ABSTRACT
Objectives
CAR-based immunotherapies represent a potentially curative strategy for hematological malignancies. However, there are a number of intracellular antigens that CAR-T cells are unable to target. Furthermore, CAR-T cells often suffer from insufficient expansion in part because of the immunosuppressive mechanisms. Lenalidomide (LEN), an immunomodulatory drug, can potentiate T cell functionality. Therefore, it is necessary to investigate combinatorial therapy using CAR-T cells and LEN for enhancing function.
Methods
We redirected T cells to express HLA-A*2402+-restricted-CAR capable of recognizing WT1235-243 peptide and adoptively transferred them into tumor-bearing mice to test their anti-tumor activity. Then we assessed the combinatorial efficacy using CAR-T cells and LEN in vitro and in vivo.
Results
Using an anti-WT1 CAR-T, we showed that LEN enhances CAR-T cell function in a concentration-dependent manner. Our data demonstrated that LEN improved the anti-tumor activity of CAR-T cells in vivo by increasing the infiltration of tumors with CD3+ and CD8+ T cells. Proteomics studies supported LEN enhanced the efficacy of CAR-T cells, including T-cell activation, mitochondrial activity and immune synapse formation.
Conclusion
These results demonstrate that lenalidomide potentiates WT1 CAR-T activity and paves the way to evaluate the combination of LEN with CAR-T for a planned clinical trial.
Introduction
CAR-based immunotherapies have provided a new class of effective therapeutics for hematological malignancies [Citation1–4]. Despite these promising results, there are a number of intracellular antigens overexpressed by tumor cells which to date have not been readily targetable. One example of an intracellular tumor-associated antigen is Wilms Tumor 1 (WT1). WT1 was identified as a high-priority antigen target because it has an important role in cell growth and differentiation; is overexpressed in leukemias, lymphomas, and solid tumors; and has limited expression in normal tissues [Citation5]. We generated a T-cell receptor-mimic (TCRm) CAR against WT1 utilizing a previously described scFv that recognizes the WT1235-243 peptide in the context of HLA-A*2402 on the cell surface [Citation6]. The scFv was identified using phage display technology and reactive to WT1235-243/HLA-A*2402 but not to HLAA*2402 or an irrelevant peptide/HLA-A*2402 [Citation6–8]. Lenalidomide (LEN) is an immunomodulatory drug currently approved for the treatment of multiple myeloma and mantle cell lymphoma, while it is clinically tested in the therapy of acute myeloid leukemia in combination with 5-azacitidine [Citation9,Citation10]. Both in vitro and in vivo studies demonstrated that LEN has known activity in the activation and expansion of normal T cells, and preferentially stimulates CD8+ T cells [Citation11–13].
Herein we investigated the effects of LEN on WT1 CAR-T cells in vitro and in vivo, and demonstrate that combining LEN with WT1 CAR-T cells has the potential for synergistic effects on the WT1+/HLA-A*2402+ tumor cells.
Methods
Lentivirus vector construction and preparation of virus solutions
The lentivirus CAR construct was modified from the previously described WT1-specific scFv chimeric immunoreceptor [Citation6]. A scFv of monoclonal antibody specific to WT1235-243/HLA-A*2402 complex in the VH-VL orientation, a Cλ hinge domain, and a CD28 transmembrane domain with novel construct of CD3ζ and GITR signaling domains were inserted into a pCDH lentivirus vector (Figure S1). Lentiviruses were prepared by transfecting the 293 T cells with packaging plasmids RRE, REV and pVSVG and lentiviral vectors pCDH containing CAR insert using Lipofectamine™ 3000 reagent (Invitrogen), supernatants were then used directly to infect primary human T cells.
T cell transduction
Briefly, PBMC were isolated by density gradient centrifugation over Ficoll-Paque (GE Healthcare). Then T cells were isolated from PBMC using EasySep™ human T cell isolation kit (STEMCELL) at room temperature. T cells were stimulated with anti-CD3/CD28/CD2 (STEMCELL) and cultured with immunoCult™-XF T cell expansion medium (STEMCELL) supplemented with 500 IU/mL human recombinant IL-2 (PeproTech) and 2% autologous human plasma. For infections, supernatants were then used directly to infect primary human T cells with antibodies to CD3/CD28/CD2, and spin-infected (90 min, 800 g, RT) in the presence of 5 mg/uL polybrene (Sigma), after 24 h the cells were replated in new flasks without antibodies and were further grown in T cell expansion medium with fresh 500 IU/mL IL-2-containing medium added every 2 days. On days 7–10, these cells were used for experiments.
Stable overexpression of HLA-A*2402 in K562 cells
To generate K562-A24, HLA-A*2402 cDNA was cloned into a pLVX-IRES-Puro (Clontech) lentivirus vector (Figure S2) [Citation14]. Lentiviruses were prepared by transfecting the 293 T cells with packaging plasmids psPAX2 and pCMV-VSV-G (Addgene). The transfection was performed using Lipofectamine™3000 reagent and transfected cells were selected and maintained in media containing 0.5 mg/mL puromycin.
In vitro functional assays and IFNγ production
Target cells co-cultured with differing amounts of CAR-T cells in RPMI 1640 medium supplemented with 5%FBS without phenol red for 24 h. The culture supernatants were harvested and analysed using a CytoTox® 96 Non-Radioactive Cytotoxicity Assay kit (Promega). In addition, the levels of IFNγ in the supernatants were determined by ELISA assay kit (Bioswamp) according to the dilution of IFNγ standard. All data are represented as a mean of triplicate wells (±SD).
Flow cytometry analysis
Cells (1 million) in 50 μL PBS were incubated with Fc Receptor Blocking Solutions, followed by incubation with various surface antibodies, such as PE-conjugated HLA-A*2402 modified WT1 Tetramer (MBL), FITC anti-human CD3, APC anti-human CD4 and APC anti-human CD8a or PE/Cy7 anti-human CD8 (Biolegend). The cells were washed twice with washing buffer before and after cell staining. Then the cells were analyzed with a flow-cytometer (InvitrogenTM). Single cell suspension was prepared from tumors by cutting them into small pieces with a blade and gentle dissociation by adding Collegenase IV and DNase I for digestion. Assay details are provided in the Supplemental Methods.
In vivo anti-tumor activity
NOD-Prkdcem26Cd52Il2rgem26Cd22/Nju mice, known as NCG mice, were purchased from the GemPharmatech. Co., LTD (Nanjing, China). Mice were fed a standard diet, housed under specific pathogen-free conditions, and used at 8–10 weeks of age. All procedures were approved by the Institutional Animal Use and Care Committee of Peking University. Tumor xenografts were established by subcutaneous s.c. injection over each flank in 50ul volume mixed with 50ul Matrigel (BD Bioscience). NCG mice were s.c. injected bilaterally with 3 × 106 K562 and K562-A24 tumor cells. These NCG mice bearing 4-day-old tumors received intravenously two doses of 5 × 106 CAR-T cells at days 4 and 8, followed with or without daily LEN treatment (10 mg/kg in 0.1 mL PBS intraperitoneally). 14 days post CAR-T cell infusion, mice were sacrificed. Tumors were excised and weighted, then the tumor tissue was minced and dissociated by adding digestion medium and analyzed by flow cytometry. All animal work was performed under approved ethical guidelines.
Statistical analysis
Analyses were performed using Prism (GraphPad). Comparisons of 2 groups were performed by 2-tailed parametric or nonparametric (Kolmogorov–Smirnov test) student t tests for unpaired data. P values < 0.05 were considered statistically significant.
Proteomics sample preparation and processing
WT1 CAR-T cells were treated with 10 μM LEN daily for 3 days. Cells were then washed twice and processed as previously with some modifications for iTRAQ proteomic assays, peptide fractionation and liquid chromatography-electrospray tandem mass spectrometry analysis (the method details and data availability of proteome analysis are provided in Supplemental methods) [Citation15,Citation16].
Results
Establish K562-A24 cells stably expressing HLA-A*2402
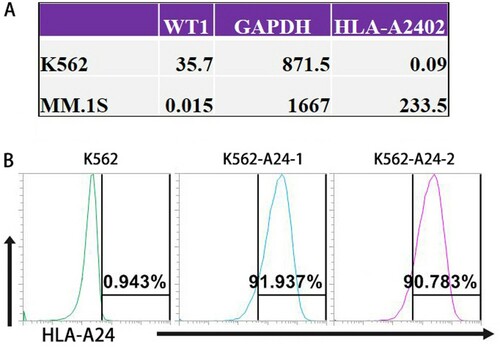
Exploring a TRON Cell Line portal (http://celllines.tron-mainz.de) integrating HLA type, predicted neo-epitopes, virus and gene expression [Citation17], we assessed HLA-A*2402 and WT1 mRNA expression of those tumor cell lines. K562 cells express high levels of WT1, but MM.1S cells do not ((A)). Meanwhile, K562 cells lack HLA classes I and II expressions on their cell surface [Citation14], and MM.1S cells have higher expression of HLA-A*2402 (HLA-A24+/WT1-). Therefore, we established K562-A24 cells expressing HLA-A*2402 and conducted flow cytometry to characterize surface HLA-A24 expression on K562-A24 cells. K562-A24 cells (HLA-A24+/WT1+) are highly (91%) HLA-A24-positive, but K562 cells do not (HLA-A24-/WT1+) ((B)).
Figure 1. Establish K562-A24 cells stably expressing HLA-A*2402. (A) The mRNA expression levels of HLA-A*2402 and WT1 in K562 cells and MM.1S cells. (The data were generated by a TRON Cell Line portal, http://celllines.tron-mainz.de). (B) K562 and K562-A24 cells were stained with phycoerythrin-conjugated antibodies specific to HLA-A24. The percentages of positive cells are presented after exclusion of dead cells with Aqua.
Generation of WT1 CAR-T cells
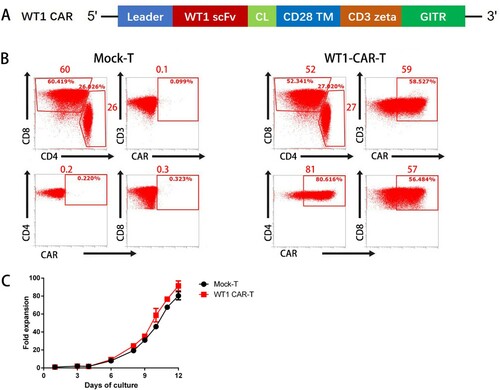
We isolated T cells from healthy donors, followed by activation and transduction with a lentivirus encoding second-generation CAR specific for WT1, containing the intracellular signaling domains of CD3z and GITR ((A)). T cells expressed CAR, as judged by WT1-236Y/HLAA*2402 tetramer binding. The data shows that 58.5% of CD3+ T cells had the WT1 CAR on the surface. CARs were differentially expressed in 80.6% of CD4+ and 56.5% of CD8+ T cells ((B)). WT1 CAR-T cells showed similar expansion compared to mock T cells and exhibited an expansion more than 90-fold ((C)).
Figure 2. Design and characterization of a WT1/HLA-A*2402-specific CAR. (A) Schematics of a lentiviral vector encoding WT1/HLA-A*2402-specific CAR. (B) Expression of CAR on human T cells. Human T cells stimulated with anti-CD3/CD28/CD2 were transduced with the lentiviral vector as depicted in panel A. Four days after the transduction, cells were stained with phycoerythrin-labeled HLA-A*2402 tetramers presenting WT1 along with FITC anti-CD3, APC anti-CD4 and PE/Cy7 anti-CD8. Mock-transduced T cells served as background staining. (C)Fold expansion of the Mock-T and WT1 CAR-T cells at different time points (n≥3).
WT1 CAR-T cells mediate specific cytolytic activity
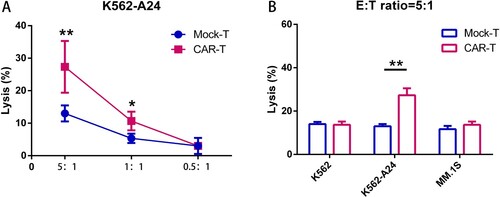
To analyze the cytotoxic capacity, we co-cultured CAR-T cells with K562-A24 target cells for 24 h; specific lysis was analyzed ((A)). At an E:T ratio of 5:1, WT1 CARs showed a mean killing of 30%. As shown in (B), WT1 CAR-T cells exhibited specific and efficient killing of K562-A24 cells (HLA-A24+/ WT1+), but not with tumor cell lines lacking of WT1 or HLA-A*2402, such as K562 (HLA-A24-/ WT1+) and MM.1S (HLA-A24 +/ WT1-).
Figure 3. WT1 CAR-T cells react to tumor cell lines endogenously expressing WT1 in an HLA-A*2402–restricted manner. (A) CAR T cells cocultured with K562-A24 cells in triplicate at the indicated E:T ratios. Cytotoxicity was measured using a CytoTox® 96 Non-Radioactive Cytotoxicity assay kit. All data are shown as means ± SD. *P < 0.05, and **P < 0.01. (B) CAR T cells cocultured with K562, K562-A24 or MM.1S cells at an E:T (Effector:Target) ratio of 5:1 for 24 h. Supernatants were collected to detect cytotoxic activity. *P < 0.05, and **P < 0.01.
WT1 CAR-T cells exhibit specific antitumor activity in vivo
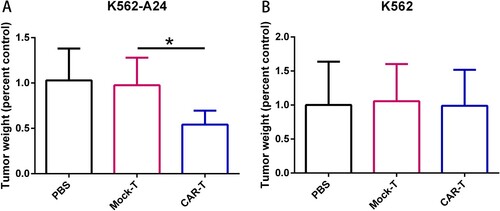
We proceeded to evaluate the antitumor activity of CAR-T cells in vivo using immunodeficient NCG mice transplanted subcutaneously with K562-A24 (HLA-A24+/ WT1+) ((A)) or K562 (HLA-A24-/ WT1+) ((B)). After tumor growth occurred for 4 days, two doses of 5 × 106 CAR-T cells specific for WT1 on days 4 and 8 were intravenously transferred into tumor-bearing mice. As shown in (A), WT1 CAR-T cells suppressed the growth of K562-A24 cells, but not K562 cells, and exhibited significantly higher antitumor activity as compared to mock T cells. These results suggested that WT1 CAR-T Cells exhibit specific antitumor activity in vivo.
Figure 4. Adoptive transfer of WT1-specific CAR-T cells suppressed tumor growth in an antigen-specific manner. (A) Tumor weights in NCG mice (n=4) transferred with PBS, CAR-T cells or Mock-T cells. NCG mice were inoculated s.c. with K562 and K562-A24 (3×106 cells). Four days following tumor inoculation, mice received two doses of 5 million CAR-T cells, Mock-T cells or PBS. 18 d later mice were sacrificed and the tumors were excised weighted. Error bars represent SD of the mean. *P < 0.05. s.c., subcutaneous.
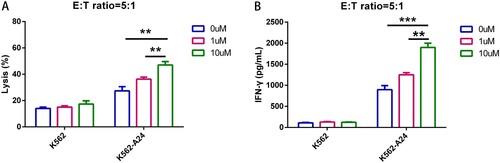
LEN enhances the effectiveness of WT1 CAR-T cells in a dose-dependent manner
LEN has immunomodulatory properties, being able to promote the activation and expansion of normal T cells, which results in antitumor activities [Citation11]. In order to enhance the effectiveness of CAR-based immunotherapy, we expanded WT1 CAR-T cells in the presence of 0, 1 and 10 μM LEN. We found that at an E:T ratio of 5:1, LEN-treated WT1 CAR-T cells dose-dependently exhibited better effector function against K562-A24 (HLA-A24+/ WT1+) ((A)). Consistently, WT1 CAR-T cells produced higher IFNγ upon interaction with K562-A24 in the presence of 10 μM LEN ((B)). Overall, these observations showed that LEN enhances the effectiveness of WT1 CAR-T cells.
Figure 5. LEN enhances the effectiveness of WT1 CAR-T Cells in a Dose-dependent manner. (A) WT1 CAR T cells treated with different concentrations of lenalidomide were co-cultured with K562 and K562-A24 at a E:T ratio of 5 to 1 for 24 h. Supernatants were collected to detect cytotoxic activity (A) and cytokine production (B).
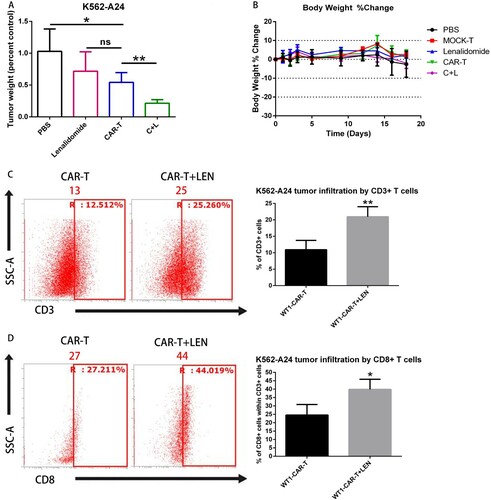
LEN Improves WT1-targeted anti-tumor activity in vivo
We investigated the synergistic effects of WT1 CAR-T cells and LEN against K562-A24 in our established xenograft mouse model. It has been reported the growth of tumors was completely suppressed when treatment with CAR-T cells was initiated early after tumor inoculation, we decided to modify the approach and delay the treatment initiation [Citation5,Citation6,Citation9,Citation11]. In the experiment shown in , we transplanted NCG mice with 3 million K562-A24 cells. On days 4 and 8 mice received intravenous injection of WT1 CAR-T cells (5 million each) followed by 10 mg/kg LEN daily intraperitoneal (IP) injection. 14 days post CAR-T cell infusion, mice were euthanized, and tumors were excised and analyzed. Although 10 mg/kg LEN daily injection alone did not induce tumor regression, WT1 CAR-T cell infusion in conjunction with LEN induced the slowest tumor progression as compared to the group treated with CAR-T cells alone ((A)). We monitored the weight of animals and did not observe any weight loss throughout the treatment ((B)). Interestingly, flow cytometry analysis uncovered that tumors in mice receiving LEN and WT1 CAR-T combinatorial treatment had the higher infiltration of CD3+ and CD8+ T cells compared to the group treated with WT1 CAR-T cells ((C,D)). Our data showed that LEN markedly augmented CAR-T mediated suppression of tumor growth in vivo.
Figure 6. LEN enhances antitumor response in vivo. (A) NCG mice were inoculated s.c. with K562 and K562-A24. Four days following tumor inoculation, mice received two doses of 5 million CAR-T cells specific for WT1 at days 4 and 8 and daily i.p injections of 10 mg/kg lenalidomide.18 d later mice were sacrificed and the tumors were excised weighted. (C, D) The infiltration of tumors by T cells was analyzed by flow cytometry in a cell suspension prepared from excised tumors using antibodies to CD3+ (C) and CD8+(D), one representative mouse is shown. Average from four replicates are depicted. C + L: CAR-T+ Lenalidomide.
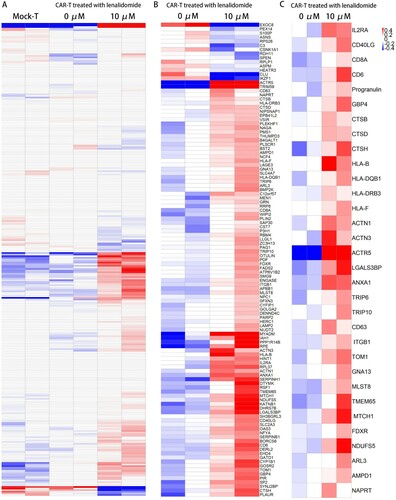
LEN enhances proteomic profiling of CAR-T cells function
We analyzed the proteome of Mock-T and the WT1 CAR-T cells treated with and without LEN. As compared to the Mock-T cells, T cells transduced with the WT1 CAR were found to have highly similar changes in protein expression pattern ((A)), indicating that WT1 CAR had little effect on the cells, which is in consistent with our findings in the growth of CAR-T cells. More than 100 proteins were differentially expressed among the LEN-treated and untreated CAR-T cells ((B)) (102 progressively upregulated and 14 downregulated). As expected, CAR-T cells treated with LEN showed greater upregulation of protein profiles related to T-cell activation and MHC class I and II molecules expression ((C)). The upregulation of the expression of key proteins involved in T-cell activation, such as IL2RA, CD40LG, CD8A and CD6, and the concurrent increase in the expression of the MHC class I and II molecules-associated proteins, such as HLA-B, HLA-F, HLA-DQB1 and HLA-DRB3 ((C)). Proteins associated with immune synapse, such as ACTIN1, ACTN3, ACTR5, ANXA1, LGALS3BP, TRIP6 and TRIP10, are up-regulated together with proteins associated with enhanced homing capacities such as ITGB1, TOM1 and MLST8 [Citation11,Citation18]. Moreover, the expression levels of the proteins associated with mitochondrial activity, including TMEM65, FDXR, NDUFS5 and MTCH1, were significantly higher in WT1 CAR-T cells treated with LEN. Thus, our proteomic data showed that LEN is useful to improve the function of CAR-T cells.
Figure 7. Proteomic profiles of WT1 CAR-T cells with and without LEN. (A) Heat map of proteins with differential expression (P < 0.05) between Mock-T cells and CAR-T cells treated with and without lenalidomide. As compared to the Mock-T cells, WT1 CAR-T were found to have highly similar changes in protein expression pattern. (B) Heat map of proteins with differential expression (P value < 0.05) between WT1 CAR-T cells treated with and without lenalidomide. (C) Heat map of selected proteins with differential expression (P < 0.05) between CAR-T cells related to T cell activation, expression of the MHC class I and II molecules, synapse and the mitochondrial activity (n = 2 replicates per group).
Discussion
In recent clinical trials, CAR-T cells targeting CD19 have achieved breakthroughs in the treatment of hematological malignancies [Citation1–4]. However, the wider application of this therapeutic approach is constrained by the rarity of cell surface antigens exclusively or abundantly expressed on tumor cells. In the present study, we used a CAR, that is reactive to a peptide portion of the intracellular onco-protein WT1 in the context of HLA-A*2402. We demonstrate that T cells modified to express this CAR specifically exhibit efficient cytotoxic activity in vitro and in vivo. We also show that combining CAR T-cell therapy and LEN can produce a synergistic effect in the anti-tumor activity of CAR T cells through increased cytotoxicity and improved persistence.
To validate the potential use of WT1 CAR T cells, we co-cultured CAR-T cells with K562-A24 target cells and demonstrated that CAR-T cells exhibited antigen-dependent effector function as indicated by cytotoxic activity and cytokine production, supporting a selective targeting for WT1-expressing tumor cell lines in an HLA-A*2402-dependent manner.
The mechanisms of antitumor activity of LEN are broad and may affect many cell types in the organism. Unanswered question, however, remains the true nature of T cells activation by LEN. To further characterize these different functional patterns, we compared the proteomic profiling of CAR-T cells treated with and without LEN. Significantly, we found that CAR-T cells treated with LEN showed greater upregulation of protein expression profiles associated with T-cell activation, as well as MHC class I and II molecules expression when compared with WT1 CAR-T alone. This acts as enhanced costimulatory signals to promote T cell activation. Our data also revealed the expression levels of the proteins associated with mitochondrial activity were significantly higher in LEN treated WT1 CAR-T cells. TMEM65 plays a role in the regulation of mitochondrial respiration [Citation11,Citation19]. This, on the one hand, leads to enhanced mitochondrial function. Taken as a whole, our data showed that LEN provides additional costimulatory signals to T cells and enhances T cells function. These effects may be attributed to greater T cells activity and persistence in the presence of LEN.
In the murine model tumor growth was inhibited without obvious regression upon either single treatment, while the combination of CAR T cell with LEN was markedly more effective, resulting in the delayed tumor growth by increasing infiltration of tumors with CD3+ and CD8+ T cells, as compared to the other treatment groups. Our proteomic data showed that the expression levels of the proteins, including ITGB1, TOM1 and MLST8, were significantly higher in WT1 CAR-T cells treated with LEN, indicating LEN might reduce the activation-induced cell death of activated T cells.
We monitored the weight of animals and did not observe any weight loss throughout the treatment. Although previous clinical trials of WT1 vaccine and TCR-T cells therapy targeting WT1 have shown no on-target/off-tumor toxicity toward those healthy tissues, CAR-T cells are more easily activated by normal cells when they express even low levels of target epitopes, which may result in on-target/off-tumor toxicity [Citation5–8]. In brief, more rigorous toxicity testing including cross-reactivity study should be designed before clinic.
Collectively, this study provides that combination therapy with LEN and WT1 CAR-T may synergistically evoke a potent and durable antitumor response. Because LEN exerts a costimulatory effect on T cell responses and the WT1 protein is highly expressed in both leukemia and various solid tumors, the presented data will provide the best insights into the future of this approach for the treatment of relapsed and refractory WT1+/HLA-A*2402+ tumors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28.
- Magnani CF, Gaipa G, Lussana F, et al. Sleeping beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. 2020;130(11):6021–6033.
- Tong C, Zhang Y, Liu Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020;136(14):1632–1644.
- Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575.
- Rafiq S, Purdon TJ, Daniyan AF, et al. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia. 2017;31(8):1788–1797.
- Akahori Y, Wang L, Yoneyama M, et al. Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination. Blood. 2018;132(11):1134–1145.
- Tawara I, Kageyama S, Miyahara Y, et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130(18):1985–1994.
- Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25(7):1064–1072.
- Otáhal P, Průková D, Král V, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2015;5(4):e1115940.
- Wei A, Tan P, Perruzza S, et al. Maintenance lenalidomide in combination with 5-azacitidine as post-remission therapy for acute myeloid leukaemia. Br J Haematol. 2015;169(2):199–210.
- Wang X, Walter M, Urak R, et al. Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin Cancer Res. 2018;24(1):106–119.
- Lapenta C, Donati S, Spadaro F, et al. Lenalidomide improves the therapeutic effect of an interferon-α-dendritic cell-based lymphoma vaccine. Cancer Immunol Immunother. 2019;68(11):1791–1804.
- Nguyen-Pham TN, Jung SH, Vo MC, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine Multiple myeloma. J Immunother. 2015;38(8):330–339.
- Britten CM, Meyer RG, Kreer T, et al. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2002;259(1-2):95–110.
- Howden AJ, Hukelmann JL, Brenes A, et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat Immunol. 2019;20(11):1542–1554.
- Perez-Riverol Y, Csordas A, Bai J, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47(D1):D442–D450.
- Scholtalbers J, Boegel S, Bukur T, et al. TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression. Genome Med. 2015;7:118.
- Gou H, Liang JQ, Zhang L, et al. TTPAL promotes colorectal tumorigenesis by stabilizing TRIP6 to activate Wnt/β-catenin signaling. Cancer Res. 2019;79(13):3332–3346.
- Nishimura N, Gotoh T, Oike Y, et al. TMEM65 is a mitochondrial inner-membrane protein. PeerJ. 2014;2:e349.
