patients responded to DSI, MSC or UCB therapy (Table S3). Among these 10 patients, 5 (50%) patients succumbed to the disease, suggesting that SGF is associated with poor prognosis in MC.
Figure 3. Therapeutic effect of patients with MC with cytopenia or SGF following allo-HSCT. (A) Patient response to different treatments. (B) Patient response to increase immunosuppressant intensity and cellular therapy. donor stem cell infusion (DSI), mesenchymal stem cell (MSC) and umbilical cord blood stem cell (UCB) were part of cellular therapy. Inside circle, cellular therapy; outside circle, increase immunosuppressant intensity.
Discussion
A relatively high incidence of MC has been reported in patients receiving HSCT for AA[Citation11,Citation14,Citation16], and MC has been found to be associated with GF [Citation12,Citation20]. In the present study, the probability of SGF was associated with the time that had elapsed from HSCT, as well as the number of RHCs present in the recipient at MC onset. However, MC is not like SGF, in the sense that MC can exist stably for a long time in non-malignant hematological diseases with hematological recovery[Citation17]. The present study showed that certain patients were able to maintain effective hematopoiesis under 60-80% chimerism of the donor. For those patients, the OS rate was not significantly affected, which was consistent with previous studies [Citation21]. For patients with MC without cytopenia, in particular, the prognosis is favorable, but it is recommended that these patients should be followed up closely, and attention should be paid to the immunosuppressant (IS) serum concentration. The coexistence of donor and recipient hematopoietic cells in the recipient’s body may be due to bidirectional immune tolerance. However, for patients in whom MC occurs early after transplantation and the RHCs reach level 3, the disease progresses quickly, and timely treatment is therefore of the essence.
The impact of allo-HSCT and MC has been found to be associated with the conditioning regimen and donor source [Citation11,Citation20]. The present study suggested that donor/recipient sex-mismatching was a risk factor for MC, especially male donor to female patients in MSD-HSCT. In this study, among the 48 patients in groups 2 and 3, 7 patients were F-F or M-M, accounting for 14.6%, and 30 patients were M-F, accounting for 62.5%, 11 patients were F-M, accounting for 22.9%. Another study also demonstrated that patients with donor/recipient sex-mismatching had a decreased survival and an increased risk of rejection. These may be associated with the h-Y gene on the Y chromosome, and women can develop a rejection reaction against the h-Y antigen [Citation22]. Recent studies have suggested that fludarabine (Flu)-based conditioning regimens are superior to the traditional CTX + ATG regimen in matched donor transplantation [Citation23,Citation24]. Particularly in old patients or patients with a high risk of GF, Flu-based conditioning regimens combined with low-dose cyclophosphamide are more conducive to the survival of patients [Citation2,Citation25]. In the present study, the incidence rate of MC in patients treated with the FCA conditioning regimen was lower than that in patients treated with the CTX + ATG conditioning regimen. The MC rate was high in CTX + ATG-treated patients following transplantation, with an incidence rate of 53.57%. In addition, a high incidence of MC was reported following MSD-HSCT, which may be due to the use of non myeloablative conditioning regimen. This study showed that the incidence of MC in patients receiving BUCY + ATG(h) regimen prior to Haplo-HSCT was low, but the mortality rate was high in MC with SGF, which may be associated with the presence of a donor-specific antibody in the recipient when HLA is incompatible [Citation14,Citation26].
In allo-HSCT for malignant diseases such as leukemia, the decrease in IS has been reported to favor the transition from MC to DC. However, that is not the case in AA. Studies have reported that MC and SGF appear as a result of low cyclosporine concentration or a reduction in cyclosporine after partial AA transplantation [Citation17]. Kako S et al. [Citation27] investigated the clinical course of patients with SGF, with a particular focus on the use of post-transplant ISs. SGF developed prior to the tapering of ISs in 14/19 of patients. Therefore, the decrease in IS may be a high-risk factor for MC.
The present results were consistent with these reports. In this study, a total of 16/58 patients (27.6%) patients with 58 MC had a low IS concentration for >2 weeks within 2 months from the onset of MC. Therefore, the decrease in IS concentration may be a high-risk factor for MC. On the other hand, increase immunosuppressant intensity is effective for MC patients with a slow disease progression and RHC levels 1 and 2. In these patients, residual recipient lymphocytes in the PB might attack donor-derived hematopoietic cells in the BM. An increase in IS may reverse this condition. In addition, DSI, MSC or UCB were used for the treatment of progressive MC and SGF. However, DSI may aggravate the occurrence of graft-versus-host disease. At present, there is no uniform standard for the time and cell number required for DSI. In the present study, MC patients with cytopenia who received DSI had a more effective response rate of 55%. The prognosis of patients with SGF following Haplo-HSCT was poor, even though some patients underwent secondary transplantation. Thus, timely identification and intervention is necessary for these patients.
There were several limitations to the present study. First, this was a retrospective study including a small number of patients who developed MC in a single center. Second, the timing and source for chimerism analysis was at each physician’s discretion, and therefore varied. This led to inevitable deviation in the conclusions drawn. However, even with these limitations, the present study included a large number of patients with SAA with detailed chimerism analysis results, which provided important insights into the current state of MC and SGF following HSCT in AA.
Conclusion
In conclusion, SGF was associated with poor prognosis, early (within 3 months after transplantation) presence of MC and increased levels of recipient chimerism. Donor/recipient sex-mismatching and CTX + ATG regimen based-MSD-HSCT were risk factors for MC. In addition, patients who received a reduced-intensity conditioning regimen were more likely to develop MC. Of note, cellular therapy combined with enhanced immunosuppression may improve the effective response rate of patients with progressive MC.
Ethics approval and consent to participate
This study was conducted according to the principals of the Declaration of Helsinki and was approved by the Ethics Committee of Guangzhou First People's Hospital. All participants were provided with written informed consent.
Patient consent for publication
Not required.
Availability of data and materials
All supporting data are included in the manuscript and supplemental files. Additional data are available upon reasonable request to the corresponding author.
Authors’ contributions
YPZ: Contributed to the concept development, study design, and helped write the manuscript. YLZ: Collected the clinical information, interpreted the data, and wrote the manuscript. YML, LLW, MZ, CXW, WJM, XWC, SLX, RQZ and SQW: Contributed to the diagnosis and treatment of patients and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bacigalupo A, Passweg J. Diagnosis and treatment of acquired aplastic anemia [J]. Hematol Oncol Clin North Am. 2009;23(2):159–170.
- Killick S B, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia [J]. Br J Haematol. 2016;172(2):187–207.
- Dufour C, Pillon M, Passweg J, et al. Outcome of aplastic anemia in adolescence: a survey of the severe aplastic anemia Working Party of the European group for blood and Marrow transplantation [J]. Haematologica. 2014;99(10):1574–1581.
- Dufour C, Pillon M, Socie G, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant [J]. Br J Haematol. 2015;169(4):565–573.
- Ebens C L, Defor T E, Tryon R, et al. Comparable outcomes after HLA-matched sibling and alternative donor hematopoietic cell transplantation for children with Fanconi anemia and severe aplastic anemia [J]. Biol Blood Marrow Transplant. 2018;24(4):765–771.
- Bacigalupo A, Socie G, Hamladji R M, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis [J]. Haematologica. 2015;100(5):696–702.
- Xu L P, Jin S, Wang S Q, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant [J]. J Hematol Oncol. 2017;10(1):25.
- Xu Z L, Zhou M, Jia J S, et al. Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia [J]. Bone Marrow Transplant. 2019;54(8):1319–1326.
- Stikvoort A, Sundin M, Uzunel M, et al. Long-Term stable mixed chimerism after hematopoietic stem cell transplantation in patients with Non-malignant disease, shall We Be tolerant? [J]. PLoS One. 2016;11(5):e0154737.
- Satwani P, Cooper N, Rao K, et al. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases [J]. Bone Marrow Transplant. 2008;41(2):173–182.
- Mccann S, Passweg J, Bacigalupo A, et al. The influence of cyclosporin alone, or cyclosporin and methotrexate, on the incidence of mixed haematopoietic chimaerism following allogeneic sibling bone marrow transplantation for severe aplastic anaemia [J]. Bone Marrow Transplant. 2007;39(2):109–114.
- Bader P, Niethammer D, Willasch A, et al. How and when should we monitor chimerism after allogeneic stem cell transplantation? [J]. Bone Marrow Transplant. 2005;35(2):107–119.
- Kong Y, Shi MM, Zhang YY, et al. N-acetyl-L-cysteine improves bone marrow endothelial progenitor cells in prolonged isolated thrombocytopenia patients post allogeneic hematopoietic stem cell transplantation [J]. Am J Hematol. 2018;93(7):931–942.
- Mattsson J, Ringden O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation [J]. Biol Blood Marrow Transplant. 2008;14(1 Suppl 1):165–170.
- Shamshad G U, Ahmed S, Bhatti F A, et al. Mixed donor chimerism in non-malignant haematological diseases after allogeneic bone marrow transplantation [J]. J Coll Physicians Surg Pak. 2012;22(12):765–768.
- Andreani M, Testi M, Lucarelli G. Mixed chimerism in haemoglobinopathies: from risk of graft rejection to immune tolerance [J]. Tissue Antigens. 2014;83(3):137–146.
- Lawler M, Mccann S R, Marsh J C, et al. Serial chimerism analyses indicate that mixed haemopoietic chimerism influences the probability of graft rejection and disease recurrence following allogeneic stem cell transplantation (SCT) for severe aplastic anaemia (SAA): indication for routine assessment of chimerism post SCT for SAA [J]. Br J Haematol. 2009;144(6):933–945.
- Ashizawa M, Akahoshi Y, Nakano H, et al. A combination of fludarabine, half-dose cyclophosphamide, and anti-thymocyte globulin is an effective conditioning regimen before allogeneic stem cell transplantation for aplastic anemia [J]. Int J Hematol. 2014;99(3):311–317.
- Marsh J C, Ball S E, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia [J]. Br J Haematol. 2009;147(1):43–70.
- Kako S, Kanda Y, Onizuka M, et al. Allogeneic hematopoietic stem cell transplantation for aplastic anemia with pre-transplant conditioning using fludarabine, reduced-dose cyclophosphamide, and low-dose thymoglobulin: A KSGCT prospective study [J]. Am J Hematol. 2020;95(3):251–257.
- Huss R, Deeg H J, Gooley T, et al. Effect of mixed chimerism on graft-versus-host disease, disease recurrence and survival after HLA-identical marrow transplantation for aplastic anemia or chronic myelogenous leukemia [J]. Bone Marrow Transplant. 1996;18(4):767–776.
- Stern M, Passweg J R, Locasciulli A, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia [J]. Transplantation. 2006;82(2):218–226.
- Bacigalupo A, Socie G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party [J]. Haematologica. 2010;95(6):976–982.
- Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen [J]. Haematologica. 2009;94(9):1312–1315.
- Chaudhry Q UN, Iftikhar R, Satti T M, et al. Outcome of fludarabine-based conditioning in high-risk aplastic anemia patients undergoing matched related donor transplantation: A single-center study from Pakistan [J]. Biol Blood Marrow Transplant. 2019;25(12):2375–2382.
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT [J]. Bone Marrow Transplant. 2013;48(4):537–543.
- Kako S, Yamazaki H, Ohashi K, et al. Mixed chimerism and secondary graft failure in allogeneic hematopoietic stem cell transplantation for aplastic anemia [J]. Biol Blood Marrow Transplant. 2020;26(3):445–450.
