ABSTRACT
Objective
Circular RNA plasmacytoma variant translocation 1 (circ-PVT1) has been reported to be an oncogene and serves as a prognostic biomarker in several solid cancers and hematological malignancies. However, no study has been performed on the tumorigenesis role of circ-PVT1 in acute myeloid leukemia (AML). Thus, this study aimed to evaluate the correlation of circ-PVT1 with disease risk, clinical characteristics, cytogenetics/molecular genetics, and prognosis of AML.
Methods
A total of 68 de novo AML patients, 30 disease controls and 30 health donors were enrolled in this study. Circ-PVT1 expression in bone marrow (BM) was determined. Complete remission (CR) status after induction therapy, event-free survival (EFS) and overall survival (OS) were evaluated in AML patients.
Results
Circ-PVT1 expression was different among AML patients, disease controls and health donors, which was highest in AML patients, followed by disease controls and lowest in health donors. Meanwhile, circ-PVT1 could distinguish AML patients from health donors and disease controls by receiver operating characteristic curve analysis. Furthermore, circ-PVT1 was correlated with BM blasts and FLT3-ITD mutation, but not other clinical features, such as French-American-Britain subtypes in AML patients. Moreover, circ-PVT1 expression was lower in AML patients with CR compared with those without CR. Besides, high circ-PVT1 expression was correlated with shorter EFS and OS in AML patients. After adjustment by multivariate Cox’s regression analysis, higher circ-PVT1 expression was an independent factor in predicting shorter EFS and OS for AML patients.
Conclusion
Circ-PVT1 potentially serves as a biomarker for evaluating the prognosis of AML patients.
Introduction
Acute myeloid leukemia (AML), characterized by clonal growth of myeloid hematopoietic precursors in the bone marrow (BM), is one of the most common acute leukemias in adults, with estimated new cases of 5,690 and recent deaths of 6,620 in the United States in 2021 [Citation1–3]. Standard treatments of AML include induction therapy (a '3 + 7' regimen based on anthracyclines and cytarabine combined therapy), consolidation chemotherapy and hematopoietic stem cell transplantation [Citation4, Citation5]. Even though approximate 60%–70% of AML patients are expected to attain complete remission (CR) after appropriate induction therapy, their 3-year survival rate is still low, which ranges from 25% to 71% [Citation2, Citation6, Citation7]. In recent decades, great efforts have been made to identify novel and effective biomarkers for prognosis evaluation of AML patients, such as cytogenetics and molecular abnormalities. Therefore, finding out more biomarkers might further improve AML outcome via individualized therapy realization [Citation8].
Plasmacytoma variant translocation 1 (PVT1), located on the 8q24 region of the human chromosome, is highly expressed in BM of diversified hematological malignancies, including AML [Citation9]. Interestingly, PVT1 inhibition reduces cell viability and mobility, while inducing apoptosis of AML cells [Citation10, Citation11]. Circular RNA PVT1 (circ-PVT1), derived from circularization of exon 3 of PVT1 locus, plays a vital role in the tumorigenesis of hematological malignancies, such as acute lymphoblastic leukemia (ALL). For instance, circ-PVT1 is highly expressed in BM of ALL patients compared with health controls. Meanwhile, silencing circ-PVT1 inhibits the proliferation, while promotes the apoptosis of ALL cell lines via regulating c-Myc and BCL-2 [Citation12].
However, no investigation has been conducted on the tumorigenesis role of circ-PVT1 in AML. Thus, this study aimed to evaluate the correlation of circ-PVT1 expression with disease risk, clinical features, cytogenetics/molecular genetics, and prognosis of AML.
Methods
Subjects
From January 2017 to December 2019, 68 de novo AML patients were consecutively screened in this study. The main inclusion criteria were as follows: (1) diagnosed as AML according to the World Health Organization (WHO) classification of AML [Citation13]; (2) aged above 18 years; (3) had cryopreserved BM specimens, which were collected before induction treatment; (4) remission evaluation data were recorded after induction treatment. The main exclusion criteria were: (1) exposed to radiotherapy or chemotherapy before being diagnosed as AML; (2) history of malignant solid tumors or other hematological malignancies; (3) complicated with bone marrow lesions such as bone marrow failure syndromes; (4) pregnant or lactating women. In addition, 30 patients with non-proliferative hematologic disorders and 30 health donors who had cryopreserved BM were also included in this study. Written informed consents were provided by all participants or their legal guardians. This study was approved by the Institutional Research Ethics Committee.
Data collection
For AML patients, demographics and clinical characteristics were collected, including age, gender, white blood cell (WBC) level, percentage of BM blasts, cytogenetics features, molecular abnormalities, French-American-British (FAB) classification and risk stratification. The risk stratification was referred to the criterion issued by National Comprehensive Cancer Network (NCCN) (version 2. 2013) [Citation14].
Circ-PVT1 determination
For AML patients, disease controls and health donors, BM specimens were collected. Then, bone marrow mononuclear cells (BMMCs) were separated using human bone marrow monocytes separation solution (Beijing Biolabo Technology Co., Ltd, Beijing, China), which were used for the determination of circ-PVT1 expression by Reverse Transcription quantitative Polymerase Chain Reaction (RT-qPCR) assay. TRIzol™ Reagent (Invitrogen™, U.S.A.) was processed to extract total RNA from BM specimens. After that, linear RNA was removed from the total RNA by RNase R (Epicenter, U.S.A.). iScript™ cDNA Synthesis Kit (Bio-Rad, U.S.A.) was treated for cDNA synthesis. Then the QTaq™ DNA Polymerase Mix (Clontech, China) was applied for cDNA amplification. The 2-ΔΔCt method was used for calculating the circ-PVT1 expression with GAPDH as an internal reference. The primers applied in the RT-qPCR procedures were referred to a previous study [Citation12].
Treatment and evaluation of response
AML patients received standard IA or DA regimen for induction therapy according to the NCCN guideline (version 2. 2013) [Citation14]. The IA regimen included idarubicin 12 mg/(m2*day), day 1–3, intravenous drip for 30 min, cytarabine (Ara-c) 200 mg/(m2*day), day 1-7, every 12 h (q12h), hypodermic injection. The DA regimen included daunorubicin 90 mg/(m2*day), day 1–3, intravenous drip for 30 min, Ara-c 200 mg/(m2*day), day 1–7, every 12 h (q12h), hypodermic injection. Response assessment was commonly performed between day 21 and 28 after the start of induction therapy. Meanwhile, remission status was assessed according to the criterion submitted by NCCN (version 2. 2013) [Citation14], in which CR was defined as (meeting all the following conditions): absolute neutrophil count >1000/mcL, platelets ≥ 100,000/mcL, BM blasts less than 5%, transfusion independence and no residual evidence of extramedullary disease. Partial remission (PR) was defined as: decrease of at least 50% in the percentage of blasts to 5% to 25% in the BM aspirate and the normalization of blood counts, as noted above. After induction therapy, patients with CR continued the original regimen for consolidation therapy, followed by consolidation chemotherapy or hematopoietic stem cell transplantation (HSCT), and those without CR received reinduction therapy, followed by consolidation chemotherapy or HSCT.
Follow-up and survival assessment
AML patients were followed up until withdrawal from the study, death or the end of this study (December 2020). In addition, diseases status was recorded for assessment of event-free survival (EFS) and overall survival (OS). EFS was defined as the time from initiation of induction therapy to treatment failure (defined as failure to achieve CR or PR), or relapse from CR, or death. OS was defined as the time from initiation of induction therapy to death [Citation15].
Statistical analysis
SPSS 24.0 (IBM, Armonk, New York, U.S.A.) was applied for statistical analysis. GraphPad Prism 8.01 (GraphPad Software Inc., San Diego, California, U.S.A.) was used for graph making. Data were presented as mean ± standard deviation (SD), median with interquartile range (IQR) and counts with frequency. Comparison of data was determined by Mann–Whitney U test or Kruskal–wallis H sum rank test. Correlation analysis was determined by Spearman’s rank correlation test. Receiver operating characteristic (ROC) analysis was performed to evaluate the ability of circ-PVT1 in distinguishing AML patients from disease controls or health donors, as well as in discriminating AML patients with CR from non-CR after induction therapy. AML patients were classified as four groups according to the quartile of circ-PVT1 expression, in which, 0–25th, 25th–50th, 50th–75th and 75th–100th quartile of circ-PVT1 expression were classified as quartile 1, quartile 2, quartile 3 and quartile 4 group, respectively. Survival analysis was illustrated using the Kaplan-Meier curve and determined by log-rank test. Cox’s proportional hazard regression was carried out for prognostic factor analysis, in which classifications of cytogenetics accounting for higher than 10% were included in the further multivariate Cox’s regression analysis, and hazard ratio with 95% confidence interval (CI) was presented using forest plot. P value<0.05 was considered as statistically significant.
Results
Baseline characteristics of AML patients
In AML patients, the mean age was 56.2 ± 13.2 years. There were 25 (36.8%) females and 43 (63.2%) males. As to the FAB classification, 4 (5.9%), 24 (35.3%), 17 (25.0%) and 23 (33.8%) AML patients presented with M1, M2, M4 and M5 AML, respectively. Regarding the molecular abnormalities, 19 (27.9%), 18 (26.5%), 8 (11.8%) and 6 (8.8%) patients manifested with NPM1 mutation, FLT3-ITD mutation, WT1 mutation and isolated biallelic CEBPA mutation, respectively. Respecting the risk stratification, 13 (19.1%), 32 (47.1%) and 23 (33.8%) patients exhibited with better, intermediate and poor risk according to the NCCN guideline (version 2. 2013) [Citation14]. Other specific baseline characteristics of AML patients were shown in .
Table 1. Characteristics of AML patients.
Correlation of circ-PVT1 expression with AML risk
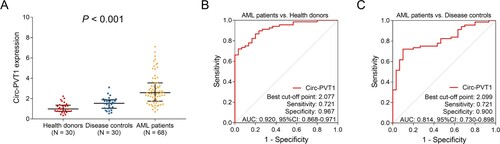
Circ-PVT1 expression was different among AML patients, disease controls and health donors (P < 0.001). In detail, circ-PVT1 expression was highest in AML patients (median (IQR): 2.584 (1.752–3.540)), followed by disease controls (median (IQR): 1.541 (1.050–1.886)), and lowest in health donors (median (IQR): 0.986 (0.752–1.358)) (A). Meanwhile, the ROC curve analysis showed that circ-PVT1 could distinguish AML patients from disease controls with an area under curve (AUC) of 0.814 (95%CI: 0.730–0.898), a sensitivity of 0.721 and a specificity of 0.900 at the best cut-off point (B). Besides, circ-PVT1 could also discriminate AML patients from health donors with an AUC of 0.920 (95%CI: 0.868–0.971), a sensitivity of 0.721 and a specificity of 0.967 at the best cut-off point (C).
Figure 1. Circ-PVT1 expression in AML patients, disease controls and health donors. Comparation of circ-PVT1 among health donors, disease controls and AML patients (A). The value of circ-PVT1 in distinguishing AML patients from health donors (B). The value of circ-PVT1 in distinguishing AML patients from disease controls (C). PVT1, plasmacytoma variant translocation 1; AML, acute myeloid leukemia.
Correlation of circ-PVT1 expression with characteristics of AML patients
As to the correlation of circ-PVT1 expression with clinical features of AML patients, circ-PVT1 expression was positively associated with BM blasts (P = 0.034) (). Regarding the correlation of circ-PVT1 expression with cytogenetics and molecular abnormalities of AML patients, circ-PVT1 was correlated with FLT3-ITD mutation (P = 0.037) (), while expression of circ-PVT1 was not associated with other clinical features, cytogenetics or molecular abnormalities, such as FAB classification (P = 0.137) and NCCN risk stratification (version 2. 2013) (P = 0.072).
Table 2. Correlation between circ-PVT1 and clinical features in AML patients.
Table 3. Correlation between circ-PVT1 and cytogenetics/molecular abnormalities in AML patients.
Correlation of circ-PVT1 expression with treatment response
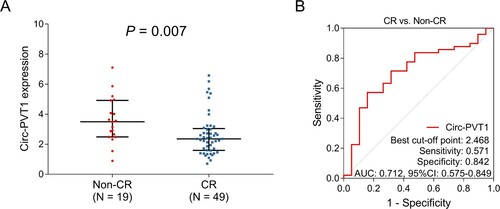
Circ-PVT1 expression was negatively associated with CR in AML patients (P = 0.007) (A). In addition, the ROC curve analysis exhibited the potential of circ-PVT1 in distinguishing AML patients with CR from those without CR with an AUC of 0.712 (95% CI: 0.575–0.849); meanwhile, the sensitivity and specificity of the best cut-off point were 0.571 and 0.842, respectively (B).
Figure 2. Circ-PVT1 expression correlated with CR in AML patients. Comparison of circ-PVT1 expression between CR and non-CR AML patients (A). The value of circ-PVT1 in discriminating AML patients with CR from those without CR (B). PVT1, plasmacytoma variant translocation 1; CR, complete remission; AML, acute myeloid leukemia.
Correlation of circ-PVT1 expression with survival and prognostic factors in AML patients
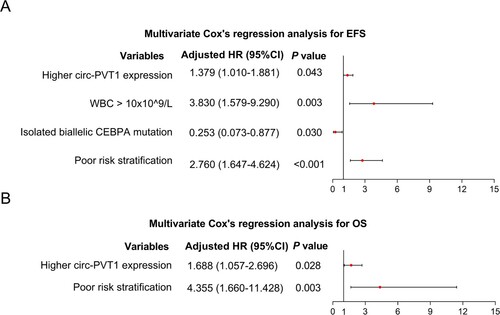
To determine the correlation of circ-PVT1 expression with survival, log-rank test was conducted, which showed that higher circ-PVT1 expression was correlated with shorter EFS (P = 0.017) (A) and OS (P = 0.026) (B). For further eliminating the confounding factors, multivariable Cox’s regression analysis was performed, which disclosed that factors including higher circ-PVT1 expression (adjusted hazard ratio (HR)) (95%CI): 1.379 (1.010–1.881) (P = 0.043), WBC > 10 × 10^9/L (adjusted HR (95%CI): 3.830 (1.579–9.290) (P = 0.001)), and poor risk stratification (adjusted HR (95%CI): 2.760 (1.647–4.624) (P < 0.001)) were independently associated with shorter EFS, while isolated biallelic CEBPA mutation (adjusted HR (95%CI): 0.253 (0.073–0.877) (P = 0.014)) was an independent factor in predicting prolonged EFS (A). In addition, higher circ-PVT1 expression (adjusted HR 1.688 (1.057–2.696) (P = 0.028)) and poor risk stratification (adjusted HR (95%CI): (95%CI): 4.355 (1.660–11.428) (P = 0.003)) were independently associated with shorter OS (B).
Figure 3. Circ-PVT1 expression correlated with EFS and OS in AML patients. Comparison of EFS (A) and OS (B) among four subgroups divided according to the quartiles of circ-PVT1 expression as follows: 0-25th, 25th -50th, 50th -75th and 75th -100th quartile of circ-PVT1 expression was classified as quartile 1, quartile 2, quartile 3 and quartile 4 group, respectively. PVT1, plasmacytoma variant translocation 1; AML, acute myeloid leukemia; EFS, event-free survival; OS, overall survival.
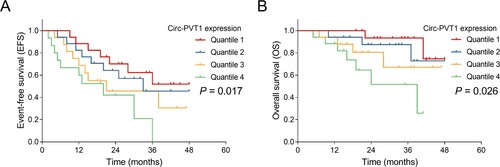
Figure 4. Independent prognostic factors in AML patients. Multivariate Cox’s regression analysis for the independent factors in predicting EFS (A) and OS (B) in AML patients. AML, acute myeloid leukemia; EFS, event-free survival; HR, hazard ratio; CI, confidence interval; PVT1, plasmacytoma variant translocation 1; WBC, white blood cell; OS, overall survival.
Discussion
Extensive studies disclose that circ-PVT1 is highly expressed in various tumors, which promotes the proliferation, while inhibits the apoptosis via stimulating multiple ways [Citation16, Citation17]. For instance, in ovarian cancer, circ-PVT1 is up-regulated, and promotes ovarian cancer cell malignant phenotype via regulating miR-149-5p/FOXM1 axis [Citation16]. Meanwhile, circ-PVT1 is highly expressed in ALL cell lines, whose knockdown inhibits cell growth and induces cell apoptosis through modification of c-Myc and BCL-2 protein expression [Citation12]. However, a review shows that the PVT1 expression in general AML patients is controversial, meanwhile, no study has been reported on the oncogenic role of circ-PVT1 in AML [Citation18]. In this study, we found that circ-PVT1 expression was highly expressed in AML patients, besides, circ-PVT1 expression could distinguish AML patients from disease donors and health controls by the ROC curve analyses. The potential explanations of these results might be that: (1) acting as a role of facilitating tumorigenesis, circ-PVT1may reflect the proliferation speed of cells, meanwhile, the malignant proliferation speed of AML cells is faster than that in the normal myeloid hematopoietic precursors; thereby, circ-PVT1 is highly expressed in AML patients; (2) circ-PVT1 is derived from PVT1 locus though a circularization effect, the latter has been reported to be up-regulated in AML patients [Citation11], therefore, the elevated PVT1 expression may imaginably enhance the expression of circ-PVT1, which causes a high circ-PVT1 expression in AML patients.
Preceding evidence shows that high circ-PVT1 expression is correlated with tumor size, lymph node metastasis and TNM stage in solid tumors [Citation19, Citation20]. For instance, in non-small cell lung cancer (NSCLC) patients, high circ-PVT1 expression is correlated with elevated tumor size and TNM stage [Citation19]. Nevertheless, no study has been performed on the correlation between the circ-PVT1 expression and clinical characteristics in hematologic malignancies. In this study, we found that circ-PVT1 expression was correlated with BM blasts and FLT3-ITD mutation. The underlying interpretation for the correlation between circ-PVT1 expression and BM blasts could be that: circ-PVT1 may promote the proliferation of AML cells via various cancer-related signaling pathways, therefore inducing the elevation of BM blasts [Citation12]. Meanwhile, a possible explanation for the correlation of circ-PVT1 expression with FLT3-ITD mutation might be that: circ-PVT1 locates on the PVT1 locus, while the location of PVT1 in the human chromosome is near to the protooncogene of MYC, and a study discloses that PVT1 expression is positively related to the expression of MYC [Citation21]; besides, another study shows that the circ-PVT1 is highly expressed in the AML patients with MYC-containing amplicons compared with normal karyotype [Citation22]. As far as we know, the protooncogene of MYC is involved in the progression of various tumors through the translation of c-MYC protein, and it is suggested that c-MYC expression is elevated in the myeloid progenitor of FLT3-ITD mutation mice but not wild-type mice [Citation23, Citation24], therefore, circ-PVT1 expression is related to FLT3-ITD mutation. Thus, considering data mentioned above, circ-PVT1 is correlated with BM blasts and FLT3-ITD mutation in AML patients.
As shown in previous studies, higher circ-PVT1 expression is related to worse survival in various solid tumor patients [Citation20, Citation25]. For instance, upregulation of circ-PVT1 indicates an unfavorable prognosis in osteosarcoma patients [Citation25]. However, there was no investigation conducted before to explore the prognostic value of circ-PVT1 in hematologic malignancies, especially in AML. Our study showed that higher circ-PVT1 expression was correlated with a shorter EFS and OS of AML patients. One possible reason is that higher circ-PVT1 expression may reduce the sensitivity of AML cell lines to chemotherapy and further cause a poor prognosis, which is similar to the previous report that higher circ-PVT1 expression may contribute to doxorubicin and cisplatin resistance [Citation20, Citation26]. However, this hypothesis needs to be further verified. Besides, we also notice that in AML patients, the FLT3-ITD mutation indicates an adverse prognosis, meanwhile, our study showed that circ-PVT1 expression was higher in those patients with FLT3-ITD mutation, thus we hypothesized that the elevated expression of circ-PVT1 may be correlated with a poor prognosis via its relation to FLT3-ITD [Citation27]. Therefore, the multivariate Cox’s regression analysis was performed in this study, which showed that higher circ-PVT1 expression was independent of other parameters (such as FLT3-ITD) in predicting shorter EFS and OS for AML patients.
Although this study was the first research that evaluated the correlation of circ-PVT1 expression with disease risk, clinical features, cytogenetics/molecular genetics, and prognosis of AML, there were still some limitations: (1) In the present study, we only excluded the M3 subtype AML patients, while due to the relatively small sample size, no M0 and M6 subtype AML patients were enrolled in this study. Thus, a study with big sample size to discovery this aspect was needed; (2) The BM samples were only collected before this study but not during the follow-up period, while the change of circ-PVT1 during the treatment period was not clear. Therefore, further study was needed; (3) We only enrolled the de novo AML patients but not the relapsed/recurrent (R/R) AML patients, thus, these results might not be suitable for R/R AML patients.
Circ-PVT1 is upregulated and related to aggravated disease features, poor treatment response, worse survival in AML, indicating its potential as a biomarker for predicting prognosis of AML patients.
Acknowledgements
Tao Chen analysed the data, and contributed to writing. Yazhou Yao and Yinsuo Zheng drafted the manuscript and helped in shaping the manuscript. Fengyun Chen designed the study, provided valuable suggestions and reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
- Adult Acute Myeloid Leukemia Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD)2002.
- Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 1 [database on the Internet]2021. Available from: https://www.nccn.org.
- Pollyea DA, Bixby D, Perl A, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 2021;19(1):16–27.
- Morsink LM, Sandmaier BM, Othus M, et al. Conditioning intensity, pre-transplant flow cytometric measurable residual disease, and outcome in adults with acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation. Cancers (Basel). 2020;12(9):2339.
- Tiribelli M, Michelutti A, Cavallin M, et al. Impact of concomitant aberrant CD200 and BCL2 overexpression on outcome of acute myeloid leukemia: a cohort study from a single center. Turk J Haematol. 2021;38(2):119–125.
- Kamal AM, Nabih NA, Elleboudy NS, et al. Expression of immune check point gene TIM-3 in patients newly diagnosed with acute myeloid leukemia: significance and impact on outcome. Oncol Lett. 2021;21(4):325.
- Izadifard M, Pashaiefar H, Yaghmaie M, et al. Expression analysis of PVT1, CCDC26, and CCAT1 long noncoding RNAs in acute myeloid leukemia patients. Genet Test Mol Biomarkers. 2018;22(10):593–598.
- Ghadiri A, Sharifi M, Mehrzad V, et al. Reduce proliferation of human bone marrow cells from acute myeloblastic leukemia with minimally differentiation by blocking lncRNA PVT1. Clin Transl Oncol. 2020;22(11):2103–2110.
- Cheng J, Song Y, Xu J, et al. LncRNA PVT1 promotes the malignant progression of acute myeloid leukaemia via sponging miR-29 family to increase WAVE1 expression. Pathology. 2021;53(5):613–622.
- Hu J, Han Q, Gu Y, et al. Circular RNA PVT1 expression and its roles in acute lymphoblastic leukemia. Epigenomics. 2018;10(6):723–732.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia Version 2 [database on the Internet]2013. Available from: https://www.nccn.org.
- Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474.
- Li M, Chi C, Zhou L, et al. Circular PVT1 regulates cell proliferation and invasion via miR-149-5p/FOXM1 axis in ovarian cancer. J Cancer. 2021;12(2):611–621.
- Bian Q. Circular RNA PVT1 promotes the invasion and epithelial-mesenchymal transition of breast cancer cells through serving as a competing endogenous RNA for miR-204-5p. Onco Targets Ther. 2019;12:11817–11826.
- Ghetti M, Vannini I, Storlazzi CT, et al. Linear and circular PVT1 in hematological malignancies and immune response: two faces of the same coin. Mol Cancer. 2020;19(1):69.
- Qin S, Zhao Y, Lim G, et al. Circular RNA PVT1 acts as a competing endogenous RNA for miR-497 in promoting non-small cell lung cancer progression. Biomed Pharmacother. 2019;111:244–250.
- Mai S, Zhang Z, Mi W. Upregulation of circ_PVT1 and circ_001569 indicate unfavorable prognosis in colorectal cancer. Ann Clin Lab Sci. 2021;51(1):55–60.
- Carramusa L, Contino F, Ferro A, et al. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol. 2007;213(2):511–518.
- Abbate AL, Tolomeo D, Cifola I, et al. MYC-containing amplicons in acute myeloid leukemia: genomic structures, evolution, and transcriptional consequences. Leukemia. 2018;32(10):2152–2166.
- Basit F, Andersson M, Hultquist A. The Myc/Max/Mxd network is a target of mutated Flt3 signaling in hematopoietic stem cells in Flt3-ITD-induced myeloproliferative disease. Stem Cells Int. 2018;2018:3286949.
- Wu G, Suo C, Yang Y, et al. MYC promotes cancer progression by modulating m(6) a modifications to suppress target gene translation. EMBO Rep 2021;22(3):e51519.
- Yan M, Gao H, Lv Z, et al. Circular RNA PVT1 promotes metastasis via regulating of miR-526b/FOXC2 signals in OS cells. J Cell Mol Med. 2020;24(10):5593–5604.
- Wang X, Zhang Y, Li W, et al. Knockdown of cir_RNA PVT1 elevates gastric cancer cisplatin sensitivity via sponging miR-152-3p. J Surg Res. 2021;261:185–195.
- Ebian HF, Elshorbagy S, Mohamed H, et al. Clinical implication and prognostic significance of FLT3-ITD and ASXL1 mutations in Egyptian AML patients: a single-center study. Cancer Biomark. 2021. DOI:https://doi.org/10.3233/CBM-210024
