ABSTRACT
Objective
To assess real-world treatment patterns in patients with immune thrombocytopenia (ITP) who received thrombopoietin receptor agonists (TPO-RAs) in Germany.
Methods
This was a longitudinal, retrospective study using anonymized patient-level data (IQVIA healthcare prescription database, covering 82% of German statutory prescriptions). Eligible patients (aged ≥18 years) had received ≥1 TPO-RA prescription (romiplostim/eltrombopag) from July 2016 to June 2019 (treatment duration ≥30 days). ITP medication use was assessed for 18 months prior to, during and for ≥6 months after TPO-RA treatment.
Results
A total of 3553 patients (median age 64 years) were included. Median persistence on TPO-RAs was 12 months (range 1–34). In the periods before, during and after TPO-RA treatment, oral corticosteroids were the most commonly used therapy (64.4%, 43.4% and 36.1% of patients, respectively); median cumulative doses across each period were 2521.9, 2000.0 and 2277.8 mg. The median total duration of corticosteroid use before, during and after TPO-RA therapy was 15, 18 and 32 weeks, respectively. The total median cumulative corticosteroid dose was 6799.7 mg.
Conclusion
We identified a potential overuse of corticosteroids in patients with ITP in Germany. Earlier use of TPO-RA therapy after a short course of corticosteroids could avoid side effects associated with long-term use.
Introduction
Immune thrombocytopenia (ITP) is a rare, autoimmune disease characterized by low platelet counts [Citation1,Citation2]. The incidence of adult ITP is estimated at 3.3–3.9 per 100,000 individuals/year [Citation3,Citation4], with 5000–13,000 patients in Germany requiring treatment annually [Citation5]. ITP is characterized by three phases: newly diagnosed (≤3 months after diagnosis), persistent (3–12 months after diagnosis) and chronic (disease duration >12 months) [Citation1]. It is a heterogeneous disorder with variable presentation. Some patients experience no clinical symptoms, while others experience increased bleeding tendency [Citation5,Citation6]. The ability to perform daily tasks, emotional well-being, work productivity and health-related quality of life (HRQoL) are frequently impaired [Citation7–9].
Corticosteroids are the standard first-line treatment for adults with newly diagnosed ITP, and they increase platelet counts in the short term [Citation5]. Where rapid platelet increase is required, or when high-dose corticosteroids should be avoided, intravenous immunoglobulin is recommended [Citation5,Citation6,Citation10,Citation11]. Although a small proportion of patients may be maintained on low daily doses of corticosteroids (e.g. ≤5 mg/day), in most cases, the risk of side effects from long-term use outweigh the potential benefits [Citation6]. High relapse rates suggest that corticosteroids do not shorten disease course [Citation5,Citation11–13] and subsequent therapies are often required [Citation6,Citation10].
Second-line interventions in Europe include the thrombopoietin receptor agonists (TPO-RAs) romiplostim and eltrombopag, the recently approved spleen tyrosine kinase inhibitor fostamatinib, and splenectomy (after 12 months) [Citation5,Citation6,Citation10,Citation11,Citation14]. TPO-RAs are a standard second-line treatment in Germany [Citation5,Citation11]. A plethora of agents are reserved for third-line use [Citation5,Citation6,Citation10,Citation11].
The evidence for TPO-RAs in ITP is robust [Citation6], with clinical studies demonstrating tolerability, higher rates of platelet response, and reduced bleeding and concomitant ITP medication use compared with placebo in adults with chronic ITP [Citation15]. Efficacy extends beyond the chronic setting; a pooled analysis of nine romiplostim clinical studies showed increased platelet counts versus placebo/standard of care regardless of disease duration, and higher rates of treatment-free remission in newly diagnosed and persistent ITP [Citation16]. Eltrombopag has also been associated with high response rates in newly diagnosed ITP [Citation17,Citation18].
While the preferred sequencing of ITP treatment is well defined on an international and German level, a translational delay from empirical guidelines to real-world clinical practice is well recognized. Indeed, contrary to guidelines, persistent overuse of corticosteroids has been observed [Citation19–21]. The present study evaluated treatment patterns prior to, during and after the use of TPO-RAs in adult patients with ITP in the German healthcare setting.
Methods
Study design and data source
We conducted a longitudinal, retrospective analysis of anonymized computerized prescription data from the IQVIA statutory healthcare prescription database (IMS® LRx), which includes dispensed German statutory healthcare prescriptions at the patient level [Citation22]. The database contains information on patient demographics, prescribers and prescriptions. The database does not include prescriptions of privately insured patients, and the coverage of hospital pharmacies is low. For compliance with data protection legislation, patient data were de-identified by a pharmaceutical data collection center before delivery to IQVIA. Coverage was approximately 82% of German statutory prescriptions dating back to 2015 (approximately 90% of the German population).
Cohort selection
Inclusion criteria were: ≥1 prescription of TPO-RA (romiplostim/eltrombopag) between 1 July 2016 and 30 June 2019, no TPO-RA prescription in the 18 months before the index date (date of first TPO-RA prescription, which was between 1 July 2016 and 30 June 2019), and ≥30 days in the TPO-RA treatment phase. Patients with known cessation of TPO-RA therapy (6 months without TPO-RA post-index date) and observable for those 6 months were included in the exploratory analysis. As TPO-RAs are a standard second-line therapy in Germany [Citation5,Citation11], the TPO-RA prescription-based dataset allowed the targeted selection of a cohort of patients with ITP of whom the majority presumably relapsed after, or did not respond to, first-line therapy.
Exclusion criteria included patients receiving TPO-RAs for other approved indications, such as aplastic anemia or chronic hepatitis C–associated thrombocytopenia, by excluding patients receiving relevant comedications such as antithymocyte globulin, cyclosporine or (peg)interferon α-2A/α-2B before/during treatment with TPO-RA, and patients aged <18 years or with unknown age on the index date. Patients who had received both TPO-RAs in their database history were also excluded from the main analyses.
Treatment phases
Three treatment phases (pre-TPO-RA, during TPO-RA, post–TPO-RA cessation) were analyzed; the timing of these phases were patient specific. The period 1 July 2016 to 30 June 2019 constituted the index time window during which all index dates (i.e. dates of first TPO-RA prescription) were required to occur. The entire coverage of the database was between 1 January 2015 and 31 December 2019. A pre–TPO-RA treatment phase, which comprised the 18 months before the first TPO-RA prescription, was used to assess ITP medications and to ensure that a patient had not received TPO-RAs before the defined index time window. As noted, diagnosis date was unknown, hence patients may have been diagnosed with ITP before or at any point during this 18-month window. However, as long-term remissions after first-line therapy with corticosteroids are rare (∼5–30% [Citation23]), it was assumed that most patients included in this analysis would be newly diagnosed with the start of their first-line treatment within the 18-month window, which allowed subsequent assessment through the treatment pathway. The TPO-RA treatment phase lasted from the index date until the expiry of the last TPO-RA prescription or to the end of the study period. In patients who stopped TPO-RA treatment for at least 6 months, a post–TPO-RA treatment phase was analyzed, comprising the maximum available duration post–TPO-RA cessation up to database end.
Study objectives
The primary objectives were to assess patient demographics and ITP medications in the 18-month pre–TPO-RA phase, and to assess ITP medications during the period of TPO-RA treatment. An additional exploratory objective was to assess the period following TPO-RA cessation (to the end of observation and for at least 6 months).
ITP medications
ITP medications were corticosteroids (prednisone/prednisolone/methylprednisolone/dexamethasone), immunoglobulins including anti-D immunoglobulin, or other (azathioprine/cyclosporine/cyclophosphamide/dapsone/hydroxychloroquine/mycophenolate/rituximab).
Statistical analyses
Results are presented descriptively; no statistical testing was conducted. ITP treatment in the 18 months prior to TPO-RA therapy was displayed as the cumulative incidence of drug use. The proportion of patients treated with each drug was analyzed before, during and after TPO-RA therapy. Total corticosteroid dosage (mg) over the analytical timespan was calculated and converted to prednisone equivalents (1 mg prednisone ≡ prednisolone 1 mg/methylprednisolone 0.8 mg/dexamethasone 0.15 mg [Citation24]). The duration of corticosteroid use was calculated by summing individual durations.
Results
Study cohort
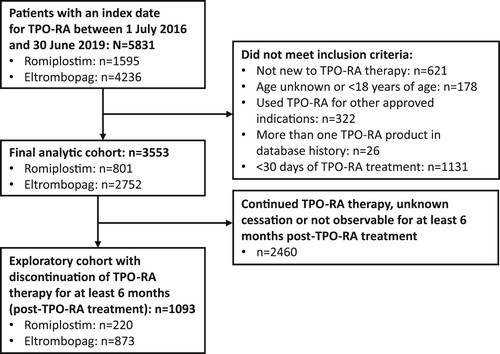
Overall, there were 5831 patients with an index date for TPO-RA between 1 July 2016 and 30 June 2019 (either romiplostim or eltrombopag). Of these, 621 had prior TPO-RA therapy in their database, 178 did not meet the age requirements, 322 had used TPO-RA for other approved indications as indicated by relevant comedications, 26 had more than one TPO-RA product in their database history and 1131 had been treated with TPO-RA for <30 days. In total, 3553 patients met all the eligibility criteria and were included in the analysis of the primary endpoints ().
Figure 1. Study flow diagram. ITP, immune thrombocytopenia; TPO-RA, thrombopoietin receptor agonist.
The median age of patients was 64 years, and the proportion of males and females was similar (). Baseline demographics were similar between romiplostim and eltrombopag groups.
Table 1. Demographic parameters of included participants.
ITP medications prior to TPO-RA treatment
At 18 months prior to TPO-RA therapy, 12% of patients were receiving corticosteroids (A). The cumulative proportion of patients receiving corticosteroids rose fairly linearly over the next 12 months, before an exponential rise during the 6 months before TPO-RA therapy. In total, 64.4% of all patients were treated with corticosteroids at some point in the 18-month period prior to the start of TPO-RA treatment (; A). A total of 7.0% of patients were treated with immunoglobulins at some point during the 18 months prior to initiation of TPO-RA (B).
Figure 2. Cumulative incidence of (A) all patients treated with corticosteroids at any point during the 18 months prior to the index date and (B) all patients treated with immunoglobulin at any point during the 18 months prior to the index date. Gray lines represent 95% confidence intervals.
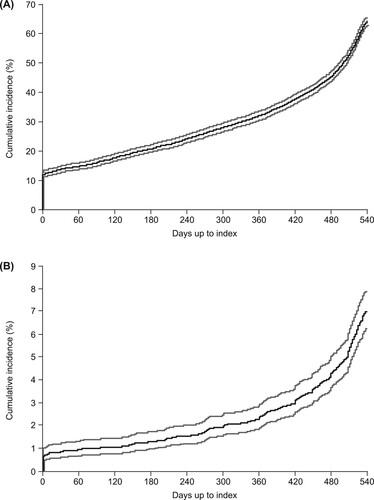
Table 2. Corticosteroid use before, during and after TPO-RA treatment.
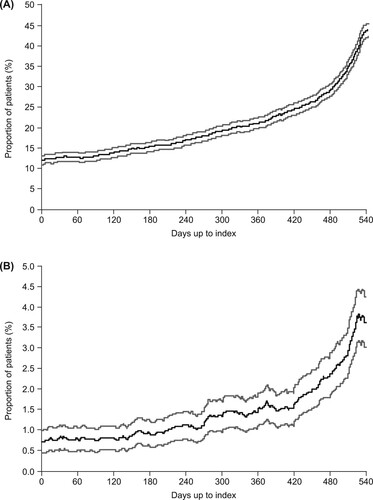
The proportions of patients receiving corticosteroid and immunoglobulin therapy at each timepoint are shown in . These data are not cumulative and present a ‘snapshot’ of the proportion of patients being treated at each timepoint over the 18-month period. Immediately prior to TPO-RA treatment, 43.9% of all patients were receiving corticosteroids (A) and 3.6% were receiving immunoglobulins (B).
Figure 3. Proportion of patients receiving (A) corticosteroids on each day prior to the index date and (B) immunoglobulins on each day prior to the index date. Gray lines represent 95% confidence intervals.
While approximately 36% of patients did not receive corticosteroids in the 18 months prior to TPO-RA therapy, other treatments, such as rituximab and azathioprine, were rarely used during this timeframe (in 1.9% and 2.4% of patients, respectively); all other ITP treatments were each used in <2% of patients. Approximately 25% of patients did not receive any relevant ITP medication prior to TPO-RA treatment (data not shown).
TPO-RA treatment
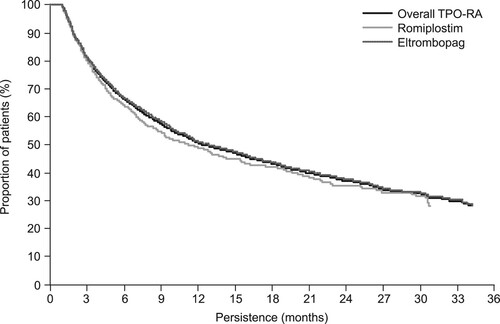
A total of 801 patients received romiplostim and 2752 received eltrombopag. The median overall persistence on TPO-RA therapy was 369 days (). Persistence on the two TPO-RAs was similar.
ITP comedications during TPO-RA treatment
Oral corticosteroids were the most commonly used comedication during the TPO-RA treatment phase (43.4% of patients; ). The proportion of patients treated with corticosteroids on commencement of TPO-RA treatment (index date) was 33.7% (A), compared with 43.9% immediately prior to index (A), indicating that nearly a quarter of patients treated with corticosteroids at the end of the pre–TPO-RA phase were taken off this treatment upon initiation of TPO-RA therapy. The proportion of patients treated with corticosteroids declined to 12.2% at 2 years (720 days) after index (A).
Figure 5. Proportion of patients receiving (A) corticosteroids on each day after the index date during the thrombopoietin receptor agonist (TPO-RA) treatment period and (B) immunoglobulins on each day after the index date during the TPO-RA treatment period. Gray lines represent 95% confidence intervals.
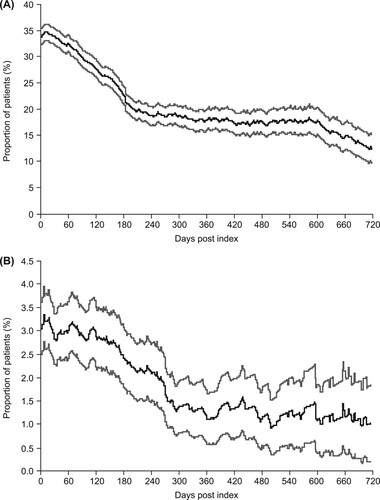
The proportion of patients treated with immunoglobulin on the index date (i.e. on commencement of TPO-RA treatment) was 3.0% (B), which was slightly lower than that immediately prior to index (3.6%; B). The proportion of patients treated with immunoglobulin then declined to approximately 1.0% after 300 days (B).
The use of other ITP medications remained low during the TPO-RA treatment phase (cumulative proportion of patients treated: rituximab, 1.5%; azathioprine, 1.3%; all other ITP medications, each <1%).
ITP medication post–TPO-RA treatment
Corticosteroids were the most commonly used ITP medication during the post–TPO-RA treatment phase (used by 36.1% of patients at some point in the post-observation period), followed by intravenous immunoglobulins (5.0%) and rituximab (2.7%). The proportion of patients receiving corticosteroid treatment immediately after cessation of TPO-RA treatment was 22.2% and remained reasonably constant throughout the post–TPO-RA treatment phase (A).
Figure 6. Proportion of patients receiving (A) corticosteroids on each day after thrombopoietin receptor agonist (TPO-RA) treatment ceased and (B) immunoglobulins on each day after TPO-RA treatment ceased. Gray lines represent 95% confidence intervals.
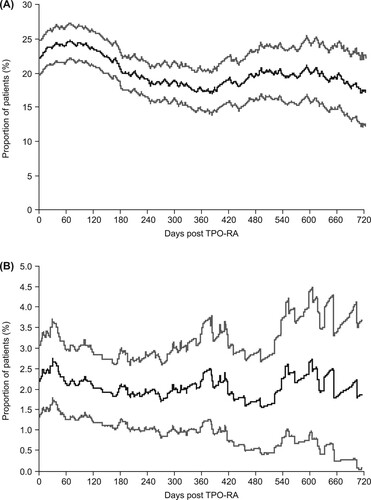
The proportion of patients receiving immunoglobulin was also reasonably constant throughout the post–TPO-RA treatment phase (approximately 1.5–2.5%; B) and the share of other ITP medications varied between approximately 2.0% and 4.0% (data not shown).
Overall, 28.1% of patients received treatment for ITP in the 6 months following the cessation of TPO-RA therapy.
Corticosteroid intake (all phases)
The median total duration of corticosteroid use before, during and after TPO-RA therapy was 15, 18 and 32 weeks, respectively (); corresponding median cumulative corticosteroid doses during these phases were 2521.9, 2000.0 and 2277.8 mg, respectively. Due to the differing observation times for the three periods, these numbers cannot be directly compared. Of the corticosteroid medications, predniso(lo)ne was the most prescribed (used by 93.6% of patients receiving corticosteroids), compared with dexamethasone (used by 24.3% of patients) ().
Discussion
Corticosteroids were the most commonly used medication prior to TPO-RA therapy, reflecting their key role as first-line therapy in newly diagnosed ITP [Citation5,Citation6,Citation10,Citation11]. In the periods before, during and after TPO-RA treatment, oral corticosteroids were prescribed in 64.4% (18-month period), 43.4% (median of approximately 9 months) and 36.1% (median of approximately 14 months) of all patients, respectively.
In support of our data, a retrospective analysis of data (gathered via questionnaires) from 26 German hematology centers reported that corticosteroids were the most commonly prescribed first-line treatment for ITP received by 45% of patients (41% underwent a watch and wait approach); 36% and 28% of patients received corticosteroids in the second and third line, respectively [Citation21]. Thus, our study builds upon published data, using a robust and generalizable statutory healthcare prescription database covering approximately 90% of the German population.
The timeframe of our observation was fixed at 18 months prior to TPO-RA therapy, and the exact date of diagnosis during or before this period was unknown. This partly explains the increase in both cumulative and actual corticosteroid prescriptions over time that we observed prior to TPO-RA therapy, as the number of ITP diagnoses would be expected to accumulate over time (and hence also the number of corticosteroid initiations). Although most patients respond to corticosteroids, only 20–30% of patients maintain a response beyond 1–2 years [Citation25]. This corresponds well with our results, with 25% of patients not receiving any ITP medication and 36% of patients not receiving corticosteroids in the 18 months prior to TPO-RA treatment. However, in the more recent FLIGHT trial, 56% of patients treated with first-line corticosteroids did not require second-line treatment during a mean follow-up of 18 months [Citation26].
The International Consensus Report guidelines note that most adult patients relapse after cessation of corticosteroids [Citation6]. Although a minority may be maintained on low doses of corticosteroids for prolonged periods, subsequent therapy (e.g. TPO-RAs) is recommended for most. In our study, while the proportion of patients receiving corticosteroids decreased by nearly a quarter upon subsequent initiation of TPO-RA treatment, a substantial cohort of patients continued to receive corticosteroids. Most (93.6%) received predniso(lo)ne, while 24.3% received dexamethasone, suggesting a difference in clinical practice from the recommendations by the American Society of Hematology (ASH) and the German Society of Hematology and Medical Oncology (DGHO) [Citation10,Citation11]. Both guidelines suggest either predniso(lo)ne or dexamethasone, but ASH recommends that an initial course of dexamethasone may be preferred if a high value is placed on rapidity of platelet responses [Citation10,Citation11].
The median duration of corticosteroid use during the 18 months prior to TPO-RA therapy was 15 weeks, which substantially exceeded the recommended ≤6 weeks (and maximum of 8 weeks) per the International Consensus Report, ASH and DGHO guidelines [Citation6,Citation10,Citation11]. Long-term overuse of corticosteroids in patients with ITP is a global problem; analysis of a US claims database found that patients with ITP were prescribed corticosteroids for a median of approximately 11 weeks during the first year, with more than 90% receiving ≥5 mg/day prednisone or equivalent [Citation19].
We report median total cumulative corticosteroid use prior to, during and after TPO-RA therapy of ≥2000 mg in each of these three periods, which exceeds guideline recommendations. The International Consensus Report guidelines, for example, recommend 1 mg/kg predniso(lo)ne (maximum dose 80 mg) for 2–3 weeks, followed by rapid taper, aiming to stop at 6 weeks; this would equate to an approximate recommended cumulative corticosteroid dose of 1370–2000 mg for a 70-kg person [Citation6,Citation27]. Due to differences in the duration of each treatment phase, it is not possible to directly compare the cumulative doses prior to, during and after TPO-RA.
The use of high corticosteroid doses is concerning. The most commonly reported adverse effects of ITP treatment are associated with corticosteroids and include insomnia, mood swings and weight gain [Citation26,Citation28]. Infections and diarrhea/gastrointestinal side effects are also frequent [Citation26]. Dose–response relationships are well documented for many corticosteroid-related adverse events [Citation29], supporting the guideline recommendations to limit dose and duration [Citation5,Citation6,Citation10].
TPO-RAs, such as romiplostim and eltrombopag, are potentially important corticosteroid-sparing therapies for ITP and may improve HRQoL and reduce the risk of corticosteroid side effects [Citation30–35]. Eltrombopag is indicated for patients with ITP lasting ≥6 months from diagnosis and who are refractory to other treatments [Citation36]. Romiplostim is indicated for the treatment of ITP in adult patients who are refractory to other treatments (e.g. corticosteroids, immunoglobulins) [Citation37]; this includes patients with newly diagnosed or persistent disease. A retrospective analysis of two 6-month phase 3 clinical trials of romiplostim in adult patients with ITP showed that corticosteroid use was reduced from 30% during weeks 1–4 to 26% during weeks 21–24 in patients treated with romiplostim, and remained above 30% in placebo-treated patients [Citation30]. Although there was insufficient power to demonstrate statistical significance, these differences were deemed clinically relevant. Moreover, the study indicated that romiplostim-treated patients were spared 7 weeks of corticosteroid treatment for every 100 weeks of romiplostim therapy, compared with placebo-treated patients. Continuous long-term treatment (up to 5 years) with romiplostim has been demonstrated to maintain platelet counts with infrequent side effects [Citation32–34]. Similarly, the phase 2/3 EXTEND study showed that long-term use of eltrombopag was effective in maintaining platelet counts and reducing bleeding in patients with ITP of more than 6 months’ duration [Citation35]. In EXTEND, 29% of patients were taking corticosteroids at baseline; this reduced to 18% at study end. Thus, earlier second-line use of TPO-RAs may be beneficial for patients rather than prolonging the corticosteroid treatment phase.
In this study, the mean and median duration on TPO-RAs was 12.6 and 9.3 months, respectively; persistence was similar between the two TPO-RAs. While persistence on TPO-RAs was approximately 30% at the end of the 36-month study period, it should be noted that the date of first TPO-RA prescription was between 1 July 2016 and 30 June 2019, and the resultant duration of the observation period was variable (<36 months in most patients).
The present study confirms the minor roles of other medications, such as immunoglobulins and rituximab, in clinical practice in Germany. Immunoglobulins are recommended for the management of severe bleeding episodes but not for continuous treatment [Citation5,Citation6,Citation11], and were used in 7% of patients prior to TPO-RA treatment. While the International Consensus Report states that rituximab has robust evidence as a subsequent ITP therapy [Citation6], DGHO guidelines now recommend rituximab as third line [Citation5,Citation11] or for life-threatening bleeding [Citation11]. In our study, rituximab use was highest post–TPO-RA therapy, but was still only used by 2.7% of patients. This is in contrast to US claims data (2011–2017), which showed that rituximab was used in 24.5% of patients with ITP across all lines of treatment [Citation19], possibly reflecting healthcare/insurance differences between Germany and the US. However, ASH guidelines (from 2019) suggest that TPO-RAs be used instead of rituximab as subsequent therapy [Citation10].
Strengths of the present study include the use of the German IMS® LRx database, which covers 82% of statutory prescriptions in Germany. In addition to the limitations discussed, it should first be reiterated that the database does not contain information on diagnoses, disease duration or therapy line (however, coverage among this population is high, and care was taken to exclude potential other diagnoses). Second, previous treatment with corticosteroids leading to a long-lasting (>18 months) remission would not have been detected. Finally, as prescription data do not necessarily translate to usage of prescribed medicines, it is possible that the corticosteroid duration/dose may have been overestimated.
Conclusions
The pattern of corticosteroid use in German patients with ITP indicates an overuse of corticosteroids in clinical practice. This may lead to serious side effects and a negative impact on HRQoL. The duration and dosing of corticosteroid therapy should be considered within the framework of recent guidelines [Citation5,Citation6,Citation10,Citation11]. Earlier TPO-RA use may be beneficial to avoid the side effects associated with long-term corticosteroid use.
Geolocation information
Germany.
Author contributions
H.R., A.L. and M.S. designed the study; H.R. collected the study data. All authors analyzed or interpreted the data, reviewed and critically revised the manuscript, approved the final draft and agree to be accountable for all aspects of the work.
Ethical considerations
The German IMS® LRx longitudinal prescription database (IQVIA, Frankfurt am Main, Germany) used in this study is fully anonymized and complies with relevant regulations for protecting patient privacy.
Acknowledgements
Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact checking and referencing, was provided by Josh Lilly and Emma McConnell at Aspire Scientific Ltd (Bollington, UK). Funding for medical writing support for this article was provided by Amgen Ltd (Uxbridge, UK).
Disclosure statement
O.M. has received honoraria for advisory boards, lectures, training courses and/or special publications from Amgen, Grifols, Novartis and Swedish Orphan Biovitrum. H.R. is an employee of IQVIA. A.L. and M.S. are employees of and hold stocks in Amgen GmbH.
Data availability statement
The data that have been used in this study are confidential.
Additional information
Funding
Notes on contributors
Oliver Meyer
Oliver Meyer is an Assistant Professor and Head of General Immunohematology and Platelet Immunology at the Institute for Transfusion Medicine, Charité Medical School, Berlin. He received his MD from Justus-Liebig University Giessen, Germany, before pursuing a PhD on specific inhibition of Fc-mediated phagocytosis by drug and antibody-treated erythrocytes and platelets at the Free University of Berlin. His work focusses on autoimmune thrombocytopenia (ITP), autoimmune hemolytic anemia, and bleeding disorders, and he is involved in phase 2–4 clinical trials in patients with ITP. He is a reviewer for prominent journals, including the British Medical Journal, Haematologica, British Journal of Haematology, and Platelets.
Hartmut Richter
Hartmut Richter has an academic background in aquatic sciences and aquaculture. In 2006, he switched his professional interests to pharmaceutical market research when he joined IQVIA (formerly IMS Health) as a database analyst. The main focus of his work is more IT-related than pharmaceutical, consisting of SAS programming and the implementation of complex statistics. With regard to the medical aspects, his work has covered a wide range of disease areas with a preponderance of respiratory studies (asthma, COPD, allergic rhinitis).
Andrea Lebioda
Andrea Lebioda is leading the health economics and outcomes research team at Amgen, Germany. Before joining the Value, Access & Policy department at Amgen in 2014, Andrea worked as a consultant in HEOR at IQVIA, Munich, Germany. She holds a Master of Science in health economics from the University of Bayreuth, Germany.
Markus Schill
Markus Schill is a Senior Medical Advisor in hematology/oncology at Amgen, Germany. He received his MD from the University of Heidelberg, Germany, and began his career in gastroenterology at Munich Municipal Hospital Bogenhausen, Germany. After this, he served as a resident in anesthesiology at the Munich Municipal Hospital Neuperlach. He moved into the pharmaceutical industry at GlaxoSmithKline in 2003 as Medical Advisor in anesthesiology, later becoming Senior Medical Advisor in hematology/oncology.
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi:https://doi.org/10.1182/blood-2008-07-162503.
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. doi:https://doi.org/10.3390/jcm6020016.
- Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180. doi:https://doi.org/10.1002/ajh.21616.
- Abrahamson PE, Hall SA, Feudjo-Tepie M, et al. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83(2):83–89. doi:https://doi.org/10.1111/j.1600-0609.2009.01247.x.
- Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia – current diagnostics and therapy: recommendations of a joint working group of DGHO, OGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(Suppl 5):1–30. doi:https://doi.org/10.1159/000492187.
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:https://doi.org/10.1182/bloodadvances.2019000812.
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) world impact survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. 2021;96(2):199–207. doi:https://doi.org/10.1002/ajh.26036.
- McMillan R, Bussel JB, George JN, et al. Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol. 2008;83(2):150–154. doi:https://doi.org/10.1002/ajh.20992.
- Bussel J, Ghanima W, Cooper N, et al. Higher symptom burden in patients with immune thrombocytopenia experiencing fatigue: results from the ITP World Impact Survey (I-WISh). Presented at: 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; 2020. Abstract 2668. Available from: https://ash.confex.com/ash/2020/webprogram/Paper136546.html.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi:https://doi.org/10.1182/bloodadvances.2019000966.
- German Society of Hematology and Medical Oncology. Onkopedia Leitlinie Immunthrombozytopenie (ITP). October 2020. [cited 2021 Aug 19]. Available from: https://www.onkopedia.com/de/onkopedia/guidelines/immunthrombozytopenie-itp/@@guideline/html/index.html.
- Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: multicentric trial of the Cooperative Latin American Group on Hemostasis and Thrombosis. Blood. 1984;64(6):1179–1183.
- Neunert CE. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv. 2017;1(24):2295–2301. doi:https://doi.org/10.1182/bloodadvances.2017009860.
- European Medicines Agency. Tavlesse (fostamatinib) summary of product characteristics; 2020. [cited 2021 Aug 19]. Available from: https://www.ema.europa.eu/en/documents/product-information/tavlesse-epar-product-information_en.pdf.
- Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719841735. doi:https://doi.org/10.1177/2040620719841735.
- Kuter DJ, Newland A, Chong BH, et al. Romiplostim in adult patients with newly diagnosed or persistent immune thrombocytopenia (ITP) for up to 1 year and in those with chronic ITP for more than 1 year: a subgroup analysis of integrated data from completed romiplostim studies. Br J Haematol. 2019;185(3):503–513. doi:https://doi.org/10.1111/bjh.15803.
- Tripathi AK, Shukla A, Mishra S, et al. Eltrombopag therapy in newly diagnosed steroid non-responsive ITP patients. Int J Hematol. 2014;99(4):413–417. doi:https://doi.org/10.1007/s12185-014-1533-y.
- González-López TJ, Fernández-Fuertes F, Hernández-Rivas JA, et al. Efficacy and safety of eltrombopag in persistent and newly diagnosed ITP in clinical practice. Int J Hematol. 2017;106(4):508–516. doi:https://doi.org/10.1007/s12185-017-2275-4.
- Bussel J, Tkacz J, Manjelievskaia J, et al. Long-term overuse of corticosteroids in patients with immune thrombocytopenia: a real-world analysis of a US claims database. Presented at: 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; 2020. Abstract 849. Available from: https://ash.confex.com/ash/2020/webprogram/Paper133757.html.
- Platzbecker U, Heßling J, Hurtz H-J, et al. Retrospective analysis of the disease management of patients with immune thrombocytopenia. Oncol Res Treat. 2017;40(Suppl 3):90. doi:https://doi.org/10.1159/000479566.
- Kubasch AS, Kisro J, Heßling J, et al. Disease management of patients with immune thrombocytopenia—results of a representative retrospective survey in Germany. Ann Hematol. 2020;99(9):2085–2093. doi:https://doi.org/10.1007/s00277-020-04173-5.
- Richter H, Dombrowski S, Hamer H, et al. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:Doc14. doi:https://doi.org/10.3205/000218.
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995–1008. doi:https://doi.org/10.1056/NEJMra010501.
- Nicolaides NC, Pavlaki AN, Alexandra M, et al. Glucocorticoid therapy and adrenal suppression; 2018. [cited 2021 Aug 19]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279156/.
- Cuker A, Prak ET, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost. 2015;41(4):395–404. doi:https://doi.org/10.1055/s-0034-1544001.
- Bradbury CA, Greenwood R, Pell J, et al. A multicentre randomised trial of first line treatment pathways for newly diagnosed immune thrombocytopenia: standard steroid treatment versus combined steroid and mycophenolate. The FLIGHT trial. Blood. 2020;136(Suppl 2):LBA–2. doi:https://doi.org/10.1182/blood-2020-143563.
- Pell J, Greenwood R, Ingram J, et al. Trial protocol: a multicentre randomised trial of first-line treatment pathways for newly diagnosed immune thrombocytopenia: standard steroid treatment versus combined steroid and mycophenolate. The FLIGHT trial. BMJ Open. 2018;8(10):e024427. doi:https://doi.org/10.1136/bmjopen-2018-024427.
- Mathias SD, Gao SK, Miller KL, et al. Impact of chronic immune thrombocytopenic purpura (ITP) on health-related quality of life: a conceptual model starting with the patient perspective. Health Qual Life Outcome. 2008;6:13. doi:https://doi.org/10.1186/1477-7525-6-13.
- Sarnes E, Crofford L, Watson M, et al. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413–1432. doi:https://doi.org/10.1016/j.clinthera.2011.09.009.
- Michel M, te Boekhorst PA, Janssens A, et al. Reduced corticosteroid use in adult patients with primary immune thrombocytopenia receiving romiplostim. Hematology. 2011;16(5):274–277. doi:https://doi.org/10.1179/102453311x13025568942005.
- Khelif A, Saleh MN, Salama A, et al. Changes in health-related quality of life with long-term eltrombopag treatment in adults with persistent/chronic immune thrombocytopenia: findings from the EXTEND study. Am J Hematol. 2019;94(2):200–208. doi:https://doi.org/10.1002/ajh.25348.
- Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–2171. doi:https://doi.org/10.1182/blood-2008-04-150078.
- Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi:https://doi.org/10.1016/S0140-6736(08)60203-2.
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–423. doi:https://doi.org/10.1111/bjh.12260.
- Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. doi:https://doi.org/10.1182/blood-2017-04-748707.
- European Medicines Agency. Revolade (eltrombopag) summary of product characteristics; 2018. [cited 2021 Aug 19]. Available from: https://www.ema.europa.eu/en/documents/product-information/revolade-epar-product-information_en.pdf.
- European Medicines Agency. Nplate (romiplostim) summary of product characteristics; 2021. [cited 2021 Aug 19]. Available from: https://ec.europa.eu/health/documents/community-register/2021/20210122150420/anx_150420_en.pdf.