ABSTRACT
Objectives
Immune thrombocytopenia (ITP) is an autoimmune disease. T helper cell 17 (Th17) cells are increased in peripheral blood of ITP patients. NOTCH signaling is involved in Th17 cell differentiation and function. Besides, lncRNA Plasmacytoma variant translocation 1 (PVT1) was decreased in experimental autoimmune encephalomyelitis, and overexpressing PVT1 inhibited Th17 cell differentiation. Here, we aimed to investigate the effect of lncRNA PVT1 on ITP and its related mechanism.
Methods
The number of Th17 cells and Treg cells was carried out using flow cytometry. PVT1 levels were detected by quantitative real-time PCR. Interleukin-17 (IL-17) levels and transforming growth factor-β (TGF-β) levels were detected by enzyme-linked immunosorbent assay. Protein levels of retinoid acid-related orphan receptor γ t (RORγt), forkhead box P3 (Foxp3), and NOTCH1 were carried out by western blot. NOTCH1 ubiquitylation was detected by ubiquitination assay.
Results
PVT1 was down-regulated and Th17 cells were up-regulated in ITP patients. Overexpression of PVT1 decreased the number of Th17 cells, and also decreased the levels of IL-17, RORγt, and NOTCH1. Besides, PVT1 could bind to NOTCH1 and mediated NOTCH1 degradation by increasing its ubiquitination. Additionally, excessive expression of PVT1 could increase the levels of PVT1, reduce the amount of Th17 cells, as well as the levels of IL-17, RORγt, and NOTCH1, while co-overexpressing NOTCH1 reversed the results.
Conclusion
PVT1 was down-regulated in ITP patients. Overexpressing PVT1 might reduce Th17 cell differentiation by down-regulating NOTCH1, and further alleviated the development of ITP.
Introduction
Immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenic purpura, is an autoimmune disease caused by a variety of causes. The typical symptom of ITP is thrombocytopenia, which may be manifested by purpura of the skin and bleeding of the mucosa, or no signs of bleeding. ITP is the most common acquired hemorrhagic disease caused by autoimmune dysfunction, with an adult prevalence of about 3.3–3.9 per 100,000 individuals, and the prevalence is increasing year by year [Citation1, Citation2]. Evidence has shown that cellular immune abnormalities play an important role in the occurrence and development of ITP [Citation3].
It is generally believed that T cell immune dysfunction promotes the development of ITP, and the role of T helper (Th) cells and their cytokines in the pathogenesis of ITP has been emphasized [Citation4]. Regulatory T (Treg) cells and Th17 cells are newly discovered subsets of CD4+ T cells. The balance of Treg/Th17 regulates the autoimmune response and plays a key role in regulating T cell function. It has been documented that the proportion of Th17 cells and the level of Interleukin-17 (IL-17) in the peripheral blood of ITP patients increased, while the proportion of Treg cells and the level of transforming growth factor-β (TGF-β) decreased [Citation3]. Therefore, exploring the changes of Treg/Th17 balance in ITP will provide a more theoretical basis for the treatment of ITP.
The Notch signaling pathway is currently recognized as an extremely vital signal transduction system for regulating cell differentiation, mainly composed of four Notch receptors (Notch1, Notch2, Notch3, Notch4) and five ligands (Delta family and Jagged family) [Citation5]. The Notch signaling pathway also contributes to the functional differentiation of CD4+ T cells [Citation6]. Toshihiro et al. found in a mouse model of lung granuloma that the Notch pathway promoted the differentiation of Th17 cells [Citation7]. These findings suggest that the Notch pathway is an important regulatory signal of Th17 cell differentiation. Gawdat RM et al. found that the levels of Notch1 and its target gene Hes1 in peripheral blood of Egyptian children with ITP were elevated [Citation8]. However, the upstream regulatory factors of the Notch1 pathway in Th17 differentiation remain unclear.
LncRNA Plasmacytoma variant translocation 1 (PVT1) is located at 8q24. 21, and studies have found that lncRNA PVT1 is not only strongly associated with the onset and development of cancer but also has an abnormal expression in autoimmune diseases [Citation9]. Eftekharian et al. find that the expression of PVT1 is decreased in the blood of patients with multiple sclerosis [Citation10]. The expression of PVT1 is also decreased in experimental autoimmune encephalomyelitis, and overexpression of PVT1 can inhibit Th17 cell differentiation [Citation11]. However, the effect of PVT1 on ITP has not been reported.
Here, we speculate that decreasing PVT1 leads to Treg/Th17 immune imbalance by regulating the expression of Notch1, and then participates in the development of ITP.
Materials and methods
Cell isolation and culture
Twelve ITP patients and twelve healthy people were enrolled in our study and signed the informed consent. For the isolation of peripheral blood mononuclear cells (PBMCs), 4 mL of peripheral blood were collected, diluted, and added to a centrifuge tube containing 4 mL of the Lymphocyte Separation Medium (Human) (Solarbio, China) and centrifuged for 25 min at 800 g. The PBMC layer was between the plasma and the separation fluid. Then the cells were transferred to a clean centrifuge tube, washed with phosphate-buffered solution (PBS), and centrifuged for 10 min at 250 g. The supernatant was discarded and the cell precipitates were resuspended in 5 mL PBS, centrifuged at 250 g for 10 min. Then the precipitates were resuspended and cryopreserved with cryomedium (A2644601, Thermo Fisher Scientific, USA). The study was approved by the Ethics Committee of 2nd Affiliated Hospital, School of Medicine, Zhejiang University.
CD4+ T cell isolation and Th17 cell differentiation
CD4+ T cells were isolated from peripheral blood of healthy people according to the Naïve CD4+ T Cell Isolation Kit Ⅱ, human (Miltenyi Biotec, Germany). To induce the differentiation of Th17 cells, cells were cultured in a 1640 medium supplemented with 20 ng/mL Interleukin-6 (IL-6), 10 ng/mL Interleukin-1β (IL-1β), 3 ng/mL TGF-β, and 10 ng/mL Interleukin-23 (IL-23).
Flow cytometry
Th17 cell proportions and Treg cell proportions were carried out using flow cytometry. In vivo, for Th17 detection, PBMCs were incubated with the anti-CD4-FITC antibody and anti-IL-17-PE antibody (BD Biosciences). For Treg detection, PBMCs were incubated with the anti-CD25-APC antibody and anti-Foxp3-PE antibody (BD Biosciences). In vitro, CD4+ T cells were stained with the anti-IL-17-PE antibody (BD Biosciences). For Treg detection, CD4+ T cells were stained with the anti-Foxp3-PE antibody (BD Biosciences). The negative control is the sample without antibody marker, which is used to adjust the voltage of FSC/SSC and fluorescence channel. The gate was set by homotype control. Data was collected using the flow cytometer (BD Biosciences, USA).
Quantitative real-time PCR (qRT-PCR)
RNA was collected using Trizol reagent (Takara, Japan) followed by a reverse transcription of the cDNA using the PrimeScript RT reagent kit (Takara, Japan) [Citation12]. The levels of PVT1 were identified via the SYBR Premix Ex Taq (Takara, Japan). Primers were as follows:
PVT1: (F) 5’- GCCCCTTCTATGGGAATCACTA-3’, (R) 5’- GGGGCAGAGATGAAATCGTAAT-3’.
GAPDH: (F) 5’- TGCACCACCAACTGCTTAG -3’, (R) 5’- GGATGCAGGGATGATGTTC -3’.
Cell transfection
The sh-PVT1, Lenti-PVT1, Lenti-PVT1+Lenti-NOTCH1, and their negative controls (GenePharma) were cloned into a lentiviral vector and transferred into 293 cells. Then the obtained virus particles were transfected into CD4+ T cells [Citation13].
ELISA (enzyme-linked immunosorbent assay)
Levels of IL-17 and TGF-β were detected through human IL-17a ELISA Kit (ab83688) and Human TGF beta 1 ELISA Kit (ab100647) as instructed. Data were obtained from the enzyme-labeled instrument (Multiskan FC, Thermo Fisher Scientific, USA).
Western blot
The CD4+ T cells were treated with the RIPA lysis buffer (Beyotime, China) followed by centrifuged to obtain the supernatant. The sample concentration was determined via the BCA Kit (Thermo Fisher Scientific, USA) before separating them through SDS-PAGE and transferring them to the polyvinylidene fluoride membranes (PVDF, Millipore, USA). After that, the membrane was blocked with 5 percent Albumin from bovine serum (BSA) followed by an incubation during one night at four degrees Celsius with the Anti-RORγt antibody (PM080, 1:1000), Anti-FOXP3 antibody (ab20034, 1:1000), Anti-NOTCH1 antibody (ab52627, 1:1000), and the Anti-beta Actin antibody (ab8226, 1:1000). After that, the membranes were incubated with the second antibody for 2 h and the blots were visualized with the ECL system (Beyotime, China).
Ubiquitination analysis
The 293T cells were transfected with HA-Ub, FLAG-NOTCH1, NC-pcDNA, or pcDNA-PVT1 followed by MG132 treatment (10 μM, 4 h). Cells were then collected in RIP Lysis Buffer and incubated with protein A/G magnetic beads coated with anti-NOTCH1 antibody. The levels of HA-Ub were then detected by western blot.
Statistical analysis
Graphpad Prism v8.0.2 (GraphPad, San Diego, CA, USA) was used for analysis. Data were expressed as mean ± standard deviation. Differences between the two groups were conducted through Student's t-test and differences among multiple groups were conducted through one-way analysis of variance (ANOVA) followed by the LSD post hoc test. P < 0.05 was considered statistically significant [Citation14].
Results
LncRNA PVT1 was lowly expressed in PBMCs of ITP patients.
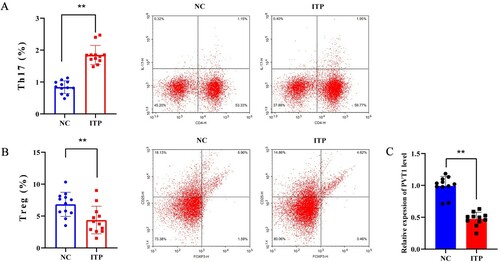
Firstly, the proportions of Th17 cells (CD4+IL-17+ cells) and Treg cells (CD25+FOXP3+ cells) in PBMCs from ITP patients (n = 12) and healthy people (n = 12) were determined using flow cytometry. The results showed that Th17 cells were increased and Treg cells were decreased in the ITP group compared with the NC group (A, B). The levels of PVT1 were also detected and the qRT-PCR result showed that PVT1 was lowly expressed in the ITP group compared with NC (C).
Figure 1. LncRNA PVT1 was lowly expressed in PBMCs of ITP patients. PBMCs were collected from ITP patients (n = 12, the ITP group) and healthy people (n = 12, the NC group). (A-B) Representative dot plots of Th17 cells (CD4+IL-17+ cells) and Treg cells (CD25+FOXP3+ cells) in NC group and ITP group. (C) The levels of PVT1 in PBMCs were determined by qRT-PCR. ** P < 0.01 vs the NC group.
Overexpression of lncRNA PVT1 inhibited Th17 cell differentiation.
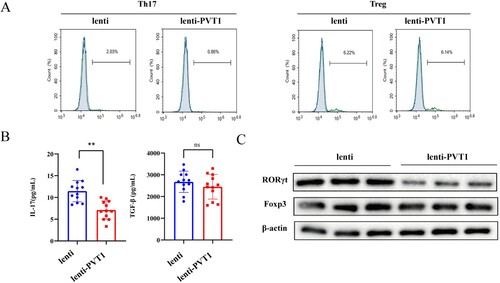
In order to examine the effect of PVT1 on Th17 cells, CD4+ T cells isolated from peripheral blood of healthy people were transfected with lentivirus-mediated overexpression of PVT1 before induction of Th17 cell differentiation. A showed that overexpressing PVT1 reduced Th17 cells (IL-17+ cells) while the number of Treg cells (FOXP3+ cells) did not change. ELISA results showed that IL-17 was reduced after overexpression of PVT1, while TGF-β expression did not change significantly (B). We further detected the levels of RORγt (a transcription factor for Th17) and Foxp3 (a transcription factor for Treg cells). Western blot results showed that overexpressing PVT1 reduced RORγt expression, while Foxp3 expression did not change (C). Results indicated that Th17 cell differentiation could be inhibited via overexpression of PVT1.
Figure 2. Overexpression of lncRNA PVT1 inhibited Th17 cell differentiation. The lenti or lenti-PVT1 was transfected into CD4+ T cells followed by induction of Th17 cell differentiation. n = 3. (A) The proportions of Th17 cells (IL-17+ cells) and Treg cells (FOXP3+ cells) in CD4+ T cells were analyzed by flow cytometry. (B) Levels of IL-17 and TGF-β were detected by ELISA. (C) The protein levels of RORγt (a transcription factor for Th17) and Foxp3 (a transcription factor for Treg cells) were determined by western blot. ** P < 0.01 vs the NC-pcDNA group.
The relationship between lncRNA PVT1 and NOTCH1.
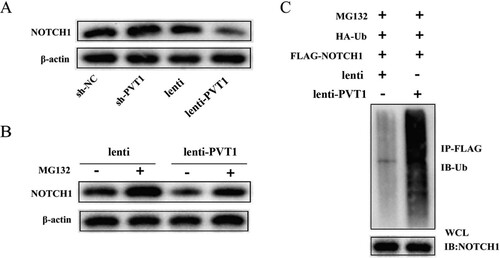
Through Bioinformatics software, PVT1 was found to probably bind to NOTCH1. The lentivirus sh-PVT1 (or sh-NC), lenti-PVT1 (or lenti) was transfected into CD4+ T cells before Th17 cells were induced to differentiate. A showed that NOTCH1 levels were increased after interference with PVT1 and NOTCH1 levels were decreased after overexpression of PVT1. Then, CD4+ T cells transfected with pcDNA-PVT1 or NC-pcDNA were treated with MG132 and the results showed that the degradation of Notch1 induced by overexpression of PVT1 was reversed by MG132 treatment (B). Besides, the ubiquitination of NOTCH1 was increased after overexpression of PVT1 (C). Data indicated that PVT1 might mediate the degradation of NOTCH1 by promoting its ubiquitination.
Figure 3. The relationship between lncRNA PVT1 and NOTCH1. CD4+ T cells were transfected with sh-NC, sh-PVT1, lenti, or lenti-PVT1. n = 3. (A) Protein levels of NOTCH1 were detected by western blot. (B) CD4+ T cells transfected with pcDNA-PVT1 or its control were treated with MG132. Protein levels of NOTCH1 were detected by western blot. (C) The HA-Ub, FLAG-NOTCH1, NC-pcDNA, or pcDNA-PVT1 was transfected into 293 cells before MG132 treatment. The cell lysate was co-immunoprecipitated with anti-FlAG-NOTCH1 antibody (IP: FlAG-NOTCH1) and co-immunoblotted with anti-ubiquitin antibody (IB: HA-Ub).
LncRNA PVT1 affected th17 cell differentiation by down-regulating notch1.
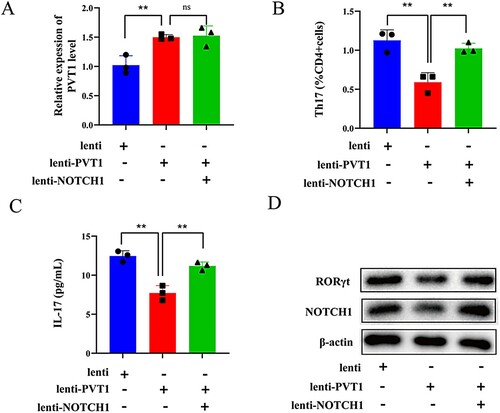
To further investigate whether PVT1 affects the differentiation of Th17 cells by down-regulating NOTCH1, CD4+ T cells were transfected with Lenti-PVT1, Lenti-PVT1 + Lenti-NOTCH1, and Lenti. PVT1 levels were increased in the Lenti-PVT1 group and Lenti-PVT1 + Lenti-NOTCH1 group (A). Th17 cells (IL-17+ cells) were down-regulated after overexpression of PVT1 while proportions of Th17 cells were up-regulated after co-overexpression of NOTCH1 (B). C showed that IL-17 levels were decreased after overexpression of PVT1 while the results were reversed after co-overexpression of NOTCH1. Western blot results showed that overexpressing PVT1 decreased protein levels of RORγt and NOTCH1 (D). Results indicated that PVT1 might affect the differentiation of Th17 cells by down-regulating NOTCH1.
Figure 4. LncRNA PVT1 affected Th17 cell differentiation by down-regulating Notch1. CD4+ T cells were divided into three groups (n = 3 per group): Lenti, Lenti-PVT1, and Lenti-PVT1 + Lenti-NOTCH1. (A) PVT1 levels were detected by qRT-PCR (B) The number of Th17 cells (IL-17+ cells) was detected by flow cytometry. (C) IL-17 levels were determined by ELISA. (D) Protein levels of RORγt and NOTCH1 were determined by western blot. ** P < 0.01 vs the lenti group or lenti-PVT1 group.
Discussion
The etiology and pathogenesis of immune thrombocytopenia (ITP) are not fully understood, and treatment methods include drug therapy (glucocorticoid, intravenous gamma globulin infusion, platelet prophylaxis drugs, rituximab), surgical treatment (splenectomy), etc., but the risk of infection is high and it is easy to relapse [Citation15]. At present, there is no radical cure for ITP, and the main treatment principle is to individualize the patients to improve the platelet count to a safe level, thus reducing mortality. Therefore, the study of its mechanism is beneficial for better treatment of ITP. In this study, we found that lncRNA PVT1 was lowly expressed in ITP patients compared with healthy people, and Th17/Treg proportion was higher than that in healthy people. Besides, we also found that overexpressing PVT1 inhibited Th17 cell differentiation by down-regulating NOTCH1.
A recent study declares that immune dysfunction of T cells plays a major part in the occurrence and development of ITP [Citation16]. The dynamic balance of Treg/Th17 jointly maintains the homeostasis of autoimmunity in vivo. Th17 cells differentiate and mature under the action of cytokines such as IL-6 and TGF-β, and induce an autoimmune response by secreting IL-17 [Citation17, Citation18]. Foxp3, a transcription factor of Treg cells, is critical for maintaining its differentiated phenotype and function. Treg cells inhibit the expression of IL-17 by secreting IL-10 and TGF-β, which plays an immunosuppressive role [Citation19]. Studies have demonstrated that the imbalance of Treg/Th17 cells is involved in ITP pathogenesis and inhibition of Th17 cell differentiation can ameliorate ITP [Citation4, Citation20–22]. Similarly, in our study, Th17 cells were up-regulated and Treg cells were down-regulated in CD4+ T cells from ITP patients compared with the NC group, indicating that the increase of Th17 cells resulted in an increased inflammatory response and a weakened immunosuppression function, further promoted the onset of ITP.
Over the past few years, it has been reported that lncRNAs are involved in the pathogenesis of ITP [Citation23]. Ayoub et al. indicated that lncRNAs metastasis associated in lung denocarcinoma transcript 1 (MALAT1) and THRIL could be potential markers of ITP [Citation24]. LncRNA MEG3 can induce the imbalance of Treg cells and Th17 cells in ITP [Citation25]. LncRNA Plasmacytoma Variant Translocation 1 (PVT1) has been reported to be associated with autoimmune diseases [Citation26]. In the present study, PVT1 was lowly expressed in ITP patients. In addition, overexpression of PVT1 could inhibit the differentiation of Th17 cells. Besides, overexpressing PVT1 also inhibits the levels of IL-17 (Th17 cell markers) and RORγt (a transcription factor for Th17). Results indicated that overexpression of PVT1 might become a therapeutic target for ITP.
The Notch signaling pathway can regulate the fate of T lymphocytes and participate in T cell differentiation [Citation27, Citation28]. In addition, the Notch signaling pathway also inhibits Treg cell differentiation and regulates Treg/Th17 balance [Citation29, Citation30]. Furthermore, the NOTCH pathway is involved in ITP, and inhibition of Notch signaling activation can reverse the imbalance of Treg/Th17 cells in ITP, suggesting that blocking the NOTCH1 pathway may be an effective target for the treatment of ITP [Citation31, Citation32]. Here, we found that NOTCH1 was decreased after overexpression of PVT1. Apart from that, overexpressing PVT1 induced the degradation of NOTCH1 and increased its ubiquitination. We also found that PVT1 could bind to NOTCH1. Collectively, increasing PVT1 expression might alleviate ITP by inhibiting the Notch1 pathway as well as the differentiation of Th7 cells.
Taken together, this present study firstly investigated the role of lncRNA PVT1 in ITP and found that PVT1 was lowly expressed in ITP. Besides, we also found that PVT1 could bind to NOTCH1 and induce the degradation of NOTCH1 by increasing its ubiquitination. Furthermore, overexpressing PVT1 decreased the number of Th17 cells as well as the level of IL-17, NOTCH1, and RORγt. In conclusion, lncRNA PVT1 might down-regulated Th17 cell differentiation via decreasing NOTCH1 levels. PVT1 might be a potential target for ITP treatment.
Conflict of interest
All authors declare that they have no conflict of interest.
Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Swinkels M, Rijkers M, Voorberg J, et al. Emerging concepts in immune thrombocytopenia. Front Immunol. 2018;9:880.
- Lv Y, Ruan G, Liu Y, et al. Aberrant expression of NLRP3, NLRC4 and NLRP6 inflammasomes in patients with primary immune thrombocytopenia. Thromb Res. 2019;176:101–103.
- Yazdanbakhsh K, Zhong H, Bao WJ. Immune dysregulation in immune thrombocytopenia. Semin Hematol. 2013, 50 Suppl 1(0 1): S63–S67.
- Wang Q, Li J, Yu T, et al. Disrupted balance of CD4 T-cell subsets in bone marrow of patients with primary immune thrombocytopenia. Int J Biol Sci. 2019;15(13):2798–2814.
- Kawaguchi K, Kaneko S. Notch signaling and liver cancer. Notch Signaling in Embryology and Cancer. 2021;1287:69–80.
- Yin X, Wei H, Wu S, et al. DAPT reverses the Th17/Treg imbalance in experimental autoimmune uveitis in vitro via inhibiting notch signaling pathway. Int Immunopharmacol. 2020;79:106107.
- Ito T, Schaller M, Hogaboam C, et al. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived notch ligand delta-like 4. J Clin Invest. 2009;119(1):33–46.
- Gawdat R, Hammam A, Ezzat DJ. Correlation of Notch1/Hes1 genes expression levels in Egyptian paediatric patients with newly diagnosed and persistent primary immune(idiopathic) thrombocytopenic purpura. Indian Journal of Hematology and Blood Transfusion. 2016;32(3):362–367.
- Ghetti M, Vannini I, Storlazzi C, et al. Linear and circular PVT1 in hematological malignancies and immune response: two faces of the same coin. Mol Cancer. 2020;19(1):69.
- Eftekharian M, Ghafouri-Fard S, Soudyab M, et al. Expression analysis of Long Non-coding RNAs in the blood of multiple sclerosis patients. J Mol Neurosci. 2017;63:333–341.
- Wu L, Xia J, Li D, et al. Mechanisms of M2 macrophage-derived exosomal Long Non-coding RNA PVT1 in regulating Th17 cell response in experimental autoimmune encephalomyelitisa. Front Immunol. 2020;11:1934.
- Xu K, Xu Q, Wu Z, et al. LncRNA NEAT1 is involved in temozolomide resistance by regulating MGMT in glioblastoma multiforme. Clin Surg Res Commun. 2018;1:24–30.
- Wang L, Yang J, Wang H, et al. Highly expressed ribosomal protein L34 predicts poor prognosis in acute myeloid leukemia and could be a poential therapy target. APT. 2020;2:32–37.
- Roman MG, Flores LC, Cunningham GM, et al. Thioredoxin overexpression in mitochondria showed minimum effects on aging and age-related diseases in male C57BL/6 mice. APT. 2020;2:20–31.
- Moulis G, Lapeyre-Mestre M, Adoue D, et al. [Epidemiology and pharmacoepidemiology of immune thrombocytopenia]. Rev Med Interne. 2017;38(7):444–449.
- Lin X, Xu A, Zhou L, et al. Imbalance of T Lymphocyte subsets in adult immune thrombocytopenia. Int J Gen Med. 2021;14:937–947.
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238.
- Tang Z, Wang Y, Xing R, et al. Deltex-1 is indispensible for the IL-6 and TGF-β treatment-triggered differentiation of Th17 cells. Cell Immunol. 2020;356:104176.
- Cheng L, Liu C, Li F, et al. The prediction value of Treg cell subtype alterations for glucocorticoid treatment in newly diagnosed primary immune thrombocytopenia patients. Thromb Res. 2019;181:10–16.
- Feng Y, Xiao Y, Yan H, et al. Sirolimus as rescue therapy for refractory/relapsed immune thrombocytopenia: results of a single-center, prospective, single-Arm study. Front Med (Lausanne). 2020;7:110.
- Li J, Tian J, Lu J, et al. LncRNA GAS5 inhibits Th17 differentiation and alleviates immune thrombocytopenia via promoting the ubiquitination of STAT3. Int Immunopharmacol. 2020;80:106127.
- Li J, Xia Y, Fan X, et al. Extracellular vesicles derived from miR-199a-5p-modified adipose-derived mesenchymal stem cells alleviate immune thrombocytopenia by inhibiting T helper 17 differentiation. Lab Invest. 2021;101(3):318–327.
- Li T, Gu M, Liu P, et al. Abnormal expression of Long noncoding RNAs in primary immune thrombocytopenia: A microarray related study. Cell Physiol Biochem. 2018;48(2):618–632.
- Ayoub S, Hefzy E, Abd El-Hmid R, et al. Analysis of the expression profile of long non-coding RNAs MALAT1 and THRIL in children with immune thrombocytopenia. IUBMB Life. 2020;72(9):1941–1950.
- Li J, Hu S, Wang Z, et al. Long non-coding RNA MEG3 inhibits microRNA-125a-5p expression and induces immune imbalance of treg/Th17 in immune thrombocytopenic purpura. Biomed Pharmacother. 2016;83:905–911.
- Fu J, Shi H, Wang B, et al. LncRNA PVT1 links Myc to glycolytic metabolism upon CD4 T cell activation and sjögren's syndrome-like autoimmune response. J Autoimmun. 2020;107:102358.
- Amsen D, Helbig C, Backer RJ. Notch in T cell differentiation: All things considered. Trends Immunol. 2015;36(12):802–814.
- Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97(4):1235–1294.
- Qin L, Zhou Y, Wu H, et al. Notch signaling modulates the balance of regulatory T cells and T helper 17 cells in patients with chronic hepatitis C. DNA Cell Biol. 2017;36(4):311–320.
- Jiao W, Wei J, Kong Y, et al. Notch signaling promotes development of allergic rhinitis by suppressing Foxp3 expression and Treg cell differentiation. Int Arch Allergy Immunol. 2019;178(1):33–44.
- Ma D, Dai J, Zhu X, et al. Aberrant expression of notch signaling molecules in patients with immune thrombocytopenic purpura. Scand J Clin Lab Invest. 2010;89(2):155–161.
- Yu S, Liu C, Li L, et al. Inactivation of notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest. 2015;95(2):157–167.
