ABSTRACT
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of diseases which are prone to progress into acute myeloid leukemia (AML). Iron overload (IOL) caused by transfusion occurred in most MDS patients. But how IOL influences MDS progression has not been clarified yet.
Methods
Herein, we collected clinical data from 143 MDS patients to investigate the impacts of IOL on patients survival and AML transformation.
Results
We found that median survival time, 3-year survival rate, leukemia-free survival (LFS) time were significantly shorter in patients with IOL than those with non-iron overload (NIOL) (P = 0.040; P = 0.044; P = 0.037). Besides, IOL was more likely to be found in higher-risk subgroups (assessed by IPSS and WPSS) of MDS patients which also promoted 2-year AML transformation. Furthermore, the serum ferritin (SF) was significantly correlated with the overall survival (OS) of MDS patients (r = −0.311, P < 0.05). The concentrations of both intracellular iron and reactive oxygen species (ROS) in CD34+ cells of bone marrow were higher in the IOL group than the NIOL group, respectively (P = 0.0426; P = 0.0185). Moreover, ROS level was closely correlated with the percentage of bone marrow blasts (r = 0.7200, P = 0.0370). Collectively, IOL threatened the survival of MDS patients and promoted AML transformation.
Conclusion
Elevated intracellular iron and ROS in CD34+ cells of bone marrow could accelerate the abnormal proliferation of blasts.
Introduction
Myelodysplastic syndromes (MDS), a group of heterogeneous clonal diseases derived from hematopoietic stem cells (HSCs), are generally characterized by ineffective hematopoiesis, pancytopenia and potential transformation to acute myeloid leukemia (AML) [Citation1]. Anemia occurs in about 80–90% of MDS patients and results in red blood cell (RBC) transfusion dependence. Subsequently, secondary iron overload (IOL) is induced inevitably in MDS patients after receiving 20–40 units of RBCs. Recent studies suggested that IOL caused by transfusion could influence the overall survival (OS) of MDS patients in Europe while treatments by iron chelation could prolong their survival time [Citation2–8]. Moreover, IOL can also advance the transformation from MDS to AML, during which serum ferritin (SF) perhaps acts as an independent prognostic factor for MDS patients [Citation3, Citation9]. However, the relationships between parameters of IOL and survival, AML transformation of MDS patients are still unclear. We here aim to explore the possible relationships between various parameters of IOL and investigate the impacts of IOL on the survival of MDS patients, as well as the transformation of MDS to AML in a single center in China.
Patients and methods
Patients
A total of 143 patients (96 males and 47 females; median age = 56.8, ranging 15–87), diagnosed with MDS in the Department of Hematology, Tianjin Medical University General Hospital between September 2009 and January 2016, was enrolled in this study. The follow-up ended in March 2016. These patients with MDS were subdivided according to the classification of WHO in 2008 (n = 143, ). The diagnostic criteria of IOL included: (1) patients received more than 20 units of RBC transfusion. In China, 1 unit RBC contains 200 mL whole blood; (2) SF level > 1000 ng/mL; (3) patients with other symptoms or diseases, such as active inflammation, liver disease, tumor, hemolysis and alcoholism, and so on that may increase the ferritin level of MDS patients, were excluded from the statistics [Citation10, Citation11].
Table 1. Demographic and characteristics of patients.
Methods
Detection of SF concentration
The immunoturbidimetric assay antigen–antibody was employed to determine the SF concentration. The SF concentration of patients was measured when they were diagnosed as MDS. The concentration 1000 ng/mL was chosen as the threshold complying with the guidelines for the diagnosis of IOL. No patients received iron chelation treatment prior to the first SF measurement.
Reactive oxygen species (ROS) assay
Intracellular ROS level of CD34+ cell in bone marrow was detected using fluorescence-activated cell sorting (FACS). The bone marrow samples (200 μL) anticoagulated by heparin were diluted with PBS and mixed with DCFH-DA-FITC (10 mmol/L) until 1 μmol/L, then incubated in 37°C for 30 min in dark. After washed by PBS, the samples were incubated with CD34+ perCP antibody (BD Biosciences, USA) for 15 min in dark and then disposed with hemolysin for hemolysis. The mean MFI of ROS was lastly analyzed through FACS-Calibur flow cytometry (BD Biosciences) and FlowJo software (Version 7.6.1, TreeStar).
Intracellular iron determination
Intracellular iron level was obtained through an Iron Colorimetric Assay Kit (BioVision). Briefly, CD34+ cells were added into 96-well plates at 1 × 105 cell/mL and diluted with assay buffer. Then each well was dealt with iron reducer for 30 min incubation and iron probe for another 60 min incubation in dark. Finally, the absorbance was measured at 593 nm using a microplate reader.
Survival evaluation
To avoid selection bias, the survival analysis was performed only for patients diagnosed with MDS up to 6 months before being enrolled in this study. As a result, there were 110 cases included in the survival analysis. Specifically, the overall survival time was calculated based on the period from the time of diagnosis to the end of follow-up or death. The transformation time to AML was calculated in diagnosed patients according to the period from the time of diagnosis to the date of transformation to AML.
Ethics
This study was approved by the Ethics Committee of Tianjin Medical University General Hospital. All applicable international regulations concerning the ethical participation of our volunteers were followed during this research protocol. All patients have signed informed consents.
Statistical analysis
Data processing was performed in SPSS 21.0 software (Chicago, IL, USA). Continuous variables were represented as mean ± standard deviation or median (range) while frequency (percentage) was applicable to categorical variables. Chi-square test or Fisher exact test were applied to compare categorical variables and Student’s t-test was used to analyze continuous variables. Kaplan–Meier survival analysis was adopted to describe median survival and leukemia-free survival (LFS), and log-rank test was introduced to compare both curves. All P values were two-sided and P < 0.05 were considered as significantly different.
Results
Demographic and characteristics of subjects
On average, patients received a total transfusion of 39.52 ± 62.89 RBC units. The average RBC transfusion was 67.80 ± 46.62 (14–288) units in the IOL group and 3.52 ± 8.04 (0–36) units in the NIOL group.
As presented in , there were 37 cases with IOL and 106 cases with non-iron overload (NIOL) diagnosed among the 143 patients with MDS. The median ages of MDS patients were 52 in the IOL group and 61.5 in the NIOL group, respectively. There were more males than females in both the IOL group (33 males, 89.2%) and the NIOL group (63 males, 59.4%).
According to WHO classification in 2008 (), we identified RA subtype (2/5, 40%), RARS subtype (8/19, 42.11%), RCMD subtype (12/33, 36.37%), RAEB-1 subtype (4/19, 21.06%), RAEB-2 subtype (9/30, 30%), MDS-u subtype (3/25, 12.00%), MDS RN, RT, 5q- and MDS/MPN subtypes (0/12, 0.00%) in the IOL group. Obviously, the RARS subtype was most common in the IOL group. In addition, the percentage of patients diagnosed with MDS subtypes (RA, RARS, RCMD, RAEB-2) were higher than those with other subtypes (respectively: 40%, 42.11%, 36.37%, 30% vs. 25.88%, P < 0.05). The IOL patients diagnosed with MDS RN, RT, MDS/MPN, 5q-, MDS-u were less than those diagnosed with other subtypes (respectively: 0, 0, 0, 0, 12.00% vs. 25.88%, P < 0.05).
Furthermore, the frequencies of each MDS stratification in the IOL group according to IPSS () were as follows: low-risk stratification (3/13, 23.08%), Int-1 stratification (21/75, 28.00%), Int-2 stratification (9/42, 21.43%) and high-risk stratification (4/13, 30.77%). Evidently, IOL occurred most frequently in high-risk stratification. Besides, frequencies in the IOL group assessed by WPSS () were listed below: very low-risk stratification (0/9, 0.00%), low-risk stratification (6/29, 20.69%), Int-risk stratification (11/30, 36.67%), high-risk stratification (15/59, 25.43%) and very high-risk stratification (5/16, 31.25%). It was clear that IOL happened most frequently in Int risk stratification, whereas IOL never occurred in very low-risk stratification.
Correlations between various IOL indicators
SF, iron stain of bone marrow, LIC and total numbers of RBC transfusion were analyzed to investigate the relationships between various indicators of IOL. Mann–Whitney test showed that the SF level was significantly higher in the IOL group than that in the NIOL group (P < 0.0001) (). Spearman correlation analysis suggested SF was significantly related to total numbers of RBC transfusion with Spearman’s coefficient of 0.588 (P = 0.002). Meanwhile, supported by , iron stain of bone marrow was also significantly increased in the IOL group compared to the NIOL group (P = 0.021), and significantly correlated with total numbers of RBC transfusion (r = 0.258, P = 0.042). Moreover, the significant correlation between iron stain and SF level was identified by Spearman’s correlation test (r = 0.281; P = 0.034). Furthermore, LIC level was confirmed to be higher in the IOL group than that in the NIOL group (41.25 ± 12.75 μmol/L vs. 31.10 ± 12.71 μmol/L, P = 0.0424) and was correlated with the total numbers of RBC transfusion (r = 0.370, P = 0.024). Spearman’s coefficient of 0.427 clearly showed the positive correlation between LIC and SF (P = 0.021).
Table 2. Level of intracellular ROS and iron in CD34+ cells of bone marrow.
Table 3. Iron stain of bone marrow in MDS patients.
Effects of IOL on survival and leukemia transformation of MDS
The median follow-up period was 24 months (2–84 months) in 110 patients. A total of 51 (46.37%) patients remained alive including 8 cases with IOL and 43 cases with NIOL. Besides, there were 59 (53.63%) patients, involving 19 cases with IOL and 40 cases with NIOL died from various reasons.
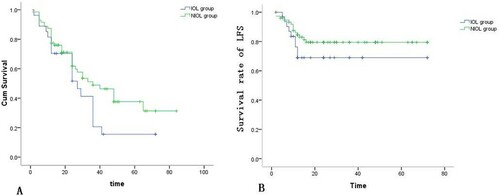
The mean OSs were 21.3 ± 12.5 (2–41) months in the IOL group and 21.4 ± 14.6 (5–65) months in the NIOL group, respectively, and no significant difference was detected between OSs of two groups. Furthermore, Kaplan–Meier analysis suggested that median survival periods of MDS patients were significantly longer in the NIOL groups (IOL group vs. NIOL group, 27 months vs. 36 months, P = 0.040). Consistently, significant lower 3-year survival rate was found in the IOL group (IOL group vs. NIOL group, 14% vs. 45%, P = 0.044). Based on results of iron stain in bone marrow, patients were divided into the high extracellular iron (HEI) group (23 cases, +++ ∼ ++++) and the normal extracellular iron (NEI) group (78 cases, - ∼ ++). As suggested, death occurred in 14 cases (60.83%) in the HEI group and 39 cases (50.0%) in the NEI group. The mean OSs were 29.9 months in the HEI group and 26 months in the NEI group, respectively. Kaplan–Meier curves indicated that the median survival was 36.0 ± 5.3 months in the HEI group and 30 ± 5.2 months in the NEI group with no significant difference between them. Besides, we found 5 cases (21.74%) in the HEI group and 10 cases (12.82%) in the NEI group with AML transformation.
Moreover, AML transformation was diagnosed in 5 (18.5%) cases in the IOL group and 14 (16.8%) cases in the NIOL group (). According to IPSS, the frequencies of AML transformation in the low-risk, Int-1 risk and high-risk subgroups were 33.3% vs. 0%, 9.52% vs. 9.26% and 50% vs. 44.4% (IOL group vs. NIOL group), respectively. Kaplan–Meier curves indicated that LFS was shorter in the IOL group (52.5 ± 5.4 months) than that in the NIOL group (59.2 ± 3.1 months) (P = 0.037) (B). Meanwhile, the frequencies of 2-year AML transformation in the IOL group and the NIOL group were 18.5% and 16.8%, respectively. Additionally, based on IPSS, the frequencies of 2-year AML transformation in the IOL group were higher than that in the NIOL group (low risk: 33.3% vs. 0%, Int risk: 9.52% vs. 9.26%, high risk: 50.0% vs. 44.4%). According to WPSS risk stratification, the frequencies of 2-year AML transformation in the high and very high-risk subgroups in the IOL group were higher than those in the NIOL group (25.0% vs. 18.2%, P = 0.042).
Association between SF and OS of MDS patients
The correlation between SF and OS of MDS patients was affirmed by Speaman’s coefficient of –0.311 (P < 0.05).
Correlations between IOL and bone marrow blasts, genetic abnormality, chromosomal abnormality, intracellular iron and ROS in CD34+ cells
Bone marrow blasts, genetic abnormality, chromosomal abnormality, intracellular iron and ROS in CD34+ cells were determined at the time of diagnosis and the follow-up period of IOL patients. As a result, the percentage of bone marrow blasts was significantly larger in the IOL group than that of the NIOL group (7.080 ± 5.408 vs. 4.470 ± 5.422, P = 0.015). Besides, leukemia-related genes were analyzed in 17 cases with IOL and 44 cases with NIOL. Both BCR-ABL fusion gene and TP53 mutation were detected in one (5.89%) patient with IOL who was finally diagnosed with PH-positive acute lymphocyte leukemia in follow-up. JAK2 mutation was detected in three (17.65%) patients with IOL and five (11.37%) patients with NIOL. TET2 mutation was detected in one (2.28%) patient with NIOL. Additionally, chromosomal abnormalities were observed in 32 patients with IOL and 90 patients with NIOL (). As shown, results of fluorescence in-situ hybridization (FISH) and conventional cytogenetic analysis displayed that Chromosome 7 abnormality was most common in the IOL group while +8 was most common in the NIOL group.
Table 4. Abnormality of chromosome of MDS.
Lastly, the concentration of intracellular iron in CD34+ cells of bone marrow from 15 cases with IOL was 0.020 ± 0.004 nmol/μL, which was higher than that of 19 cases with NIOL (P = 0.043). Similarly, the intracellular ROS level in CD34+ cells of bone marrow from the IOL group was higher than that from the NIOL group (239.36 ± 77.10 vs. 143.87 ± 67.89, P = 0.0190). Moreover, the correlation between ROS and the percentage of bone marrow blasts was statistically significant (r = 0.720, P = 0.037).
Discussion
SF is the most appropriate standard to estimate body-iron reserves [Citation9], although ferritin >1000 ng/mL is accepted as non-invasive and high-precision measure. Other IOL indices such as the total number of RBC transfusion, iron stain of bone marrow and LIC are also available and applied in clinical researches. In this retrospective clinical study, we investigated relationships between SF and other IOL indicators, and the relationships between various IOL indicators and total numbers of RBC transfusion in MDS patients from a single center of China. Three indicators (SF, LIC and iron stain of bone marrow) were all significantly elevated in patients with IOL, also significantly associated with total numbers of RBC transfusion. To our knowledge, it is the first time to confirm that iron stain of bone marrow could also be used as a reference standard for IOL diagnosis. Simultaneously, it reminds us that RBC transfusion dependence is the chief cause of IOL in MDS patients. Additionally, LIC is the most dangerous and immediate factor in the occurrence of ineffective hematopoiesis. Thus the correlation between total numbers of RBC transfusion and LIC showed that both RBC transfusion and SF could impair hematopoiesis. We also found that the RARS subtype was the most common in the IOL group of MDS patients, which was consistent with previous literature. The mechanisms of IOL are slightly different in MDS subtypes. IOL is more likely to occur in simple erythroid abnormalities or low-risk MDS patients. Active but ineffective hematopoiesis in RARS patients accelerates iron load. At the same time, ineffective hematopoiesis induces erythrocytes to secrete regulatory proteins and cytokines, such as growth differentiation factor 15, human bone morphogenetic protein 6, which significantly inhibit the expression of ferritin, resulting in the lowest ferritin level in RA/RAS patients and stimulation of increased iron absorption. Finally, patients with RARS are more prone to transfusion-dependent iron overload because of better prognosis and longer survival.
IPSS and WPSS are different scoring systems to evaluate the prognostic risk stratification of MDS patients [Citation12]. In this study, the percentage of IOL cases in the high-risk subgroup was highest according to IPSS. Besides, the frequency in the Int risk subgroup based on WPSS was higher than those of other risk subgroups. These results were also consistent with RBC transfusion dependence in these patients.
Previous studies have shown that IOL caused by transfusion seriously threatened the survival of MDS patients, including OS and leukemia transformation [Citation2, Citation5]. However, the relationships between IOL and prognosis of MDS in Chinese patients have not been discussed yet. Kaplan–Meier analysis manifested that the median survival time was significantly longer in the NIOL group than that in the IOL group. Furthermore, the 3-year survival rate was lower in patients with IOL than NIOL, suggesting that IOL led to worse survival of Chinese patients with MDS. Additionally, AML transformation rate also increased and LFS tended to be shorter in the IOL group. As shown, according to IPSS, patients with IOL had a higher transformation rate in the low-risk, Int-1 risk and high-risk groups than patients with NIOL. As far as we know, this is the first time to provide clinical evidence that IOL has an adverse impact on the survival of Chinese MDS patients in different risk subgroups based on IPSS. Moreover, our data indicated that patients with higher extracellular iron level suffered from a higher mortality and transformation rate to AML.
As is well known, pathological mechanisms of IOL on survival and AML transformation were not clarified yet. Thus we conducted extensive research on the genetic and chromosomal abnormality, intracellular iron and ROS in CD34+ cells of bone marrows. Previously, researchers suggested that genetic abnormalities were closely associated with the prognosis of MDS patients [Citation13–16]. In this work, chromosomal abnormalities happened in 65.6% of MDS patients and patients with IOL had a higher frequency of –5 related to a poor prognosis according to a study performed by Tasaka et al. [Citation17], who found that MDS patients with chromosome 5 abnormality accompanied by other chromosomal karyotype abnormalities or complex karyotype displayed poor prognosis. Besides, chromosome 7 abnormality was most common and happened more frequently in the IOL group than the NIOL group. Further, according to IPSS scoring, Chromosome 7 is a high-risk karyotype and associated with poor prognosis. As Haase et al. [Citation14] reported, patients with normal karyotype had a median survival time of over 49 months and a lower risk of leukemia transformation. However, the median survival time of patients with –7/7q– was only 12 months and about 72% of them had AML transformation. In addition, an unusual chromosome abnormality, +8, has been classified as an intermediate prognostic factor. The median survival time of the +8 abnormal group (including simple +8 and +8 combined with a chromosomal abnormality) was between that of the normal karyotype group and the complex karyotype group. In this study, our results also indicated that patients with IOL possessed a high frequency of 20q–. Although Campbell Li et al. reported that MDS patients with 20q– had a higher risk of leukemia transformation and lower survival [Citation16], the role of 20q– in MDS prognosis was still controversial.
The abnormality of genes, such as JAK2 and TP53, is generally associated with cell proliferation and apoptosis. These abnormal genes are capable of promoting the proliferation of hematopoietic cells, indicating that IOL could enhance the abnormal proliferation of bone marrow since patients with IOL own high frequencies of these abnormal genes. Further analysis showed that a higher percentage of bone marrow blasts in the IOL group was attributed to the abnormality of proliferation-associated genes. Moreover, IOL perhaps accelerated AML transformation via promoting the abnormal proliferation of hematopoietic stem cells.
All of these findings demonstrated that MDS patients with IOL were associated with poor prognosis, AML transformation risk and higher frequencies of chromosomal abnormality. Our previous study found that elevated levels of plasma iron and ROS could damage hematopoiesis of MDS patients [Citation18–21]. In this study, we further observed that both intracellular iron level and ROS level in CD34+ cells of bone marrow were significantly higher in the IOL group, suggesting that IOL contributed to highly oxidized status and damaged intracellular organic matters of CD34+ cells effortlessly. Meanwhile, the positive correlation between ROS and the percentage of bone marrow blasts indicated that IOL could accelerate the proliferation of CD34+ cells and promote AML transformation. Shaw et al. [Citation22] confirmed that intracellular iron led to DNA damage of lymphocytes in patients with IOL. Therefore, excessive intracellular iron and accumulation of ROS induced by IOL could directly damage genes and chromosomes and influence the proliferation of CD34+ cells of bone marrow in MDS patients [Citation23]. In addition, IOL might promote leukemia transformation by altering the cytogenetics of MDS patients, though further studies are still needed.
Some shortcomings of this study may lead to potential deviations of our results, such as insufficient sample size of patients with IOL, a significant difference in the proportions of patients in the low-risk and high-risk groups, which may impact on OS, PFS and leukemia conversion rates in MDS patients. Therefore, the inferior prognosis in this subgroup may be due to the underlying biology of the disease. The iron overload may be an additional factor, but to better evaluate the influence of IOL, a multivariate analysis may be helpful in future research.
Conclusion
Levels of various IOL indicators were positively related to total numbers of RBC transfusion in MDS patients with IOL. Besides, iron stain of bone marrow was a potential factor to evaluate mortality and AML transformation of MDS patients with IOL. Overall, IOL threatened the survival of MDS patients and could probably induce AML transformation. Elevated intracellular iron and ROS in CD34+ cells of bone marrow probably led to abnormality of genes and chromosomes, and thus accelerated proliferation of blasts and promote AML transformation of MDS.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Tianjin Medical University General Hospital (Ethical No. IRB2020-WZ-059). All applicable international regulations concerning the ethical participation of our volunteers were followed during this research protocol, and all cases signed informed consent.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Yihao Wang, Rong Fu and Zonghong Shao designed this program. Yanni Hua operated the experiments. Lei Huang conducted the data collection and analysis. Yihao Wang and Yanni Hua produced the manuscript which was checked by Hui Liu, Huijuan Jiang, Wei Zhang, Huaquan Wang. All the authors have confirmed the submission of this manuscript.
Additional information
Funding
References
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi:https://doi.org/10.1056/NEJMra0902908.
- Shenoy N, Vallumsetla N, Rachmilewitz E, et al. Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood. 2014;124(6):873–881. doi:https://doi.org/10.1182/blood-2014-03-563221.
- Leitch HA. Controversies surrounding iron chelation therapy for MDS. Blood Rev. 2011;25(1):17–31. doi:https://doi.org/10.1016/j.blre.2010.09.003.
- Lyons RM, Marek BJ, Paley C, et al. Comparison of 24-month outcomes in chelated and non-chelated lower-risk patients with myelodysplastic syndromes in a prospective registry. Leuk Res. 2014;38:149–154. doi:https://doi.org/10.1016/j.leukres.2013.11.004.
- Delforge M, Selleslag D, Beguin Y, et al. Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res. 2014;38:557–563. doi:https://doi.org/10.1016/j.leukres.2014.02.003.
- Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicen-ter study by the GFM (Groupe Francophone des Myélodysplasies). Leuk Res. 2010;34(7):864–870. doi:https://doi.org/10.1016/j.leukres.2009.12.004.
- Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States medicare beneficiaries. J Clin Oncol. 2010;28:2847–2852. doi:https://doi.org/10.1200/JCO.2009.25.2395.
- Lyons RM, Marek BJ, Paley C, et al. Comparison of 24-month outcomes in chelated and non-chelated lower-risk patients with myelodysplastic syndromes in a prospective registry. Leuk Res. 2014;38:149–154. doi:https://doi.org/10.1016/j.leukres.2013.11.004.
- Waszczuk-Gajda A, Mądry K, Machowicz R, et al. Red blood cell transfusion dependency and hyperferritinemia are associated with impaired survival in patients diagnosed with myelodysplastic syndromes: results from the first Polish MDS-PALG registry. Adv Clin Exp Med. 2016;25(4):633–641. doi:https://doi.org/10.17219/acem/62397.
- Xue Z, Xue Y, Zhi Z. A Chinese expert panel consensus statement on diagnosis and treatment of iron overload. Chin J Hematol. 2011;32(8):572–574.
- Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011;90(1):1–10. doi:https://doi.org/10.1007/s00277-010-1091-1.
- Leitch HA, Parmar A, Wells RA, et al. Overall survival in lower IPSS risk MDS by receipt of iron chelation therapy, adjusting for patient-related factors and measuring from time of first red blood cell transfusion dependence: an MDS-CAN analysis. Br J Haematol. 2017;179(1):83–97. doi:https://doi.org/10.1111/bjh.14825.
- P1 B, Klersy C, Boni M, et al. World Health Organization classification in combination with cytogenetic markers improves the prognostic stratification of patients with de novo primary myelodysplastic syndromes. Br J Haematol. 2007;137(3):193–205.
- Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–4395.
- Barouk-Simonet E, Soenen-Cornu V, Roumier C, et al. Role of multiplex FISH in identifying chromosome involvement in myelodysplastic syndromes and acute myeloid leukemias with complex karyotypes: a report on 28 cases. Cancer Genet Cytogenet. 2005;157(2):118–126.
- Campbell LI, Garson OM. The prognostic significance of deletion of the long arm of chromosome 20 in myeloid disoIdem. Leukemia. 1994;8(1):67–71.
- Tasaka T, Tohyama K, Kishimoto M, et al. Myelodysplastic syndrome with chromosome 5 abnormalities: a nationwide survey in Japan. Leukemia. 2008;22(10):1874–1881. doi:https://doi.org/10.1038/leu.2008.199.
- Mies A, Platzbecker U. Increasing the effectiveness of hematopoiesis in myelodysplastic syndromes: erythropoiesis-stimulating agents and transforming growth factor-β superfamily inhibitors. Semin Hematol. 2017;54(3):141–146. doi:https://doi.org/10.1053/j.seminhematol.2017.06.004.
- Angelucci E, Cianciulli P, Finelli C, et al. Unraveling the mechanisms behind iron overload and ineffective hematopoiesis in myelodysplastic syndromes. Leuk Res. 2017;62:108–115. doi:https://doi.org/10.1016/j.leukres.2017.10.001.
- Messa E, Biale L, Castiglione A, et al. Erythroid response during iron chelation therapy in a cohort of patients affected by hematologic malignancies and aplastic anemia with transfusion requirement and iron overload: a FISM Italian multicenter retrospective study. Leuk Lymphoma. 2017;58(11):2752–2754. doi:https://doi.org/10.1080/10428194.2017.1312385.
- Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016;172(4):512–523. doi:https://doi.org/10.1111/bjh.13820.
- Shaw J, Chakraborty A, Nag A, et al. Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. Eur J Haematol. 2017;99(5):399–408. doi:https://doi.org/10.1111/ejh.12936.
- Kim IH, Moon JH, Lim SN, et al. Efficacy and safety of deferasirox estimated by serum ferritin and labile plasma iron levels in patients with aplastic anemia, myelodysplastic syndrome, or acute myeloid leukemia with transfusional iron overload. Transfusion. 2015;55(7):1613–1620. doi:https://doi.org/10.1111/trf.13036.