ABSTRACT
Purpose
This study aimed to investigate the latent Epstein–Barr virus (EBV) infection status of patients with newly diagnosed Hodgkin lymphoma (HL) and to discuss the relationship between tumor cell EBV status and the prognosis of HL patients.
Patients and methods
A total of 134 previously untreated HL patients were analyzed in the study. Epstein–Barr virus encoded RNAs (EBERs) in situ hybridization was performed to detect the EBV status of tumor cells.
Results
EBV positive status correlated with sex (p=0.046) and the proportion of extranodal lesions(p=0.037). There was no obvious correlation between EBV status and overall survival (OS) or failure-free survival (FFS) in all cases, but in cases over 50 years old, EBV positive group had an inferior 5-year FFS compared with EBV negative group (38.5%±13.5% vs 90.9%±8.7%, p=0.012). In FFS multivariate analysis of this age subgroup, EBV positive status was associated with significantly inferior survival (HR, 10.10; 95% CI, 1.26–81.08; p=0.030).
Conclusion
This study demonstrates positive tumor cell EBV status is an unfavorable prognostic factor in elder HL patients.
1. Introduction
Hodgkin lymphoma (HL) is an important subtype of malignant lymphoma, and its occurrence can be affected by many factors. With improvements in diagnostic technology, changes in people's living habits, and other factors, the incidence of HL is increasing. The incidence of HL varies among different races worldwide. It is most common in Caucasians, followed by Africans and Hispanics, and least common in Asians [Citation1,Citation2]. Based on the analysis of 10,002 patients in multiple Chinese centers, the Chinese Lymphoma Pathology Research Cooperation Group found that HL accounted for 8.54% of lymphoma cases in China [Citation3].
It has been almost two centuries since the discovery of HL, but its etiology and pathogenesis are still not fully understood. The relationship between HL and Epstein–Barr virus (EBV) infection has been a recent advance in HL research [Citation4]. In 1987, Weiss et al [Citation5] detected EBV DNA in the tissues of patients diagnosed with HL using molecular hybridization. Using subsequent detection techniques, EBV DNA has been detected in R-S (Reed-Sternberg) HL cells, indicating that EBV infection occurs before the clonal proliferation of cells. Therefore, it is strongly believed that EBV plays an etiological role in at least part of the pathogenesis of HL [Citation6]. The detection rate of EBV in HL patients differs between regions and races, and the impact of EBV positivity on prognosis is still inconclusive.
The Chinese province of Fujian has a high incidence of EBV-related nasopharyngeal carcinoma [Citation7,Citation8]. This suggests that EBV is common in Fujian, and the EBV status of HL is also worth investigating. This study screened patients with HL admitted to Fujian Cancer Hospital from December 2012 to September 2019, retrospectively analyzed the latent infection status of EBV in patients with newly diagnosed HL and the impact of tumor EBV positive on the prognosis of patients, to explore whether there is a correlation between EBV status and the prognosis of HL.
2. Material and methods
2.1. Study design and general information
In this retrospective study, data of all 166 consecutive patients with HL diagnosed and treated in Fujian Cancer Hospital from December 2012 to September 2019 were collected through the electronic medical record system. All patients were confirmed by the Pathology Department of Fujian Cancer Hospital to meet the WHO diagnostic criteria of HL and had complete efficacy evaluation records. 28 patients were excluded because there were not enough specimens for EBV detection. 4 patients were excluded because of the second primary tumor. A total of 134 patients were eligible for our study. The study was approved by the Ethics Committee of Fujian Cancer Hospital (No. 2017-067-01). Ethics Committee of Fujian Cancer Hospital agreed to apply for exemption from the signature of informed consent, because all interviews were conducted on the phone and the risk of the study to the subjects was not greater than the minimum risk, and if the background of ‘research’ was separated, the behavior or procedure under the same situation did not require the signing of written informed consent. The baseline demographic characteristics and clinical information of the participants were obtained from the electronic medical record system. For the sake of confidentiality, the data is deprived of any personally identifiable information. Participants or family members were contacted on the telephone to obtain verbal consent and follow up information. All of the participants or family members who can be contacted provided verbal consent and received telephone interviews.
2.2. Epstein–Barr virus detection
All tissue wax blocks underwent EBER in situ hybridization (ISH) to determine the EBV status of the tumor. The EBER probe ISH detection kit® (Taipu bioscience Co, Ltd, Fuzhou, Fujian, China) was used. Specimens which were positive for EBV showed obvious blue-purple granules in the nucleus of Hodgkin and Reed-Sternberg cells (HRS). Patients were considered to have EBV-positive HL (EBV+HL) if HRS cells were positive; otherwise, they were considered EBV-negative HL (EBV-HL).
2.3. Age subgroup division and efficacy evaluation
The age subgroups were classified according to the four-disease model proposed by the Scotland and Newcastle Epidemiology of Hodgkin’s Disease Study Group [Citation9,Citation10]. According to the four-disease model of HL patients were divided into four age groups: 0–14, 15–34, 35–49, 50years and older. The 2014 Lugano Classification [Citation11] was used to judge the efficacy, which was divided into complete remission (CR), uncertain complete remission (CRu), partial remission (PR), No response or stable disease(SD), and disease progression(PD).
2.4. Follow-up
Most of these patients received long-term follow-up from the hospital follow-up chamber after receiving treatment and leaving the hospital for many years. In December 2020, the researchers conducted the last follow-up of all the patients in this study by telephone. Eleven patients (follow-up rate 91.8%) were lost to follow up. The median follow up time was 56.8 months (range, 6.6∼92.9 months).
2.5. Statistical analysis
SPSS 20.0 software was used for the statistical analysis. χ2 test and Fisher's exact test were used to analyze the correlation of factors. Mann–Whitney U test was used for the comparison of the efficacy evaluation and the Kaplan-Meier method was used for survival analysis. Cox proportional hazards model was used in multivariate analysis to test the independent prognostic significance of factors. P values of less than 0.05 were considered significant. Overall survival (OS) was evaluated from the date of diagnosis to death from any cause or censored at the time of last follow-up for a surviving patient. Failure-free survival (FFS) was calculated from the date of start of treatment to the date of treatment failure (progression or relapse) or death from HL, whichever was first. Patients were censored when alive and free of HL or when they died of an unrelated cause at the last follow-up examination.
3. Results
3.1. Clinical characteristics
Among 134 patients with HL, 83 were male (61.9%) and 51 were female (38.1%). The median age at diagnosis was 31 years (range, 5–74 years). Most were young patients aged between 15 and 49 years. Pathological typing was conducted in order to diagnose patients using the WHO HL diagnostic criteria. Seven patients (5.2%) had nodular lymphocyte predominant HL, and the remaining 127 had classical HL (cHL). In patients with cHL, most had nodular sclerosing HL (68/134, 50.7%), followed by mixed-cellularity HL (48/134, 35.8%). There were 7 (5.2%) patients with lymphocyte-rich HL and 1 (0.7%) with lymphocyte depleted HL. Three patients (2.2%) were diagnosed with cHL but could not be typed further.
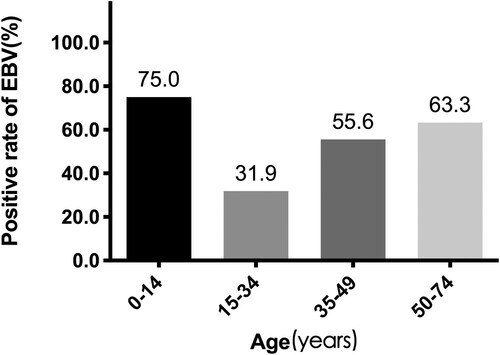
EBER-ISH was used to determine EBV tumor status in all 134 patients. 46.3% (62/134) had EBV+HL. The EBV+HL group had a higher proportion of males than the EBV-HL (71.0% (44/62) vs 54.2% (39/72), p=0.046). The rate of EBV positivity in patients aged 0–14 years, 15–34 years, 35–49 years, and over 50 years was 75.0% (6/8), 31.9% (22/69), 55.6% (15/27), and 63.3% (19/30), respectively (P=0.004). For patients aged 15–74 years, the rate of EBV positivity increased gradually with age (Figure A).
There were 15 patients with stage I disease, 60 with stage II disease, 21 with stage III disease, and 38 with stage IV disease. The most frequent initial symptom was painless swelling of the superficial lymph nodes. Before treatment, 36 patients (26.9%) had extra-lymphatic invasion, common sites of which included bone in 19 patients, lung in 9, pericardium in 3, and pleura in 5. At the time of diagnosis, 55 patients (41.0%) also had B symptoms, with fever being the most common of these (32/55). Twenty-four patients (17.9%) had bulky disease (bulky mediastinal disease or >10 cm adenopathy). Extranodal disease was significantly more likely in EBV+HL patients than in EBV-HL patients (35.5% (22/62) vs. 19.4% (14/72), p=0.037) The GHSG (German Hodgkin Study Group) criteria were used to evaluate unfavorable risk factors of the early-stage (Ann Arbor stage I∼II) HL patients, while the IPS (International Prognostic Score) was used to assess the advanced HL (Ann Arbor stage III∼IV). Among the 75 early stage patients, 50 cases (66.6%) had at least 1 adverse prognostic factor. Among the 59 advanced HL patients, 33 patients (55.9%) were in the low-risk group (IPS 0∼2) and 26 patients (44.1%) in the high-risk group (IPS≥3). There was no significant difference in unfavorable risk factors evaluation and IPS between EBV- and EBV+ groups (see Table A for further details).
3.2. Treatment selection and curative effect
Chemotherapy was the first line treatment for all 134 patients, of whom 131 (97.8%) had the ABVD regimen (doxorubicin 25 mg/m2, bleomycin 10 mg/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2 on days 1 and 15 of each 28 d cycle), and 3 (2.2%) had the BEACOPP standard dose regimen [cyclophosphamide 650 mg/m2 on day 1, doxorubicin 25 mg/m2 on day 1, etoposide 100 mg/m2 on days 1–3, procarbazine 100 mg/m2 on days 1–7, prednisone 40 mg/m2 on days 1–14, vincristine 1.4 mg/m2 (limited to 2.0 mg) on day 8 and bleomycin 10 mg/m2 on day 8 of each 21 d cycle]. The median number of chemotherapy cycles was 6 (range, 1∼8). The usual number of chemotherapy cycles for newly treated patients with stage I-II HL was 2–4, and for patients with stage IIIIV HL, 6–8. A total of 44 patients (32.8%) received local radiotherapy. Salvage chemotherapy was administered if there was tumor progression during treatment or relapse after treatment. Three patients were treated with autologous peripheral blood stem cell transplantation (PBSCT) after recurrence.
Ninety patients (67.2%) achieved CR/CRu after first-line chemotherapy; 39 (29.1%) achieved PR; 4 (3.0%) achieved SD; and 1 (0.7%) achieved PD (see Table B for details). The difference in the complete remission rate of EBV+HL and EBV-HL patients was not statistically significant (p=0.181). 26 patients (19.4%) experienced progression or relapse during follow-up. Of these, 25 received chemotherapy, and 1 discontinued treatment. The CR rate of salvage chemotherapy in the EBV+HL group was lower than that in the EBV-HL group (27.3% (3/11) vs. 57.1% (8/14)), but the sample size of each group was small, and the difference was not statistically significant.
3.3. Survival
Among 134 HL patients, 14 (10.4%) died. Eight died due to disease progression and 2 due to pulmonary infection. Four patients did not have routine visits and the cause of death was unknown (see Figure B1 for further details).
Overall survival (OS) rates for EBV+HL and EBV-HL patients were 90.5%±4.1% vs 95.4% ±2.6% at 3 years and 85.3%±5.3% vs 86.0%±5.1% at 5 years, respectively. There was no significant difference between the two groups (Figure B2, p=0.552). The results of univariate analysis of FFS are summarized in Table C. Age > 50 years, Ann Arbor stage III–IV, and bulky disease were associated with worse FFS. The 5-year FFS rates of EBV+HL and EBV-HL patients were 68.6%±6.9% and 83.3%±4.6%, respectively, and the FFS curves of the two groups intersected (Figure B3, p=0.123). However, in patients aged over 50 years, we found that the 5-year FFS rate was 38.5% (±13.5%) for EBV+HL patients vs. 90.9% (±8.7%) for EBV-HL patients (Figure B6, p=0.012). In multivariate analysis of this age subgroup, EBV status seems to be the only independent prognostic factor for FFS, with an HR of 10.10 ([95% CI, 1.26–81.08]; p=0.030; See Table D for details).
4. Discussion
Epstein–Barr virus, a gamma herpes virus identified in 1964, has a high rate of infection in the population [Citation12]. It is the first virus associated with a variety of human malignancies [Citation13]. In most infected individuals, the Epstein–Barr virus is present in a latent state. However, once the virus is activated, it can cause a number of diseases, including nasopharyngeal cancer (NPC) [Citation14], gastric cancer (GC) and infectious mononucleosis [Citation15,Citation16]. EBV-related NPC shows the most consistent correlation with EBV globally, with the highest incidence of about 20–30/100,000, especially in China and Southeast Asia [Citation17]. More specifically, EBV-related NPC incidence is higher in Fujian province than in many other regions in China [Citation7,Citation8]. Although the reasons for the high prevalence of EBV in this area are not completely clear, we speculate that at least the following factors are involved. Firstly, poor material factors, such as poor sanitary conditions or overcrowded living conditions in rural areas of Fujian, make EBV have a high prevalence in people with low socio-economic gradient. In fact, EBV acquisition itself is related to socio-economic status [Citation18–20]. A prospective study used data on children included in the UK Millennium Cohort Study and suggested that Children living in more deprived social conditions may be more likely to become EBV carriers at an earlier age [Citation21]. Secondly, Asian food culture and the use of chopsticks make it possible for family members to induce the transmission of EBV through saliva exchange [Citation22]. The findings that suggested spontaneous secretions of EBV in seropotitive adults and successful replications of the virus in human oropharyngeal epithelial cells further strengthen the role of saliva transmission in the epidemiology of EBV infection [Citation23,Citation24]. A large number of studies have shown that some lymph and hematogenous tissue tumors may be related to EBV. These include HL, Burkitt lymphoma (BL), diffuse large B cell lymphoma, and extranodal NK/T cell lymphoma [Citation25–28]. There is little or no clear association between EBV and other types of lymphoma. At present, the mechanism by which EBV acts on HL is still unclear, but some studies believe that EBV infection occurs before HL, and EBV infection may participate in the important initiation stage or promote the deterioration of B cells by inducing activated nuclear transcription factors such as LMP-1 [Citation29].
Epidemiological data shows that the incidence of EBV infection in HL patients varies in different regions and races. A meta-analysis showed that the EBV infection rate in patients with HL is significantly higher in African, Central, and South American countries than in other countries and regions [Citation30]. Japanese research data indicates that the rate of EBV detection in HL patients is 40%–60%, and a clinical study with a larger sample size reported by Asano et al. found that 47.5% of 314 patients with HL tested positive for EBV using EBER [Citation31]. The United States reports an infection rate of 20%–30% [Citation32,Citation33]. Fu et al [Citation34] reported that the EBV infection rate in the northern Chinese population is 28%–39%. Our study found that 62 of 134 (46.3%) patients with HL had an EBV positive status in tumor cell. Because this is a single-center study, the patients were mainly from Fujian Province, South China, and the EBV positivity rate in this study is similar to Southern Chinese population data reported previously [Citation35].
Jarrett et al [Citation36] analyzed the EBV status of 437 patients with cHL, and found that EBV-related HL was more common in patients ≥ 50 years of age. Zhou et al [Citation37] reported that the rates of EBER positivity in 104 children and 52 adult patients with HL were 89% and 38%, respectively (p<0.01). Among children with HL, the rate in those aged 3–10 years was higher than in those aged 11–14 years (95% vs. 54%, p<0.01). Kang et al [Citation38] evaluated the rate of EBV positivity in 207 untreated HL cases, and reported that there were two peaks, at age 0–15 years and >60 years. Our study found similar results in terms of the age structure in EBV positivity: the rate of EBV positivity increased gradually in patients aged 15–74 years. These findings may be due to the underdeveloped immune system in children or immunodeficiency in the aged, compared to a well-developed immune surveillance system in young people. The rate of EBV positivity in children in this study was higher than that in most previous reports [Citation39,Citation40]. This may be due to the low number of children with HL at our center, and may not reflect the wider population. In addition, we observed that the proportion of males in the EBV+HL group was significantly higher than that in the EBV-HL group. This finding is consistent with epidemiological data. Gulfaraz et al [Citation41] analyzed GBD10 [Citation42] data, and found that global deaths from EBV-attributed cases of malignancies including NPC, BL, HL, GC, and post-transplant lymphoproliferative disease (PTLD) were up to 2.6 times higher in men than in women, and this male predominance was common in virtually all world regions.
Since the discovery of a correlation between HL and EBV infection, researchers have been exploring whether there is a correlation between EBV status and HL prognosis. Claviez et al [Citation43] studied 842 patients with HL in the German HD-90 and HD-95 studies, and found that the EBV-HL group had a higher 10-year survival rate than the EBV+HL group. Yin et al [Citation44] reported that in 120 patients with HL, the 1-year and 2-year total survival rates in the EBV-positive group were significantly lower than those in the EBV-negative group (88.9% and 83.3% vs. 97.6% and 95.2%, respectively, p=0.031). In a study of Jarrett et al [Citation36], the OS rate of patients with EBV-positive cHL was worse in patients aged over 50 years. A large population-based study by Keegan et al [Citation45] showed that EBV-positive status was associated with worse OS and disease-specific survival (DSS) in HL patients older than 45 years. These studies are controversial because of the use of OS and DSS as clinical endpoints. Because of the high cure rate of HL, these two indicators can be affected by salvage treatment and mortality from other causes. Herling et al [Citation46] suggested that FFS is a more appropriate choice as a clinical endpoint because it relates to HL itself.
In our study, there was no overall correlation between EBV positivity and OS or FFS. However, in the subgroup aged over 50 years, the FFS was better in the EBV-HL group. These results are contrary to those of other studies. Some studies in South America, South Africa, and South Asia [Citation47–51] suggest that EBV positivity is associated with a superior prognosis, while the opposite is usually true in East Asia [Citation38,Citation44]. This suggests that the relationship between EBV status and prognosis may be affected by geographical factors. In addition, some studies did not analyze different age groups. The results of our analysis of age subgroups are consistent with those of Diespstra et al [Citation52], Jarrett et al [Citation36] and Stark et al [Citation53]. Despite the lack of pediatric patients in our study, Claviez et al [Citation43] a Biccler el's [Citation54] studies have shown that EBV-positive status in children or young HL patients is associated with a better prognosis. There are several reasons why EBV positivity may correlate with a worse prognosis in elderly HL patients. One possibility is immunosenescence. Alternatively, it may be due to the elderly patients having poor tolerance to treatment and a subset of patients being unable to tolerate sufficient chemotherapy or combined radiotherapy and chemotherapy. Additionally, elderly patients may have had complications which affected survival.
It is unclear whether EBV status affects the efficacy of salvage chemotherapy in patients with HL. In our study, the rate of CR in EBV+HL patients having salvage chemotherapy was lower than that in EBV-HL patients. As there were few patients in each group, the difference did not reach statistical significance, but the trend is worth exploring in a larger group of patients.
Several limitations existed in this retrospective cohort study. The main limitation was the retrospective design, which made it impossible to control exposure. Another problem with retrospective study is recall bias. Immortal time and selection bias might have occurred, depending on the accuracy of medical records. Moreover the study had a relatively small sample size. Despite the long-term follow-up, there were only 166 HL patients treated, and 134 patients were included ultimately. Finally, it's a single-center study and lacked data from other hospitals, that made the results could not reflect the situation in other areas. We will increase the sample size and extend the follow-up time in future studies to increase the accuracy of our conclusions.
5. Conclusion
In conclusion, HL is a malignant tumor with effective treatments and a high complete remission rate. The use of molecular pathology can result in more specific and accurate lymphoma classification, which can better guide clinical treatment. We found differences in clinical characteristics between EBV+HL and EBV-HL patients, and the results of survival analysis suggested that in older patients with HL the EBV+HL group had a worse FFS than the EBV-HL group.
Acknowledgments
This work was supported by grants from the Startup Fund for scientific research, Fujian Medical University (Grant number: 2017XQ1218) to Chang Wang. We would like to thank Editage (www.editage.cn) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Townsend W, Linch D. Hodgkin’s lymphoma in adults. J Lancet. 2012;380:836–847. doi:https://doi.org/10.1016/S0140-6736(12)60035-X.
- Glaser SL, Hsu JL. Hodgkin’s disease in Asians: incidence patterns and risk factors in population-based data. J Leuk Res. 2002;26:261–269. doi:https://doi.org/10.1016/s0145-2126(01)00126-6.
- Xiao-qiu LI, Gan-di LI, Zi-fen GAO, et al. Distribution pattern of lymphoma subtypes in China: A nationwide multicenter study of 10 002 cases. J Diagn Concepts Pract. 2012;11:111–115. http://kns.cnki.net/KCMS/detail/detail.aspx?FileName=ZDLS201202007&DbName=CJFQ2012%25%5C 2020-10-21 21:40:00.
- Flavell KJ, Linford JA, Flavell JR, et al. Detection of Epstein-Barr virus in archival Hodgkin’s disease specimens. Mol Pathol. 2000;53:162. doi:https://doi.org/10.1136/mp.53.3.162.
- Weiss LM, Strickler JG, Warnke RA, et al. Epstein-Barr viral DNA in tissues of Hodgkin’s disease. Am J Pathol. 1987;129:86–91. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2821817&query_hl=1.
- Murray P, Bell A. Contribution of the Epstein-Barr virus to the pathogenesis of Hodgkin lymphoma. Curr Top Microbiol Immunol. 2015;390:287–313. doi:https://doi.org/10.1007/978-3-319-22822-8_12.
- Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374:22–30. doi:https://doi.org/10.1016/j.canlet.2016.01.040.
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. 2014;33:381–387. doi:https://doi.org/10.5732/cjc.014.10086.
- Jarrett RF. Viruses and Hodgkin’s lymphoma. J Ann Oncol. 2002;13(Suppl 1):23–29. doi:https://doi.org/10.1093/annonc/13.s1.23.
- Armstrong AA, Lennard A, Alexander FE, et al. Prognostic significance of Epstein-Barr virus association in Hodgkin’s disease. J Eur J Cancer. 1994;30A:1045–1046. doi:https://doi.org/10.1016/0959-8049(94)90157-0.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi:https://doi.org/10.1200/jco.2013.54.8800.
- Rickinson A. Epstein-Barr virus. Virus Res. 2002;82:109–113. doi:https://doi.org/10.1016/s0168-1702(01)00436-1.
- Farrell PJ. Epstein-Barr virus and cancer. Annu Rev Pathol. 2019;14:29–53. doi:https://doi.org/10.1146/annurev-pathmechdis-012418-013023.
- Yi M, Cai J, Li J, et al. Rediscovery of NF-kappaB signaling in nasopharyngeal carcinoma: How genetic defects of NF-kappaB pathway interplay with EBV in driving oncogenesis? J Cell Physiol. 2018;233:5537–5549. doi:https://doi.org/10.1002/jcp.26410.
- Macsween KF, Crawford DH. Epstein-Barr virus-recent advances. Lancet Infect Dis. 2003;3:131–140. doi:https://doi.org/10.1016/s1473-3099(03)00543-7.
- Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003;22:5108–5121. doi:https://doi.org/10.1038/sj.onc.1206556.
- Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi:https://doi.org/10.1016/s1044579 ( 02000858.
- Condon LM, Cederberg LE, Rabinovitch MD, et al. Age-specific prevalence of Epstein-Barr virus infection among minnesota children: effects of race/ethnicity and family environment. Clin Infect Dis. 2014;59:501–508. doi:https://doi.org/10.1093/cid/ciu342.
- Dowd JB, Palermo T, Brite J, et al. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6–19, 2003–2010. PLoS One. 2013;8:e64921. doi:https://doi.org/10.1371/journal.pone.0064921.
- Ferres M, Prado P, Ovalle J, et al. [Seroprevalence of Epstein Barr virus infection in a healthy population of Santiago de Chile]. Rev Med Chil. 1995;123:1447–1452. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8733260&query_hl=1.
- Gares V, Panico L, Castagne R, et al. The role of the early social environment on Epstein Barr virus infection: a prospective observational design using the Millennium Cohort study. Epidemiol Infect. 2017;145:3405–3412. doi:https://doi.org/10.1017/S0950268817002515.
- Chen CY, Huang KY, Shen JH, et al. A large-scale seroprevalence of Epstein-Barr virus in Taiwan. PLoS One. 2015;10:e0115836. doi:https://doi.org/10.1371/journal.pone.0115836.
- Gerber P, Lucas S, Nonoyama M, et al. Oral excretion of Epstein-Barr virus by healthy subjects and patients with infectious mononucleosis. Lancet. 1972;2:988–989. doi:https://doi.org/10.1016/s0140-6736(72)92402-6.
- Miller G, Niederman JC, Andrews LL. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973;288:229–232. doi:https://doi.org/10.1056/NEJM197302012880503.
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi:https://doi.org/10.1056/NEJMra032015.
- Paschos K, Smith P, Anderton E, et al. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. 2009;5:e1000492. doi:https://doi.org/10.1371/journal.ppat.1000492.
- Ping LY, Ding N, Shi YF, et al. [Clinical characteristics and prognosis analysis of patients with LMP-1 positive Hodgkin’s lymphoma after EBV infection]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:78–84. doi:https://doi.org/10.7534/j.issn.1009-2137.2014.01.017.
- Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372. doi:https://doi.org/10.1098/rstb.2016.0271.
- Carbone A, Gloghini A, Caruso A, et al. The impact of EBV and HIV infection on the microenvironmental niche underlying Hodgkin lymphoma pathogenesis. Int J Cancer. 2017;140:1233–1245. doi:https://doi.org/10.1002/ijc.30473.
- Lee JH, Kim Y, Choi JW, et al. Prevalence and prognostic significance of Epstein-Barr virus infection in classical Hodgkin’s lymphoma: a meta-analysis. Arch Med Res. 2014;45:417–431. doi:https://doi.org/10.1016/j.arcmed.2014.06.001.
- Asano N, Oshiro A, Matsuo K, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin’s lymphoma: a clinicopathologic study. J Clin Oncol. 2006;24:4626–4633. doi:https://doi.org/10.1200/jco.2006.06.5342.
- Glaser SL, Clarke CA, Chang ET, et al. Hodgkin lymphoma incidence in California Hispanics: influence of nativity and tumor Epstein-Barr virus. Cancer Causes Control. 2014;25:709–725. doi:https://doi.org/10.1007/s10552-014-0374-6.
- Ghesquieres H, Maurer MJ, Casasnovas O, et al. Cytokine gene polymorphisms and progression-free survival in classical Hodgkin lymphoma by EBV status: results from two independent cohorts. Cytokine. 2013;64:523–531. doi:https://doi.org/10.1016/j.cyto.2013.08.002.
- FU Q, JI H, ZHANG T, et al. Association between classical Hodgkin’s lympohoma and Epstein-Barr virus infection in Northern Chinese Han population. Chin J Clin Oncol. 2014;41:629–633. http://kns.cnki.net/KCMS/detail/detail.aspx?FileName=ZGZL201410005&DbName=CJFQ2014%25%5C 2020-10-21 21:43:00.
- Zeng W, Zhou M, Lin H. [The significance of detecting Epstein-Barr virus BNLF1 fragment and its expression in Hodgkin’s disease in the Guangdong area]. Zhonghua Bing Li Xue Za Zhi. 1997;26:27–30. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10072846&query_hl=1.
- Jarrett RF, Stark GL, White J, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood. 2005;106:2444–2451. doi:https://doi.org/10.1182/blood-2004-09-3759.
- Zhou XG, Sandvej K, Li PJ, et al. Epstein-Barr virus (EBV) in Chinese pediatric Hodgkin disease: Hodgkin disease in young children is an EBV-related lymphoma. Cancer. 2001;92:1621–1631. https://doi.org/https://doi.org/10.1002/1097-0142(20010915)92:6<1621::aid-cncr1488>3.0.co;2-p.
- Kang S, Qin Y, He X, et al. [Infection status and prognostic significance analysis of Epstein-Barr virus in 207 cases with newly diagnosed Hodgkin’s lymphoma]. Zhonghua Yi Xue Za Zhi. 2015;95:2594–2598. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=26711606&query_hl=1.
- Glaser SL, Gulley ML, Clarke CA, et al. Racial/ethnic variation in EBV-positive classical Hodgkin lymphoma in California populations. Int J Cancer. 2008;123:1499–1507. doi:https://doi.org/10.1002/ijc.23741.
- Herling M, Rassidakis GZ, Medeiros LJ, et al. Expression of Epstein-Barr virus latent membrane protein-1 in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s lymphoma: associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res. 2003;9:2114–2120. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12796376&query_hl=1.
- Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infect Agent Cancer. 2014;9:38. doi:https://doi.org/10.1186/1750-9378-9-38.
- Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: a multi-investigator collaboration for global comparative descriptive epidemiology. Lancet. 2012;380:2055–2058. doi:https://doi.org/10.1016/s0140-6736(12)62134-5.
- Claviez A, Tiemann M, Luders H, et al. Impact of latent Epstein-Barr virus infection on outcome in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4048–4056. doi:https://doi.org/10.1200/jco.2005.01.701.
- Yin JJ, Liang B, Lu Y, et al. [Infection status and prognosis analysis of Epstein-Barr virus in patients with newly diagnosed Hodgkin’s lymphoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:1410–1415. doi:https://doi.org/10.7534/j.issn.1009-2137.2016.05.023.
- Keegan TH, Glaser SL, Clarke CA, et al. Epstein-Barr virus as a marker of survival after Hodgkin’s lymphoma: a population-based study. J Clin Oncol. 2005;23:7604–7613. doi:https://doi.org/10.1200/jco.2005.02.6310.
- Herling M, Rassidakis GZ, Vassilakopoulos TP, et al. Impact of LMP-1 expression on clinical outcome in age-defined subgroups of patients with classical Hodgkin lymphoma. Blood. 2006;107:1240. author reply 1241. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16434497&query_hl=1.
- Morente MM, Piris MA, Abraira V, et al. Adverse clinical outcome in Hodgkin’s disease is associated with loss of retinoblastoma protein expression, high Ki67 proliferation index, and absence of Epstein-Barr virus-latent membrane protein 1 expression. J Blood. 1997;90:2429–2436. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9310494&query_hl=1.
- Montalban C, Abraira V, Morente M, et al. Epstein-Barr virus-latent membrane protein 1 expression has a favorable influence in the outcome of patients with Hodgkin’s disease treated with chemotherapy. Leuk Lymphoma. 2000;39:563–572. doi:https://doi.org/10.3109/10428190009113386.
- Engel M, Essop MF, Close P, et al. Improved prognosis of Epstein-Barr virus associated childhood Hodgkin’s lymphoma: study of 47 South African cases. J Clin Pathol. 2000;53:182–186. doi:https://doi.org/10.1136/jcp.53.3.182.
- Naresh KN, Johnson J, Srinivas V, et al. Epstein-Barr virus association in classical Hodgkin’s disease provides survival advantage to patients and correlates with higher expression of proliferation markers in Reed-Sternberg cells. Ann Oncol. 2000;11:91–96. doi:https://doi.org/10.1023/a:1008337100424.
- Vassallo J, Metze K, Traina F, et al. Expression of Epstein-Barr virus in classical Hodgkin’s lymphomas in Brazilian adult patients. Haematologica. 2001;86:1227–1228. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11694413&query_hl=1.
- Diepstra A, van Imhoff GW, Schaapveld M, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol. 2009;27:3815–3821. doi:https://doi.org/10.1200/jco.2008.20.5138.
- Stark GL, Wood KM, Jack F, et al. Hodgkin’s disease in the elderly: a population-based study. Br J Haematol. 2002;119:432–440. doi:https://doi.org/10.1046/j.1365-2141.2002.03815.x.
- Biccler JL, Glimelius I, Eloranta S, et al. Relapse risk and loss of lifetime after modern combined modality treatment of young patients with Hodgkin lymphoma: a nordic lymphoma epidemiology group study. J Clin Oncol. 2019;37:703–713. doi:https://doi.org/10.1200/jco.18.01652.
Appendices
Figure B. Overall survival and failure-free survival among HL patients whose EBV infection status was classified 1. Overall survival and failure-free survival for the entire study population 2. Overall survival by EBV infection status in patients of all ages 3. Progression-free survival by EBV infection status in patients of all ages 4-6. Progression-free survival by EBV infection status in patients of different age subgroups.
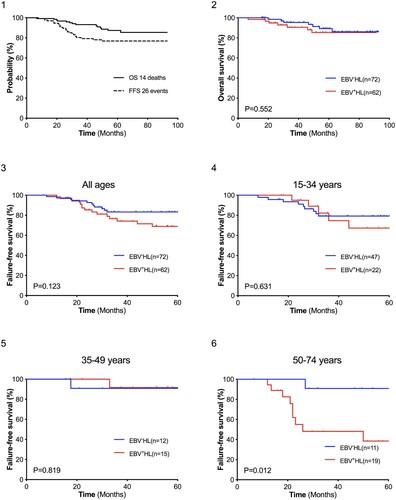
Table A. General clinical characteristics of patients with Hodgkin lymphoma.
Table B. Curative effect of treatment.
Table C. Univariate Analysis of FFS.
Table D. Multivariate Analysis of FFS using a Cox Regression Model.