ABSTRACT
Objective
Infections in ruxolitinib-treated myeloproliferative neoplasm (MPN) patients were reported frequently. This work aimed to systematically estimate the risk of infection associated with ruxolitinib in MPN patients.
Methods
The PUBMED, CNKI, EMBASE, Cochrane and CBM databases were searched to identify all related studies. Odds ratio (OR) and 95% confidence interval (CI) were used to express the difference between groups. I2 was calculated to evaluate heterogeneity. Revman software was used to conduct the analysis.
Results
Eleven randomized control trials were included in this analysis. The risk of overall infections was not different at the early stage of ruxolitinib use (OR, 95%CI: 1.23, [0.91, 1.67]). In the extension phase, overall infection was significantly lower in patients receiving ruxolitinib (OR, 95%CI: 0.53, [0.36, 0.79]). Herpes zoster infection was at higher risk both at early stage and in the extension phase (OR, 95%CI: 7.39, [1.33, 41.07]), (OR, 95%CI: 5.23, [1.46, 18.79]), respectively.
Conclusion
Our study suggested that ruxolitinib increased the risk of herpes zoster infection. However, current studies were not enough to estimate the effects of ruxolitinib on the risk of overall infection in patients with myeloproliferative neoplasm.
KEYWORDS:
1. Introduction
Myeloproliferative neoplasms, including myelofibrosis (MF), essential thrombocythemia (ET) and polycythemia vera (PV), are associated with the dysregulation of JAK-STAT (Janus kinase- signal transducer and activator of transcription) signaling pathway. JAK2, CALR and MPL were the three mainly known phenotypic drivers for their role in MPN development [Citation1]. Almost all PV patients had JAK2 mutation, and JAK2 mutation also occurred in about 55% ET patients and 65% PMF patients, respectively [Citation2]. These mutations caused hyperactivity of JAK-STAT signaling pathway. On the basis of the understanding of molecular and cellular mechanisms of MPNs, a novel targeted treatment was developed. Ruxolitinib is a potent JAK (JAK1/JAK2) inhibitor, approved by the US Food and Drug Administration for MF and PV treatment in 2011 and 2014 [Citation3], respectively and was effective in the treatment of myelofibrosis and polycythemia vera. Patients treated with ruxolitinib had reduced spleen size, improved constitutional symptoms and survival compared with placebo or best available therapy in several randomized control trials [Citation4–7].
However, JAK inhibitors also suppressed the immune system. Ruxolitinib reduced the levels of several inflammatory factors, such as IL-6 and C-reaction protein [Citation8,Citation9]. Studies suggested that ruxolitinib impaired the function of natural killer cell [Citation10], caused functional silencing of T helper cell and reduction of T regulatory cells [Citation11] and impaired virus-specific T cell response [Citation12] and dendritic cell function [Citation13]. These may potentially result in increased infections in patients who used ruxolitinib. In recent years, cases regarding infections were reported frequently in MPN patients treated with ruxolitinib [Citation14,Citation15]. Research studies on infections were limited and it’s necessary to assess the risk of infection in MPN patients treated with ruxolitinib. Therefore, this study aimed to evaluate the risk of overall infection in patients treated with ruxolitinib compared with placebo or best available therapy in the early stage of treatment or extension phase, respectively and then we analyzed the risk of herpes zoster infection.
2. Method
2.1. Search strategy
We searched PUBMED, CNKI, EMBASE and CBM databases to identify studies associated with ruxolitinib and infections, with keywords ‘myelofibrosis’, ‘polycythemia vera’, ‘essential thrombocythemia’, ‘myeloproliferative neoplasm’, ‘ruxolitinib’ and ‘infection’. The search terms were ‘((((((Myeloproliferative neoplasm) OR (myelofibrosis)) OR (polycythemia vera)) OR (essential thrombocythemia))) AND (ruxolitinib)) AND (infection)’ and ‘(((((Myeloproliferative neoplasm) OR (myelofibrosis)) OR (polycythemia vera)) OR (essential thrombocythemia))) AND (ruxolitinib)’. We aimed to identify all related randomized clinical trials. Two researchers searched the database according to the strategy. References of these retrieved studies were regarded as the supplement source to conduct a further collection.
2.2. Study selection
The eligible criteria were as follows: (1) randomized clinical trials or phase III studies which reported infections related to ruxolitinib in MPN patients; (2) both the ruxolitinib group and the control group reported infections or marked symptoms of potential infection; (3) the treatment of the control group could be any of placebo or best available therapy; (4) the samples were patients diagnosed with primary MF, ET, PV, post ET-MF, or post PV-MF.
Studies were excluded if they met any of the following criteria: (1) case report; (2) the infections were not reported in both groups; (3) research of ruxolitinib combined with other drugs; (4) enrolled samples were diagnosed with other diseases or healthy populations.
2.3. Data extraction strategy
Patients’ characteristics (sample size, age, etc.), therapy methods for both group patients and infection-related data were extracted. In the infectious data, overall infections and herpes zoster infections were extracted. For a few research studies, overall infectious events were not reported so that we regarded two marked symptoms (cough and pyrexia) as potential infections and potential infection events of these studies were included in the pooled analysis. Some clinical trials have long-term follow-up, researchers may publish their findings at different stages for several times. In this study, the early stage was defined as the beginning of treatment to the cut-off time that the first publication reported, and the extension phase was followed by the early stage. In the extension phase, most patients, primarily allocated into the control group, could cross over into the ruxolitinib group or discontinued for reasons. Results in the extension phase may report more than once. To avoid more bias, we enrolled the first extension-phase publication into analysis, when all patients in the control group cross over into the ruxolitinib group or discontinued.
2.4. Statistical analysis
Revman 5.4.1 software was used to conduct this meta-analysis. Statistic heterogeneity was estimated by I2 value. Odd ratio (OR) was calculated in the pooled analysis to express the infection risk by the random model in the case of I2 > 30%; otherwise, a fixed model was used. Publication bias was estimated by the funnel plot.
3. Results
3.1. Study characteristics
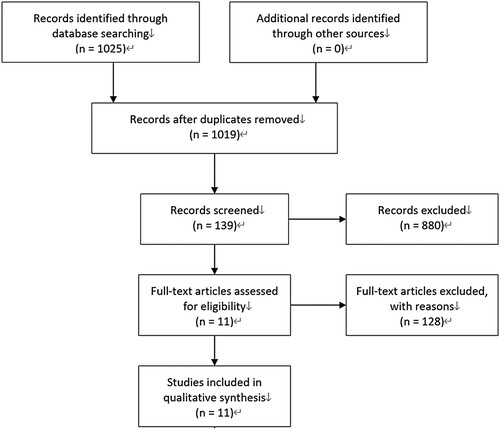
A total of 1025 publications were searched out. After careful reading of these articles, 6 randomized clinical trials and 11 publications were enrolled in this work [Citation4–7,Citation9,Citation16–21]. represents the process of study selection. There were 597 patients receiving ruxolitinib treatment and 527 receiving placebo or best available therapy (BAT). A total of 528 patients were diagnosed as MF, 481 patients were diagnosed as PV and 115 patients as ET. Crossover into the ruxolitinib group from the control group was allowed in RELIEF study, COMFORT I trial, COMFORT II trial, RESPONSE study and RESPONSE II study. MAJIC study was composed of two parts: ruxolitinib treatment in ET patients and in PV patients. However, results of the later part have not been published and the results relating to the ET patients were enrolled in this analysis. COMFORT-I trial, COMFORT-II trial, RESPONSE study and RESPONSE-II study published several extension-phase results and we included one of these publications according to our eligible criteria. gives the detailed information about the characteristics of included research studies. The results of COMFORT II published in 2016 were enrolled in the analysis for herpes zoster infection due to the lack of herpes zoster infection report in the 2013 publication.
Table 1. Characteristics of included research studies.
3.2. Risk of bias assessment
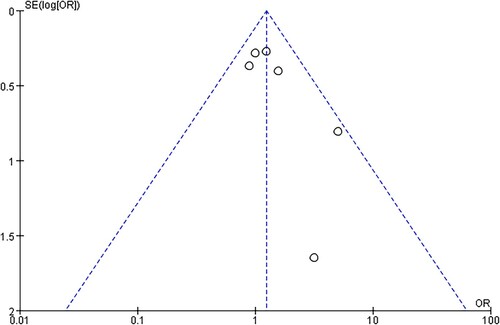
Risk of bias was assessed by Cochrane criteria. No study was at low risk of bias. depicts the detailed risk of bias. The funnel plot was relatively symmetrical, demonstrating a low risk of publication bias ().
Table 2. Assessment of bias risk.
3.3. Risk of overall infection
A total of 6 randomized control trials were enrolled in this section for analysis. These data were retrieved at the early stage of treatment. Three publications (RESPONSE II 2016, RESPONSE 2015, and MAJIC-ET 2017) reported overall infection rate in two groups, the COMFORT I trial published in 2012 reported parts of infection so that we added two marker symptoms (cough and pyrexia) of infection into the pooled analysis, and the RELIEF trial only reported the herpes zoster infection. As the results stated, patients receiving ruxolitinib treatment didn’t experience an increased risk of infection at the early stage of treatment (OR, 95%CI: 1.23, [0.91, 1.67]) ().
Figure 3. Risk of overall infection in patients receiving ruxolitinib. (A) Indicated the risk of overall infection at the early stage, and the odds ratio was calculated by the total number of patients. (B) Depicted the risk of overall infection in the extension phase, and the Odds ratio was evaluated by the patient-year of exposure.
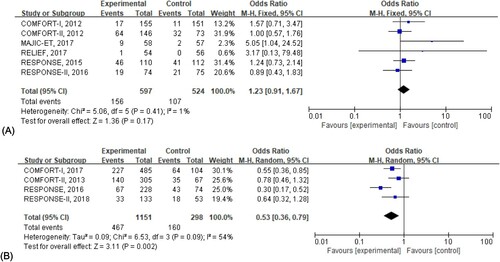
In the analysis of the extension phase, we included 4 studies and the risk of infection was evaluated by the rate of per 100 patient-year exposure. The results suggested a lower risk of infection in the ruxolitinib group with statistical significance when compared with the control group (OR, 95%CI: 0.53, [0.36, 0.79]) ().
Figure 4. Risk of herpes zoster infection in patients receiving ruxolitinib. (A) Indicated the risk of herpes zoster infection at the early stage, and the odds ratio was calculated by the total number of patients. (B) Depicted the risk of herpes zoster infection in the extension phase, and the odds ratio was evaluated by the patient-year of exposure.
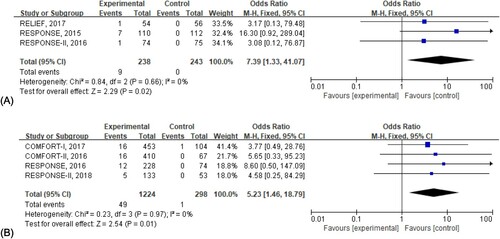
3.4. Risk of herpes zoster infection
Herpes zoster infection was reported frequently. To identify whether ruxolitinib was associated with higher herpes zoster infection, we conducted further analysis. Only 3 studies reported the herpes zoster infection at the early stage and 4 in the extension phase. As stated in the following figures, herpes zoster infection was significantly higher in patients receiving ruxolitinib both at the early stage (OR, 95%CI: 7.39, [1.33, 41.07]) and in the extension phase (OR, 95%CI: 5.23, [1.46, 18.79]).
4. Discussion
This study systematically evaluated the risk of infection associated with ruxolitinib in MPN patients, and we collected all available phase III randomized control trials. In our study, we found that the risk of overall infection was not significantly increased at the early stage of ruxolitinib treatment, while in the extension phase, a significant reduction of overall infection risk was observed. We suggested taking this conclusion carefully due to the crossover or discontinuation of control group patients, but the basic characteristics, such as age and gender, were unclear about these patients. Herpes zoster infection rate was higher with significance both at the early stage and in the extension phase compared with placebo or best available therapy, which demonstrated a higher risk of herpes zoster infection caused by ruxolitinib.
After the widespread use of ruxolitinib, a series of case reports regarding infections were published. For example, a PV patient experienced a reactivation of HBV after a 4-week treatment of ruxolitinib [Citation22], primary myelofibrosis patient suffered from Epstein–Barr virus (EBV) gastric ulcer due to severe immunosuppression caused by ruxolitinib [Citation23], and Klebsiella pneumoniae infections were also reported frequently in patients [Citation14]. Reactivation of tuberculosis and other bacterial or viral infections were also found in MPN patients using ruxolitinib [Citation15,Citation24].
Research studies show that ruxolitinib reduced the count of various immune cells and impaired their function, including dendritic cell, T helper cell, natural killer cell and so on. The ability of JAK inhibitor to inhibit multiple cytokines at once raised clinical concerns about the increased risk of infection. Rumi et al. followed up 25 patients to detect the dynamic viral load and their T cell response, suggesting that ruxolitinib treatment impaired patients’ virus-specific T cell response and caused subclinical reactivation of several virus, and this immunological consequence might be related to treat duration, treatment more than 5 years was more likely to manifest these symptoms [Citation12].
However, except for some case reports regarding infectious events, a few research studies had focused on the risk of infection caused by ruxolitinib treatment. Federico et al first systematically evaluated the risk of infection in patients treated with ruxolitinib [Citation25]; their work indicated higher risk of herpes zoster infection in patients treated with ruxoliyinib, which was consistent with our conclusion. The downside was that they did not evaluate the risk of overall infection. Our work filled up this downside and updated the information about infection risk in the extension phase.
Although ruxolitinib showed great effects on improving patients’ constitutional symptoms and survival, its adverse events can’t be ignored. Researchers pointed out the necessity to screen and treat latent tuberculosis before ruxolitinib treatment [Citation26]. Since JAK is crucial in response to IL-10, IL-6, IFN-γ and other key cytokines relating to the innate or adaptive immunity [Citation27], the risk of infection seems inevitable caused by the use of JAK inhibitor. Our study also confirmed this point. An accurate assessment of infection risk is necessary and clinically significant. In fact, the lack of literatures on infection report limited our works so that we could not accurately estimate the risk of overall infection associated with ruxolitinib use. We suggest future works give more attention on comprehensive infectious events.
Overall, ruxolitinib was effective on improving MPNs patients’ symptoms and survival while its concerns about increased risk of infection emerged. Herpes zoster infection was higher in ruxolitinib-treated patients. Clinicians ought to carefully evaluate the patient’s conditions when prescribing the drug and take proper prophylaxis methods when necessary. We also suggest more cohort studies focus on the infectious events.
Acknowledgements
We thank our hospital and all department members for their support and encouragement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood. 2017;130(23):2475–2483. DOI:https://doi.org/10.1182/blood-2017-06-782037.
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(12):1599–1613. DOI:https://doi.org/10.1002/ajh.26008.
- Passamonti F, Maffioli M. The role of JAK2 inhibitors in MPNs 7 years after approval. Blood. 2018;131(22):2426–2435. DOI:https://doi.org/10.1182/blood-2018-01-791491.
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821–829. DOI:https://doi.org/10.3324/haematol.2016.143644.
- Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–1707. DOI:https://doi.org/10.1038/leu.2016.148.
- Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017;130(17):1889–1897. DOI:https://doi.org/10.1182/blood-2017-05-785790.
- Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxycarbamide therapy versus switching to ruxolitinib in patients with polycythaemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Br J Haematol. 2017;176(1):76–85. DOI:https://doi.org/10.1111/bjh.14382.
- Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 in hibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. DOI:https://doi.org/10.1056/NEJMoa1002028.
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. DOI:https://doi.org/10.1056/NEJMoa1110556.
- Schönberg K, Rudolph J, Vonnahme M, et al. JAK inhibition impairs NK cell function in myeloproliferative neodelimplasms. Cancer Res. 2015;75(11):2187–2199. DOI:https://doi.org/10.1158/0008-5472.CAN-14-3198.
- Keohane C, Kordasti S, Seidl T, et al. JAK inhibition induces silencing of T helper cytokine secretion and a profound reduction in T regulatory cells. Br J Haematol. 2015;171(1):60–73. DOI:https://doi.org/10.1111/bjh.13519.
- Rumi E, Sant'Antonio E, Cavalloni C, et al. Impaired virus-specific T cell responses in patients with myeloproliferative neoplasms treated with ruxolitinib. Hematol Oncol. 2020;38(4):554–559. DOI:https://doi.org/10.1002/hon.2769.
- Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–1202. DOI:https://doi.org/10.1182/blood-2013-03-484642.
- Hanna RM, Khalid M, El-Nour LA, et al. Frequent Klebsiella pneumoniae urinary tract infections in a patient treated with ruxolitinib. Antibiotics. 2019;8(3):150.
- Khalid F, Damlaj M, AlZahrani M, et al. Reactivation of tuberculosis following ruxolitinib therapy for primary myelofibrosis: case series and literature review. Hematol Oncol Stem Cell Ther. 2020. DOI:https://doi.org/10.1016/j.hemonc.2020.02.003.
- Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18(1):88–99. DOI:https://doi.org/10.1016/S1470-2045(16)30558-7.
- Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. DOI:https://doi.org/10.1056/NEJMoa1409002.
- Griesshammer M, Saydam G, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann Hematol. 2018;97(9):1591–1600. DOI:https://doi.org/10.1007/s00277-018-3365-y.
- Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55. DOI:https://doi.org/10.1186/s13045-017-0417-z.
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. DOI:https://doi.org/10.1056/NEJMoa1110557.
- Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. DOI:https://doi.org/10.1182/blood-2013-02-485888.
- Kirito K, Sakamoto M, Enomoto N. Elevation of the hepatitis B virus DNA during the treatment of polycythemia vera with the JAK kinase inhibitor ruxolitinib. Intern Med. 2016;55(10):1341–1344. DOI:https://doi.org/10.2169/internalmedicine.55.5529.
- Kusano Y, Terui Y, Ueda K, et al. Epstein-Barr virus gastric ulcer associated with ruxolitinib. Ann Hematol. 2016;95(10):1741–1742. DOI:https://doi.org/10.1007/s00277-016-2748-1.
- Prakash K, Richman D. A case report of disseminated histoplasmosis and concurrent cryptococcal meningitis in a patient treated with ruxolitinib. BMC Infect Dis. 2019;19(1):287. DOI:https://doi.org/10.1186/s12879-019-3922-6.
- Lussana F, Cattaneo M, Rambaldi A, et al. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339–347. DOI:https://doi.org/10.1002/ajh.24976.
- Hirai N, Kasahara K, Yoshihara S, et al. Necessity to screen and treat latent tuberculosis before ruxolitinib treatment-ruxolitinib-associated disseminated tuberculosis: a case report and literature review. IDCases. 2020;21:e00892. DOI:https://doi.org/10.1016/j.idcr.2020.e00892.
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–20063. DOI:https://doi.org/10.1074/jbc.R700016200.