ABSTRACT
Objectives
To compare the outcomes of antihuman T lymphocyte globulin (ATG-F) and porcine antihuman lymphocyte globulin (p-ALG) as part of a conditioning regimen in hematopoietic stem cell transplantation (HSCT) for severe aplastic anemia (SAA).
Methods
we performed a retrospective analysis, evaluating the outcome of patients with SAA who received ATG-F based conditioning (n = 26) with those receiving p-ALG conditioning (n = 34).
Results
The median time to neutrophil engraftment was 11 days (range, 8 − 38) and 11 days (range, 9 − 24) in the p-ALG and ATG-F groups (P = 0.857); the median platelet engraftment time was 15 (range, 9 − 330) days and 13 (range, 10 − 56) days (P = 0.155). There were no significant differences in grades II − IV acute graft-versus-host disease (aGVHD), grades III − IV aGVHD, chronic GVHD (cGVHD), and the moderate-severe cGVHD between the ATG-F and p-ALG groups (P>0.05).
Discussion
Patients in the ATG-F group functioned significantly better on role-physical (P = 0.006), general health (P = 0.029), and physical component summary (P = 0.009). The estimated overall survival and failure free survival rates at 5 years were 88.5% ± 6.3% vs. 82.4% ± 6.5% (P = 0.515), 84.6% ± 7.1% vs. 79.4% ± 6.9%, respectively (P = 0.579). The infection rates were 61.53% and 47.05%, respectively (P = 0.265).
Conclusion
As part of the conditioning regimen, p-ALG achieved a similar efficacy as ATG-F without increasing the incidence of transplantation complications in SAA patients.
Introduction
In most cases, severe aplastic anemia (SAA) is an autoimmune disorder resulting from autoreactive cytotoxic T lymphocyte attack of the hematopoietic component of the bone marrow. The disease results in fatal complications, such as infection and/or hemorrhage [Citation1,Citation2]. Allogeneic haematopoietic stem cell transplantation (allo-HSCT) from a matched-related donor (MRD) is the initial treatment of choice for newly diagnosed SAA or very SAA (vSAA) patients who are less than 35 years of age [Citation1]. According to the current therapeutic algorithms, immunosuppressive therapy (IST) with a combination of horse antithymocyte globulin (ATG) and cyclosporin A (CsA) is the preferred first-line treatment for patients without an MRD and older patients [Citation3]. Although patients respond to IST, the long-term risks of relapse, CsA dependence, and clonal evolution are high; those unresponsive to initial IST or experiencing clonal evolution will be considered for transplantation using an alternative donor [Citation4]. Alternative HSCTs from sources of stem cells, from groups including HLA-matched unrelated donors (URDs), mismatched unrelated donors, haploidentical family donors, and unrelated umbilical cord blood (UCB) are options for individuals with no suitable MRD [Citation4–9]. URD haematopoietic stem cell transplantation (URD-HSCT) has even been considered as a first-line treatment for SAA in children and young patients by some studies [Citation2,Citation10–13]. Haploidentical haematopoietic stem cell transplantation (haplo-HSCT) as a treatment for SAA has greatly improved [Citation6,Citation14–18]. Patients who underwent haplo-HSCT as an initial therapy had primary engraftment and survival outcomes similar to SAA patients who received MRD-HSCT [Citation19–21]. In China, haplo-HSCT is recommended for newly diagnosed young SAA patients without an MRD [Citation22].
In previous studies, improved survival after transplantation was shown for both MRD and alternative donors in children and adults. Improved outcomes may have been a consequence of changes in graft-versus-host disease (GVHD) prophylaxis, changes in conditioning regimens, better donor selection, and a larger use of ATG. Conditioning regimens for HSCT in SAA with ATG led to better survival associated with a lower risk of grade II − IV acute GVHD (aGVHD) [Citation23,Citation24]. There are different species of animals utilized to produce ATG, and the available sources currently include horse ATG (h-ATG) and rabbit ATG (r-ATG). Two different r-ATG formulations, thymoglobulin-ATG (ATG-T) and fresenius-ATG (ATG-F) (Fresenius Biotech GmbH, Germany), are usually employed as prophylaxis for GVHD [Citation25]. ATG-T use resulted in fewer adverse effects compared with ATG-F, and similar clinical outcomes were observed in patients undergoing HSCT, suggesting that ATG-T has stronger immunosuppressive activity than ATG-F [Citation26–29]. On the other hand, HSCT with ATG remain to be a heavy financial burden for SAA patients even in Western countries. Anti-human T lymphocyte porcine immunoglobulin (p-ALG) (Wuhan Institute of Biological Products, China, State Medical Permit No. S10830001) was approved by the Sino Food and Drug Administration (SFDA) in 2004. It is commonly used in China to treat people with SAA due to its similar efficacy and safety as rabbit ATG; because of its lower cost (p-ALG costs only about 40% of the cost of rabbit ATG), it is widely used as a first-line therapy against acquired SAA in China [Citation30,Citation31].
As part of a component of the conditioning regimen, whether p-ALG achieves a similar efficacy as ATG-F without increasing the incidence of transplantation complications, such as GVHD, graft failure (GF), and infection in SAA patients have not been evaluated. These mechanistic differences between ATG-F and p-ALG may not generate the same results following HSCT, and the effects of p-ALG versus ATG-F in an allo-HSCT conditioning regimen for SAA are unknown. Therefore, we retrospectively analyzed the outcome of 60 SAA patients who underwent HSCT with ATG-F or p-ALG in the conditioning regimen at our centers from September 2011 to November 2017.
Materials and methods
Patients
Between September 2011 and November 2017, 60 SAA patients who underwent HSCT with ATG-F or p-ALG as a conditioning regimen at our transplantation unit were enrolled in this study. Patients met the following criteria: diagnosis and management of SAA or vSAA, as defined by the guidelines [Citation1], no response to previous immune-suppressive therapy (IST) (CsA or r-ATG/p-ALG plus CsA) or HSCT as an initial treatment, transfusion dependence; received p-ALG or ATG-F as a conditioning regimen, voluntary participation in HSCT, and absence of severe liver, renal, lung, and heart diseases. Iron chelation therapy was administered when the serum ferritin was > 1000 μg/L, to decrease the level to less than 1000 μg/L before transplantation. All of the patients and donors provided written informed consent for this protocol. This study was approved by the hospital Ethics Committee.
HLA typing and donor selection
The HLA-A, -B, -C, DRB1 and -DQB1 in the recipients were typed. Those with appropriate HLA type, age, gender, health status, and willingness were considered as qualified donors. MRD was most preferred, followed by URD, haploidentical donors or UCB units.
Conditioning regimen
The time sequences in the transplant were differentially named. For instance, the days before transplantation were marked with ‘−’, and those after the last stem cell infusion with ‘+’. A therapy based on fludarabine (FLU)/cyclophosphamide (CY) was used to deal with patients with an MRD or UCB. Protocols were as follows. FLU:30 mg/m2/day (i.v.) on days −7 and −2; CY: 50 mg/kg/day i.v. on days −4 to −3; and ATG-F (Zetbulin, ATG-F, Fresenius, Munich, Germany): 5 mg/kg/day i.v. or p-ALG (porcine, Wuhan Institute of Biological Products): 20 mg/kg/day i.v. on days −5 to −2. For those receiving haploidentical donors, a therapy was designed with busulfan (BU)/CY. The protocols were as follows. BU: 3.2 mg/kg/day intravenously (i.v.) on days −7 and −6; CY: 50 mg/kg/day i.v. on days −5 to −2; and ATG-F: 5 mg/kg/day i.v. or p-ALG: 20 mg/kg/day i.v. on days −5 to −2. For those receiving URD, FLU/CY-based or BU/CY-based regimens were introduced. Protocols were as follows: FLU:30 mg/m2/day (i.v.) on days −7 and −2; CY: 50 mg/kg/day i.v. on days −4 to −3; a therapy was designed with busulfan (BU)/CY: BU: 3.2 mg/kg/day intravenously (i.v.) on days −7 and −6; CY: 50 mg/kg/day i.v. on days −5 to −2.
Graft collection and infusion
As preparation for stem cell mobilization of haploidentical donors, granulocyte colony-stimulating factor (G-CSF) (10 mg/kg/day) was subcutaneously injected from day −4. ‘day 01’ referred to the first day of stem cell injection. On day 01, BM aspiration were employed to prepare BM grafts in the surgery room. As the mononuclear cell (MNC) count rose to 2 − 4 × 108/kg of recipient weight, the peripheral blood stem cells (PBSCs) were harvested on day 02 through apheresis accomplished by a COBESPECTRA device (Gambro BCT, Lakewood, CO, USA). In the total cells from the BM and peripheral blood (PB), the MNC count was allowed to grow to 6 − 8 × 108/kg of the recipient weight. If the count was below this level, PBSCs were supplied the next day. BM and PBSCs, once collected, were translated into the recipient.
GVHD prophylaxis and treatment strategy
The patients who were assigned to FLU/CY-based regimen received CsA for aGVHD prophylaxis. Infusion of CsA (3 mg/kg/day) sustained for over 24 h, from day −4 to the day when the infusion was replaced with oral intake (PO). The requested whole-blood trough level was kept at 200 − 300 ng/mL for 12 months after HSCT. CsA was gradually reduce till withdrawn during the next 2−3 months. In patients assigned to BU/CY-based regimen, CsA (from day −7), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) were administered for aGVHD prophylaxis. MMF (1.0 g, or 0.5 g for children, PO, twice daily) was given from day −7 to +30, then gradually reduced until day +60. MTX doses were set as 15 mg/m2/day on day +1, and at 10 mg/m2/day on days +3, +6 and +11. As CsA dropped, CsA was continued and raised to the therapeutic dose in the case of GVHD. Acute GVHD was resolved with methylprednisolone (1–2 mg/kg, once daily). Steroid refractory aGVHD was copedwith second-line immunosuppressive therapy based on including tacrolimus (FK506), CD25 monoclonal antibody, MMF and MTX.
Supportive care and post-transplantation surveillance
Once preparative regimen initiated, all patients were restricted inside sterile rooms for reverse isolation. Prior to conditioning 3 days, the gut was selectively decontaminated with fluconazole (200 mg twice daily), albendazole (200 mg once daily, 3 days) and levofloxacin (200 mg twice daily, gentamycin for children). During the conditioning and immunosuppressive period, prophylactic antibiotics, antifungal and antiviral therapies were administered. Trimethoprim/sulfamethoxazole (four tablets per day, twice weekly) was adopted for preventing Pneumocystis jiroveci infection. G-CSF (5 µg/kg/day) was given on day +7, until the confirmation of myeloid recovery [absolute neutrophil count (ANC) ≥ 2 × 109/L for 3 consecutive days]. Heparin and prostaglandin E1 were taken to prevent veno-occlusive disease. Prophylactic i.v. immunoglobulin (400 mg/kg, once weekly) was used in the first month after HSCT. Irradiated and leukodepleted blood products were given to maintain a haemoglobin (Hb) level > 60 g/L and a platelet count > 20×109/L.
After neutrophil recovery, the chimerism of donor cells was evaluated through weekly multiplex fluorescent analysis on short tandem repeat (STR) in PB for 1 year. Cytomegalovirus (CMV) and Epstein–Barr virus (EBV) viremia was confirmed using real-time PCR weekly. The case of positive CMV was treated with pre-emptive therapy based on ganciclovir or foscarnet.
Assessment of health-related quality of life (HRQoL)
As a tool for measuring HRQoL among adults, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) consists of eight subscales pertaining to physical functioning, role-physical functioning, bodily pain, general health, vitality, social functioning, role-emotional functioning, and mental health. The scores of eight subscales are summarized into the physical component summary (PCS) and the mental component summary (MCS) [Citation32,Citation33].
All the eligible patients were informed of the objective of the study. A consent form, questionnaires, and a self-addressed stamped envelope were mailed to those volunteers. Their written informed consent and questionnaires were returned as early as possible. Their clinical information was put into a medical database.
Post-transplantation evaluations
ANC > 0.5×109/L during three consecutive days indicated neutrophil engraftment. Platelet count > 20×109/L without transfusion support for seven consecutive days indicated platelet engraftment. Other deifications were as follows. Primary GF: neutrophil engraftment failure after HSCT at day +28. Secondary GF: recurrent ANC < 0.5 × 109/L after initial engraftment by loss of donor cells. Platelet recovery delay: platelet engraftment after > 30 days. Early death: death within 60 days after HSCT. Transplantation-related mortality (TRM): death without disease progression. Failure-free survival (FFS): survival without treatment failure. Death, primary or secondary GF, and relapse suggested treatment failures. Acute GVHD was scored by the 1994 Consensus Conference on Acute GVHD Grading [Citation34], and chronic GVHD (cGVHD) by the NIH Consensus Development Project on the Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: Diagnosis and Staging Working Report [Citation27].
After HSCT, recipient BM was sampled monthly during the first 3 months and every 3 − 6 months during 1 − 2 years of follow-up.
Statistical methods
The chi-square or Fisher’s exact test was used to compare categorical variables, and the Mann − Whitney nonparametric test was used for continuous variables and HRQoL. Overall Survival (OS) and FFS were calculated using the Kaplan-Meier method and compared between different patient groups using the log-rank test. The cumulative incidence function was used to calculate the incidence of TRM, aGVHD and cGVHD. SPSS 22.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. All of the P values are two-sided, and the results were considered to be statistically significant when P < 0.05.
Results
Patient, disease, and transplant characteristics
A total of 60 patients were enrolled in this study. The characteristics of the patients and donors prior to transplantation are shown in . The p-ALG group included 17 male patients and 17 female patients, the median age was 25.5 years (range: 7 − 54 years). Data from 19 vSAA patients were included, six patients with failure of IST who underwent salvage therapy, and 28 patients underwent transplantation as first-line therapy. The median interval from SAA diagnosis to HSCT was 4.0 months (range 0.8 − 240.0 months). The ATG-F groups included 13 male patients and 13 female patients, the median age was 27 years (range: 3 − 52 years). Data from 15 vSAA patients were included, one patient with paroxysmal nocturnal haemoglobinuria (PNH) clones, two patients with failure of IST that underwent salvage therapy, and 24 patients underwent transplantation as first-line therapy. The median interval from SAA diagnosis to HSCT was 2.0 months (range 1.0 − 180.0 months). Details of the graft subtypes are also presented in . The ATG-F group had the highest incidence of haplo-HSCT, followed by all, MRD, URD and UCBT. The p-ALG group had the highest incidence of MRD transplantation, followed by haplo-HSCT, URD, UCBT.
Table 1. Characteristics of SAA patients undergoing HSCT.
Hematopoietic recovery
The median values of absolute mononuclear cells (MNCs) were 10.36 × 108/kg (range 0.41 − 22.39 × 108/kg) and 13.06 × 108/kg (range 0.62 − 24.00 × 108/kg) in the p-ALG group and ATG-F groups, respectively (P = 0.142). The median values of absolute CD34+ cells were 4.23 × 106/kg (range 0.15 − 5.94 × 106/kg) and 4.15 × 106/kg (range 0.86 − 5.95 × 106/kg), respectively (P = 1.000). The median time to neutrophil engraftment was 11 days (range, 8 − 38) and 11 days (range, 9 − 24; P = 0.857) and the median time to platelet engraftment was 15 days (range, 9 − 330) and 13 days (range, 10 − 56) in the p-ALG group and ATG-F groups, respectively (P = 0.155).
In the ATG-F group, all of the patients achieved successful donor myeloid engraftment, no patient experienced primary GF, and one patient experienced secondary GF. One patient demonstrated delayed platelet recovery and one patient demonstrated platelet GF. In the p-ALG group, two patients experienced early mortality and among the evaluable 32 patients, two UCBT patients experienced hematopoietic function spontaneous recovery. The other 30 patients achieved successful donor myeloid engraftment, and no patient experienced primary or secondary GF. Four patients demonstrated delayed platelet recovery and one patient demonstrated platelet GF ().
Table 2. Clinical outcomes after HSCT.
Infections and transplantation-related toxicities
The infection rate was 61.53% in the ATG-F group and 47.05% in the p-ALG group (P = 0.265) within the first 100 days after transplantation (). The infections included febrile neutropenia (19.23% vs. 5.88%, P = 0.234), pulmonary infection (15.38% vs. 20.59%, P = 1.000), septicaemia (15.38% vs. 11.76%, P = 1.000), urinary infection (0.00% vs. 2.94%, P = 1.000), mucositis/stomatitis (11.53% vs. 8.82%, P = 0.728), viremia (0.00% vs. 2.94%, P = 0.378), upper airway (0.00% vs. 5.88%, P = 0.208), and others (3.85% vs. 8.23%, P = 0.720). No patients died due to lethal organ toxicities during the 40 days after HSCT.
GVHD incidence and severity
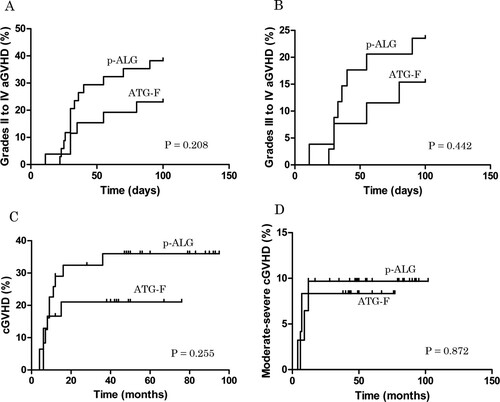
The cumulative incidence of grades II to IV aGVHD on day +100 was 23.08% ± 8.26% and 38.24% ± 8.33% in the ATG-F and p-ALG groups, respectively (P = 0.208; A). The cumulative incidence of grades III to IV aGVHD on day +100 was 15.39% ± 7.08% and 23.53% ± 7.28% (P = 0.442; B), and the cumulative incidence of cGVHD was 21.05% ± 8.38% and 35.97% ± 8.71%, respectively (P = 0.255; C). The cumulative incidence of moderate-severe cGVHD was 8.33% ± 5.64% and 9.68% ± 5.31%, respectively (P = 0.872; D).
Figure 1. The cumulative incidences of GVHD. (A) The cumulative incidence of grades II to IV aGVHD on day +100 was 23.08% ± 8.26% and 38.24% ± 8.33% in the ATG-F and p-ALG groups, respectively (P = 0.208). (B) The cumulative incidence of grades III to IV aGVHD on day +100 was 15.39% ± 7.08% and 23.53% ± 7.28% in the ATG-F and p-ALG groups, respectively (P = 0.442). (C) The cumulative incidence of cGVHD was 21.05% ± 8.38% and 35.97% ± 8.71% in the ATG-F and p-ALG groups, respectively (P = 0.255). (D) The cumulative incidence of moderate-severe cGVHD was 8.33% ± 5.64% and 9.68% ± 5.31% in the ATG-F and p-ALG groups, respectively (P = 0.872).
Health-related quality of life
As shown in , patients in the ATG-F group functioned comparably or better than the p-ALG group on HRQoL, which was indicated by comparable or higher scores in PCS, physical functioning, role-physical, general health, MCS, vitality, social functioning and mental health. Patients in the ATG-F group scored better in role-physical (P = 0.006), general health (P = 0.029) and PCS (P = 0.009) compared with the p-ALG group.
Table 3. SF-36 scores for survivors by therapy (median, range).
Relapse, treatment-related mortality, and survival
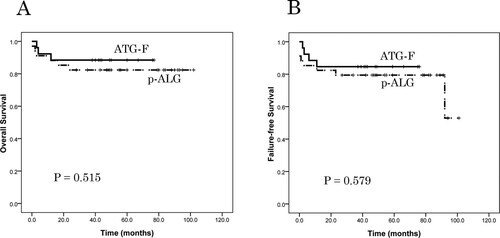
The median follow-up time among living patients was 44 months (range, 38 − 77) and 92 months (range, 28 − 102) in the ATG-F and p-ALG groups, respectively. No patients relapsed during the follow-up period in the two groups. The TRM rate was 11.54% in the ATG-F group and 15.15% in the p-ALG group (P = 0.703; ). The causes of TRM included GVHD in one case and infection in two cases; GVHD in two cases, infection in two cases, and thrombotic microangiopathy in one case, respectively. The estimated overall survival (OS) at 5 years was 88.5% ± 6.3% in the ATG-F group and 82.4% ± 6.5% in the p-ALG group (P = 0.515; A). The estimated FFS at 5 years was 84.6% ± 7.1% in the ATG-F group and 79.4% ± 6.9% in the p-ALG group (P = 0.579; B).
Figure 2. The TRM rate during follow-up. The TRM rate was 11.54% in the ATG-F group and 15.15% in the p-ALG group (P = 0.703).
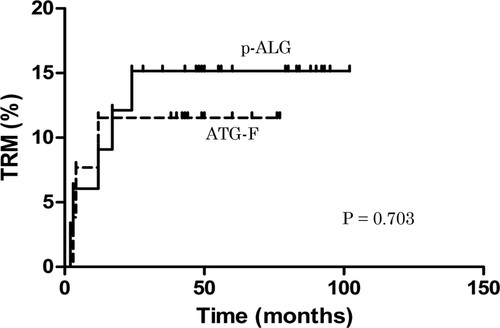
Figure 3. Patient overall survival (OS) and failure-free survival (FFS), as assessed using Kaplan-Meier analysis. (A) The probability of 5-year OS was 88.5% ± 6.3% in the ATG-F group and 82.4% ± 6.5% in the p-ALG group (P = 0.515). (B) The probability of 5-year GFFS was 84.6% ± 7.1% in the ATG-F group and 79.4% ± 6.9% in the p-ALG group (P = 0.579).
Discussion
MRD-HSCT for young and adult patients remains the treatment of first choice for SAA [Citation1]. Alternative donor types should be considered in the absence of a MRD in cases where patients fail to respond to IST. URD, haplo-HSCT, or UCBT are generally regarded as a salvage treatment option for SAA due to the high rate of GF and refractory GVHD [Citation6,Citation7,Citation14,Citation19,Citation35,Citation36]. For recipients of MRD-HSCT, Flu/Cy/ATG or Cy/ATG led to the best survival and are considered optimal transplant conditioning regimens. In recipients of URD, there were no differences in survival between regimens. Rabbit-derived ATG was associated with a lower risk of grade II to IV aGVHD but not cGVHD [Citation11]. The conditioning regimen was a combination of BU/Cy/ATG or Flu/Cy/TBI/ATG for haplo-identical transplantation [Citation6,Citation14]. ATG is an important component of conditioning regimens for preventing GVHD and prevent GF in SAA patients undergoing HSCT.
In this study, we assessed the differences between p-ALG and ATG-F formulations in the conditioning regimen for HSCT in SAA. We analyzed the efficacy, survival, and safety profiles in the two groups. The hematopoietic recovery was similar in the ATG-F group and the p-ALG group. In the ATG-F group, all of the patients achieved successful donor myeloid engraftment, one patient experienced secondary GF, one patient demonstrated delayed platelet recovery, and one patient demonstrated platelet GF. In the p-ALG group, two patients experienced early mortality, and among the evaluable 32 patients, two UCBT patients experienced hematopoietic function spontaneous recovery. The other 30 patients achieved successful donor myeloid engraftment. Four patients demonstrated delayed platelet recovery and one patient demonstrated platelet GF.
HSCT for SAA has made significant progress over the past decade; this is true especially for alternative donor transplants including URD, CBT, and haplo-identical grafts [Citation4,Citation8,Citation9,Citation37]. Although antithymocyte globulin had been widely used in HSCT to its ability to prevent acute and chronic GVHD. Most previous studies have focused on two rabbit ATG products: ATG-T and ATG-F. They exert immunomodulatory functions primarily via in vivo depletion of T-lymphocytes [Citation38]. Paiano S et al. compared two rabbit ATG products in allo-HSCT after reduced intensity conditioning (RIC) for hematologic malignancies. There were no differences in OS, disease-free survival (DFS), relapse incidence, GF, infectious complications, immune reconstitution, and acute or chronic GVHD [Citation39]. Nicola Polverelli et al. compared the two different ATG formulations in a cohort of 77 allo-HSCT patients transplanted from URD. Their results suggest a different immunological activity for the different ATG, with ATG-F ensuring a more extensive and long-lasting effect on more severe forms of cGVHD [Citation27]. Huang et al. did a retrospective analysis of patients who underwent HLA-mismatched allogeneic peripheral blood stem cell transplantation from unrelated donors (UR-PBSCT) and received pre-transplant r-ATG or ATG-F. This was the first comparison of two commonly used ATG preparations administered at fixed doses in HLA-mismatched UR-PBSCT patients, and results suggested that ATG-F is as effective as r-ATG and had fewer adverse effects [Citation26]. In our findings, a trend was observed showing that ATG-F was associated with less GVHD but more infections. The 5-year OS was 88.5% ± 6.3% and 82.4% ± 6.5% in the ATG-F and p-ALG groups, respectively (P = 0.515). The infection rate was 61.53% in the ATG-F group and 47.05% in the p-ALG group (P = 0.265). The overall mortality rate was not different between the ATG-F and p-ALG groups; infection-related deaths were 7.69% vs. 5.88%, (P = 0.781) and GVHD related deaths were 3.85% vs. 5.88%, respectively (P = 0.720). Others have noted that the timing and dose of ATG are important to outcomes of engraftment, infection rate, and survival post HSCT. Some studies have shown that higher doses of ATG are associated with lower rates of GVHD but higher rates of infection, including post-transplant lymphoproliferative disorder [Citation25,Citation40]. In addition, there are likely other unmeasured or unknown factors that may have affected GVHD rates and survival [Citation41]. This should be considered when designing clinical protocols.
For SAA patients, OS is no longer the only parameter used to determine the optimal treatment. Survival rates after HSCT have improved considerably during the past 30 years. There is a growing population of survivors, particularly in individuals who received HSCT as children. To date, no assessments on HRQoL in long-term SCT survivors for SAA have been reported. Issues of the long-term side-effects of intensive therapies, return to prior activities, and reconstitution of normal levels of functioning are of tremendous importance for patients and their relatives during the decision-making process leading to admittance for or refusal of SCT. SAA patients experience poor quality of life, including severe fatigue, poor global health status, impaired functioning, pain, and dyspnea. Although many studies have evaluated the HRQoL of patients after HSCT, to the best of our knowledge, this study was the first comparative trial focused on HRQoL in patients receiving p-ALG or ATG-F as the conditioning regimen for allo-HSCT. There are a few studies focused exclusively on recipients of HSCT for SAA; however, the instruments used to measure HRQoL were heterogeneous, making comparisons difficult. Many studies have suggested that 25%−93%, 63%−77%, and 84% of those who received MRD, URD, or HSCT, respectively, exhibited strong recovery in terms of general health, physical health, and mental health [Citation42]. Patients receiving HLA-haploidentical/partially matched related allo-HSCT can achieve desirable HQoL comparable with those receiving HLA-identical sibling allo-HSCT [Citation43]. Measured by multivariate analysis, cGVHD (especially extensive cGVHD) was the most common adverse factor affecting HRQoL, while male gender status, lower age when receiving allo-HSCT, and returning to work or school were associated with positive impacts on at least one subscale. Also, long-term survivors exhibit better HRQoL [Citation43–45]. Comparing the two groups, patients in the ATG-F group had higher scores on role-physical (P = 0.006), general health (P = 0.029), and PCS (P = 0.009) than the p-ALG group.
In conclusion, these results suggest that ATG-F and p-ALG in an allo-HSCT conditioning regimen for SAA had similar outcomes in engraftment, infection, TRM, GVHD, OS, and FFS. However, ATG-F group survivors exhibited better HRQoL than those in the p-ALG group. Our study is limited by its retrospective nature and relatively few patients. Therefore, further large-scale multi-centre cooperative studies should be conducted to completely evaluate our results.
Authors’ contributions
Depei Wu designed the research; Yanming Zhang, Limin Liu, Yejun Si analyzed the data and wrote the paper; and all authors provided patient data and gave final approval for the paper.
Ethics approval
This study was approved by the hospital Ethics Committee, and this study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All of the patients and donors provided written informed consent for this protocol.
Data availability statement
The datasets generated during and/or analyzed during current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207.
- Bacigalupo A. How i treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436.
- Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656.
- Socié G. Allogeneic bm transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematol Am Soc Hematol Educ Program. 2013;2013:82–86.
- Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of hla identical sibling versus unrelated donor transplants in severe aplastic anemia: An ebmt analysis. Haematol. 2015;100(5):696–702.
- Xu LP, Wang SQ, Wu DP, et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol. 2016;175(2):265–274.
- Liu HL, Sun ZM, Geng LQ, et al. Unrelated cord blood transplantation for newly diagnosed patients with severe acquired aplastic anemia using a reduced-intensity conditioning: high graft rejection, but good survival. Bone Marrow Transpl. 2012;47(9):1186–1190.
- Scheinberg P, Young NS. How i treat acquired aplastic anemia. Blood. 2012;120(6):1185–1196.
- Wu Y, Cao Y, Li X, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild gvhd. Stem Cell Res. 2014;12(1):132–138.
- Kennedy-Nasser AA, Leung KS, Mahajan A, et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transpl. 2006;12(12):1277–1284.
- Bacigalupo A, Socie G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: A retrospective study from the ebmt-saa working party. Haematol. 2010;95(6):976–982.
- Yagasaki H, Takahashi Y, Hama A, et al. Comparison of matched-sibling donor bmt and unrelated donor bmt in children and adolescent with acquired severe aplastic anemia. Bone Marrow Transpl. 2010;45(10):1508–1513.
- Zhang Y, Wu L, Mo W, et al. Comparable outcomes of first-line hematopoietic stem cell transplantation from unrelated and matched sibling donors in adult patients with aplastic anemia: A retrospective single-center study. Biol Blood Marrow Transpl. 2019;25(8):1567–1575.
- Bacigalupo A, Giammarco S. Haploidentical donor transplants for severe aplastic anemia. Semin Hematol. 2019;56(3):190–193.
- Im HJ, Koh KN, Choi ES, et al. Excellent outcome of haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Biol Blood Marrow Transpl. 2013;19(5):754–759.
- Gao L, Li Y, Zhang Y, et al. Long-term outcome of hla-haploidentical hematopoietic sct without in vitro t-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transpl. 2014;49(4):519–524.
- Clay J, Kulasekararaj AG, Potter V, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Transpl. 2014;20(11):1711–1716.
- Esteves I, Bonfim C, Pasquini R, et al. Haploidentical bmt and post-transplant cy for severe aplastic anemia: A multicenter retrospective study. Bone Marrow Transpl. 2015;50(5):685–689.
- Xu LP, Jin S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10(1):25.
- Prata PH, Eikema DJ, Afansyev B, et al. Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: A report from the ebmt severe aplastic anemia working party. Bone Marrow Transpl. 2020;55(6):1050–1058.
- Bacigalupo A. Alternative donor transplants for severe aplastic anemia. Hematol Am Soc Hematol Educ Program. 2018;2018(1):467–473.
- Xu L, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese society of hematology. J Hematol Oncol. 2018;11(1):33.
- Bejanyan N, Kim S, Hebert KM, et al. Choice of conditioning regimens for bone marrow transplantation in severe aplastic anemia. Blood Adv. 2019;3(20):3123–3131.
- Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematol Am Soc Hematol Educ Program. 2013;2013(1):76–81.
- Basara N, Baurmann H, Kolbe K, et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transpl. 2005;35(10):1011–1018.
- Huang W, Zhao X, Tian Y, et al. Outcomes of peripheral blood stem cell transplantation patients from hla-mismatched unrelated donor with antithymocyte globulin (atg)-thymoglobulin versus atg-fresenius: A single-center study. Med Oncol. 2015;32(2):465.
- Polverelli N, Malagola M, Turra A, et al. Comparative study on atg-thymoglobulin versus atg-fresenius for the graft-versus-host disease (gvhd) prophylaxis in allogeneic stem cell transplantation from matched unrelated donor: A single-centre experience over the contemporary years. Leuk Lymph. 2018;59(11):2700–2705.
- Penack O, Fischer L, Gentilini C, et al. The type of atg matters – natural killer cells are influenced differentially by thymoglobulin, lymphoglobulin and atg-fresenius. Transpl Immunol. 2007;18(2):85–87.
- Zheng Y, Liu Y, Chu Y. Immunosuppressive therapy for acquired severe aplastic anemia (saa): A prospective comparison of four different regimens. Exp Hematol. 2006;34(7):826–831.
- Bing H, Siyi Y, Wei Z, et al. The use of anti-human t lymphocyte porcine immunoglobulin and cyclosporine a to treat patients with acquired severe aplastic anemia. Acta Haematol. 2010;124(4):245–250.
- Liu L, Ding L, Hao L, et al. Efficacy of porcine antihuman lymphocyte immunoglobulin compared to rabbit antithymocyte immunoglobulin as a first-line treatment against acquired severe aplastic anemia. Ann Hematol. 2015;94(5):729–737.
- Ware JE, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of sf-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care. 1995;33(4 Suppl):264–279.
- Li L, Wang HM, Shen Y. Chinese sf-36 health survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57(4):259–263.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15(6):825–828.
- Yagasaki H, Kojima S, Yabe H, et al. Acceptable hla-mismatching in unrelated donor bone marrow transplantation for patients with acquired severe aplastic anemia. Blood. 2011;118(11):3186–3190.
- Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: A study by eurocord and the aplastic anemia working party of the european group for blood and marrow transplantation. Biol Blood Marrow Transpl. 2011;17(1):78–85.
- Xu LP, Liu KY, Liu DH, et al. A novel protocol for haploidentical hematopoietic sct without in vitro t-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transpl. 2012;47(12):1507–1512.
- Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: A systematic review. Leukemia. 2012;26(4):582–588.
- Paiano S, Roosnek E, Tirefort Y, et al. Comparing two types of rabbit atg prior to reduced intensity conditioning allogeneic hematopoietic sct for hematologic malignancies. Bone Marrow Res. 2015;2015:980924.
- Kekre N, Zhang Y, Zhang MJ, et al. Effect of antithymocyte globulin source on outcomes of bone marrow transplantation for severe aplastic anemia. Haematol. 2017;102(7):1291–1298.
- Baker KS, Gurney JG, Ness KK, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the bone marrow transplant survivor study. Blood. 2004;104(6):1898–1906.
- Worel N, Biener D, Kalhs P, et al. Long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transpl. 2002;30(9):619–626.
- Mo XD, Xu LP, Liu DH, et al. Patients receiving hla-haploidentical/partially matched related allo-hsct can achieve desirable health-related qol that is comparable to that of patients receiving hla-identical sibling allo-hsct. Bone Marrow Transpl. 2012;47(9):1201–1205.
- Wong FL, Francisco L, Togawa K, et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010;115(12):2508–2519.
- Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: A report from the bone marrow transplant survivor study. Blood. 2006;108(8):2867–2873.
