ABSTRACT
Objectives: Hereditary hyperferritinaemia cataract syndrome (HHCS) is an autosomal dominant disease characterized by high serum ferritin levels and juvenile bilateral cataracts. It is often caused by mutations in the iron response element (IRE) of the ferritin L-subunit (FTL) gene. Here, we report a 73-year-old woman who presented to clinic with persistently elevated serum ferritin and family history of juvenile bilateral cataracts in four generations.
Methods: Exome sequencing was used to identify the mutation of the FTL gene. Moreover, Sanger sequencing was performed to validate the mutation in the proband. We also reviewed the FLT gene mutations in published HHCS cases to provide experience for accurate diagnosis of similar patients.
Results: A heterozygous mutation at position +33 (c.-167C > T, chr19:49468598) of the FTL gene was identified in the patient.
Discussion: HHCS should be considered in the differential diagnosis of hyperferritinemia, especially in the presence of normal serum iron concentration and transferrin saturation.
Conclusion: For patients with unexplained hyperferritinemia and bilateral cataracts who have experienced early vision loss, the establishment of genetic counseling is essential to diagnose other family members who are at risk in time.
Abbreviations: FTL: ferritin L-subunit; HHCS: hereditary hyperferritinaemia cataract syndrome; IDT: integrated DNA technologies; IRE: iron response element; IRP: iron regulatory proteins; MRI: magnetic resonance imaging; SNV: single nucleotide variant; UTR: untranslated region
Background
Serum ferritin is a water-soluble protein that contains about 23% iron. It is mainly composed of the L-subunit and a few H subunits, which is roughly equivalent to the total iron storage of the human body. Therefore, serum ferritin is often used to determine the level of stored iron in the body. Hereditary hyperferritinaemia cataract syndrome (HHCS) is a rare autosomal dominant genetic disease characterized by significantly raised serum ferritin, bilateral congenital cataracts, but the transferrin saturation and serum iron in the body are normal or high, no increased iron load in the liver and other parenchymal organs, and no evidence of inflammation or tumor [Citation1].
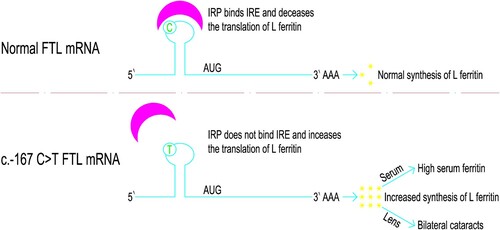
It is currently believed that the pathogenesis of HHCS is mainly the mutation in the segment of Iron Response Element (IRE) that located in the 5’ untranslated region (UTR) of the ferritin L-subunit (FTL) gene, which is the critical sequence of the stem-loop motif [Citation2]. This region can affect the binding affinity of Iron Regulatory Proteins (IRP) to IRE and deregulate the expression of the L-Ferritin, resulting in increased synthesis of L-Ferritin. The excessive ferritin subsequently accumulated in serum and crystalline lens, leading to hyperferritinemia and the early onset of bilateral cataracts [Citation3]. Mumford and colleagues found that the level of ferritin in the lens of affected individuals was 10-fold higher than that in the normal group [Citation4]. The severity of cataracts is related to the levels of serum ferritin and the clinical severity of HHCS (i.e. serum ferritin levels and cataract severity) is related to the location of the IRE mutation [Citation2,Citation5].
The clinical manifestations of HHCS are bilateral cataracts with elevated serum ferritin level, and bilateral cataract may be the only recognizable phenotypic manifestation [Citation6]. However, many clinicians often fail to identify and diagnose HHCS. They just treat the patient as a pure cataract patient and even perform eye surgery on the patient without checking the serum ferritin concentration, ignoring the genetic characteristics of the disease [Citation7]. Family history of hyperferritinemia and cataracts are important diagnostic indicators of HHCS. Genetic analysis of the FTL gene is a simple procedure that can confirm the diagnosis [Citation8]. Accurate diagnosis of HHCS can avoid unnecessary phlebectomy therapy and focus attention on early cataract detection in high-risk offspring.
Here, we report a Chinese female with HHCS associated with a heterozygous mutation at position +33 (c.-167C > T, chr19:49468598) in the FTL gene. The version of the human genome used for chr19:49468598 position is hg19. ‘+33’ refers to the traditional nomenclature, where the number of the mutational position is counted from the transcription initiation site as +1; in opposition to the official HGVS nomenclature considering +1 the A in the ATG of the starting codon (c.-167C > T). In addition, we have reviewed all the mutations of the FTL gene in reported cases of HHCS until now, in order to provide experience for accurate diagnosis of similar patients.
Case presentation
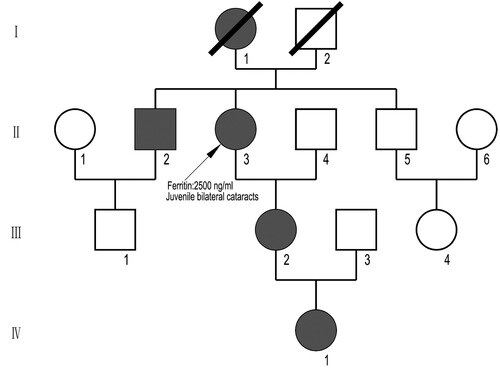
The proband was a 73-year-old woman (II-3 in ), and her overall condition was good. According to the patient, she had poor vision from childhood. At the age of twelve, she was diagnosed with congenital cataracts, with visual acuity 6/10 in the left eye and 7/10 in the right eye. She began to have blurred vision in both eyes at the age of 40. When she was 68 years old, the loss of her visual acuity progressed, and visual acuity was 5/10 in the left eye and 4/10 in the right eye. Then she underwent ultrasound phacoemulsification and intraocular lens implantation under topical anesthesia. After the surgery, her visual acuity recovered to 8/10 in the left eye and 7/10 in the right eye, with eyeglasses for correction. She was found to have elevated serum ferritin (2500 ng/ml, reference value 24–336 ng/ml) during a routine health check-up at the age of 71. Then the patient began to review serum ferritin regularly.
Figure 1. A four-generation family pedigree with HHCS. The proband is shown arrowed. Circles denote female family members and squares male family members. Black symbols indicate individuals with HHCS. Open symbols indicate non-affected individuals.
In August 2020, she visited the outpatient department of Hematology of West China Hospital for further assessment due to persistently raised serum ferritin. Repeated examination indicated that her serum ferritin concentration was significantly increased (1652 ng/ml, reference value 24–336 ng/ml). Further laboratory examination indicated that, except for a slight decrease in transferrin (2.26 g/L, reference value 2.5–4.3 g/L), other iron metabolism parameters including soluble transferrin receptor (1.16 mg/L, reference value: 0.76–1.76 mg/L), serum iron (22.4 umol/L, reference value: 7.8–32.2 umol/L), total iron-binding capacity (TIBC) (51.25 umol/L, reference value: 48.3–68.0 umol/L) and transferrin saturation (43.7%, reference value: 20%∼55%) were all normal. Evaluation of magnetic resonance imaging (MRI) of the heart and liver showed no evidence of parenchymal iron overload. She had no other aetiologies such as malignancy, inflammation, obesity, and alcohol abuse that could cause hyperferritinemia. Consequently, genetic testing was carried out on the patient.
After obtaining informed consent, the peripheral blood sample was drawn from the proband. DNA was extracted using the commercially available kits. Integrated DNA Technologies (IDT) capture kits, Kapa Library Construction Kit was employed for seizing and gathering the patient DNA in exon regions. Exome sequencing using Illumina Novaseq6000 sequencing machine was performed to detect gene mutations in the proband, including all point mutations, small insertions, and small deletions. DNA analysis detected a mutation in the IRE region of the FTL gene (c.-167C > T, chr19:49468598).
Sanger sequencing using ABI 3730 Genetic Analyzer machine was performed to validate the mutation in the proband. PCR amplification was performed using the following primer pair: forward, 2F: 5’-GGGCTGAGACTCCTATGTGC-3’, reverse, 2R: 5’-GGTTGGTTGGCAAGAAGGAG-3’.
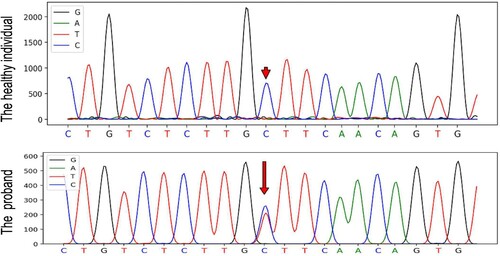
We found a heterozygous sequence variant converting C to T at chr19:49468598 (c.-167C > T) in the FTL gene in the patient (). The mutation is reported to be related to HHCS in the HGMD professional database. Her daughter and granddaughter were also confirmed to have the same genetic mutation. represents the mechanism of HHCS caused by c.-167C > T mutation of the FTL gene.
Figure 2. DNA sequence of the L-ferritin gene encompassing the c.-167C > T mutation in the proband and in a healthy individual. The mutation is indicated by red arrow.
Family history was significant in that the patient’s mother (I-1) also had early onset cataract. One of her younger brothers (II-2) was diagnosed with a congenital cataract at the age of 10 and underwent cataract surgery at the age of 19. The patient’s daughter (III-2) and granddaughter (IV-1) also suffered from eye problems at the age of 12, and were confirmed to have the same genetic mutation as the patient. The affected brother of the patient had a 35-year-old son who had no eye problems. The remaining healthy brother had a non-affected daughter. No member of this family had previously had genetic counseling. The family pedigree is outlined in .
Literature review
We identified 115 cases that reported HHCS from 1995 to 2021 via PubMed using a combination of the keywords ‘Hereditary hyperferritinaemia cataract syndrome’, ‘L-Ferritin’, and ‘Cataract’. The current case is the second reported case of HHCS in China. Since first reported in 1995, a series of point mutations and short deletions of L-ferritin related to HHCS have been reported [Citation9–57]. shows that a total of 34 single nucleotide transitions (SNT) and 5 nucleotide deletions have been reported as of September 2021. Among them, the single nucleotide transition most frequently reported is +40A > G (c.-160A > G in the HGVS nomenclature). Mutations causing HHCS in the predicted IRE structure of L-ferritin are presented in . It can be seen from the figure that most of the mutations that cause HHCS are at the upper stem and the conserved hexanucleotide of the hairpin structure of IRE.
Figure 4. Mutations causing HHCS in the predicted secondary structure of the IRE. The nucleotide deletions are represented by brackets, and the single nucleotide variants are denoted by black arrows. Genetic variants are reported following the traditional nomenclature.
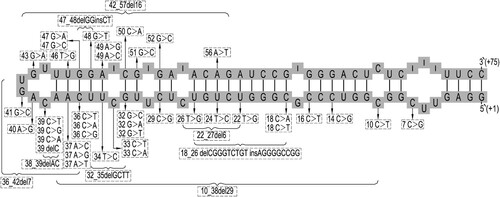
Table 1. An overview of all mutations causing HHSC (as of September 2021).
Discussion
In this report, we described a Chinese family affected with HHCS who was identified a heterozygous c.-167C > T mutation in the 5’ UTR of the FTL gene. HHCS is inherited in an autosomal dominant manner, and this heterozygous mutation is in line with the inheritance pattern of the disease. This mutation has also been reported to be associated with HHCS previously in several other pedigrees [Citation8,Citation12,Citation35,Citation45,Citation53,Citation56,Citation58].
According to a study by Australian scholars, the prevalence of HHCS in the population is at least 1/20,000 [Citation1]. Since the first case of HHCS in China was reported in 2020 [Citation55], our case is the second identified case. This may be due to the relatively low actual incidence of the disease in the Chinese population, or it may be that the understanding of the disease is still insufficient, and the rate of missed diagnosis and misdiagnosis is still high.
As the increase of L-Ferritin in affected individuals is due to the dysregulation of L-Ferritin synthesis and has nothing to do with the body iron storage, there is no real iron overload in HHCS patients. In our case, the level of serum iron and TIBC was normal and MRI of liver and heart showed no evidence of iron overload. Patients with HHCS often develop cataracts at a young age. Just as in our case, the proband, her daughter, granddaughter and younger brother all started to develop cataracts around the age of twelve. The typical cataract of HHCS has unique morphological features, which are characterized by progressive white spots along the axial and surrounding areas, accompanied by small crystal aggregates [Citation1]. Unfortunately, since the patient had undergone cataract surgery before diagnosis, we were unable to obtain the photos of patient’s eye before surgery.
Conclusion
HHCS should be considered in the differential diagnosis of hyperferritinemia. Serum ferritin levels can be effectively used to screen suspicious families for this condition. We recommend that patients with cataracts at young age should check their serum ferritin levels routinely. On the other hand, individuals with unexplained high level of serum ferritin should be referred for ophthalmological consultation. In summary, although HHCS is a rare disorder, clinicians should be aware of it in order to avoid unnecessary treatment to deplete iron, as well as enable patients to conduct appropriate genetic counseling and more appropriate management.
Ethical approval
The study protocol conforms to the World Medical Association Declaration of Helsinki.
Acknowledgements
We would like to thank the patient for participating in this study. Author contributions: Xinchuan Chen provided the clinical data. This manuscript was written by Yunfan Yang and Ting Lin and edited by Pu Kuang. All the authors critically revised and provided final approval for the manuscript.
Disclosure statement
None of the authors has a potential conflict of interest to disclose.
Additional information
Funding
References
- Craig J, Clark J, McLeod J, et al. Hereditary hyperferritinemia-cataract syndrome. Arch Ophthalmol. 2004;121:1753–1761. DOI: https://doi.org/10.1001/archopht.121.12.1753.
- Luscieti S, Tolle G, Jaranda J, et al. Novel mutations in the ferritin-L iron-responsive element that only mildly impair IRP binding cause hereditary hyperferritinaemia cataract syndrome. Orphanet J Rare Dis. 2013;8:30. DOI: https://doi.org/10.1186/1750-1172-8-30.
- Ismail A, Lachlan K, Mumford A, et al. Hereditary hyperferritinemia cataract syndrome: ocular, genetic, and biochemical findings. Eur J Ophthalmol. 2006;16:153–160. DOI: https://doi.org/10.1177/112067210601600125.
- Mumford A, Vulliamy T, Lindsay J, et al. Hereditary hyperferritinemia-cataract syndrome: two novel mutations in the L-ferritin iron-responsive element [7]. Blood. 1998;91:367–368. DOI: https://doi.org/10.1182/blood.V91.1.367.367_367_368.
- Cazzola M, Bergamaschi G, Tonon L, et al. Hereditary hyperferritinemia-cataract syndrome: relationship between phenotypes and specific mutations in the iron-responsive element of ferritin light-chain mRNA. Blood. 1997;90:814–821. DOI: https://doi.org/10.1182/blood.V90.2.814.814_814_821.
- Chang-Godinich A, Ades S, Schenkein D, et al. Lens changes in hereditary hyperferritinemia-cataract syndrome. Am J Ophthalmol. 2001;132:786–788. DOI: https://doi.org/10.1016/S0002-9394(01)01106-0.
- Cosentino I, Zeri F, Swann P, et al. Hyperferritinemia-cataract syndrome: long-term ophthalmic observations in an Italian family. Ophthalmic Genet. 2016;37:1–5. DOI: https://doi.org/10.3109/13816810.2015.1059460.
- Lachlan K, Temple I, Mumford A. Clinical features and molecular analysis of seven British kindreds with hereditary hyperferritinaemia cataract syndrome. Eur J Human Genet. 2004;12:790–796. DOI: https://doi.org/10.1038/sj.ejhg.5201252.
- Angelika M, Capanna R, Chiarelli F. A girl with persistent hyperferritinaemia. Lancet. 2005;365:1744. DOI: https://doi.org/10.1016/S0140-6736(05)66549-X.
- Arnold J, Mumford A, Lindsay J, et al. Hyperferritinemia in the absence of iron overload. Gut. 1997;41:408–410. DOI: https://doi.org/10.1136/gut.41.3.408.
- Arosio C, Fossati L, Viganò M, et al. Hereditary hyperferritinemia cataract syndrome: a de novo mutation in the iron responsive element of the L-ferritin gene. Haematologica. 1999;84:560–561.
- Balas A, Aviles M, García-Sánchez F, et al. Description of a New mutation in the L-ferritin iron-responsive element associated with hereditary hyperferritinemia-cataract syndrome in a Spanish family. Blood. 1999;93:4020–4021. DOI: https://doi.org/10.1182/blood.V93.11.4020.
- Barton J, Beutler E, Gelbart T. Coinheritance of alleles associated with hemochromatosis and hereditary hyperferritinemia-cataract syndrome. Blood. 1999;92:4480. DOI: https://doi.org/10.1182/blood.V92.11.4480.423a57b_4480_4481.
- Beaumont C, Leneuve P, Devaux I, et al. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995;11:444–446. DOI: https://doi.org/10.1038/ng1295-444.
- Bosio S, Campanella A, Gramaglia E, et al. C29g in the iron-responsive element of L-ferritin: a new mutation associated with hyperferritinemia-cataract. Blood Cells Mol Dis. 2004;33:31–34. DOI: https://doi.org/10.1016/j.bcmd.2004.04.010.
- Bowes O, Baxter K, Elsey T, et al. Hereditary hyperferritinaemia cataract syndrome. Lancet. 2014;383:1520. DOI: https://doi.org/10.1016/S0140-6736(14)60484-0.
- Burdon KP, Sharma S, Chen CS, et al. A novel deletion in the FTL gene causes hereditary hyperferritinemia cataract syndrome (HHCS) by alteration of the transcription start site. Hum Mutat. 2007;28:742. DOI: https://doi.org/10.1002/humu.9501.
- Camaschella C, Zecchina G, Lockitch G, et al. A new mutation (G51C) in the iron-responsive element (IRE) of L-ferritin associated with hyperferritinaemia-cataract syndrome decreases the binding affinity of the mutated IRE for iron-regulatory proteins. Br J Haematol. 2000;108:480–482. DOI: https://doi.org/10.1046/j.1365-2141.2000.01920.x.
- Campagnoli MF, Pimazzoni R, Bosio S, et al. Onset of cataract in early infancy associated with a 32G–>C transition in the iron responsive element of L-ferritin. Eur J Pediatr. 2002;161:499–502. DOI: https://doi.org/10.1007/s00431-002-1019-4.
- Castiglioni E, Soriani N, Girelli D, et al. High resolution melting for the identification of mutations in the iron responsive element of the ferritin light chain gene. Clin Chem Lab Med. 2010;48:1415–1418. DOI: https://doi.org/10.1515/CCLM.2010.281.
- Cazzola M, Foglieni B, Bergamaschi G, et al. A novel deletion of the L-ferritin iron-responsive element responsible for severe hereditary hyperferritinaemia-cataract syndrome. Br J Haematol. 2002;116:667–670. DOI: https://doi.org/10.1046/j.0007-1048.2001.03310.x.
- Cicilano M, Zecchina G, Roetto A, et al. Recurrent mutations in the iron regulatory element of L-ferritin in hereditary hyperferritinemia-cataract syndrome. Haematologica. 1999;84:489–492.
- Cremonesi L, Fumagalli A, Soriani N, et al. Double-gradient denaturing gradient gel electrophoresis assay for identification of L-ferritin iron-responsive element mutations responsible for hereditary hyperferritinemia-cataract syndrome: identification of the new mutation C14G. Clin Chem. 2001;47:491–497. DOI: https://doi.org/10.1093/clinchem/47.3.491.
- Cremonesi L, Paroni R, Foglieni B, et al. Scanning mutations of the 5'UTR regulatory sequence of L-ferritin by denaturing high-performance liquid chromatography: identification of new mutations. Br J Haematol. 2003;121:173–179. DOI: https://doi.org/10.1046/j.1365-2141.2003.04253.x.
- del Castillo A, Ruano M. Hereditary hyperferritinemia cataracts syndrome in a Spanish family caused by the A40G mutation (Paris) in the L-ferritin (FTL)gene associated with the mutation H63D in the HFE gene. Med Clín. 2007;129:414–417.
- Ferrante M, Geubel A, Fevery J, et al. Hereditary hyperferritinaemia-cataract syndrome: A challenging diagnosis for the hepatogastroenterologist. Eur J Gastroenterol Hepatol. 2005;17:1247–1253. DOI: https://doi.org/10.1097/00042737-200511000-00016.
- Ferro E, Capra A, Zirilli G, et al. FTL c.-168G > C mutation in hereditary hyperferritinemia cataract syndrome: a new Italian family. Pediatr Develop Pathol. 2018;21. DOI: https://doi.org/10.1177/1093526618755200.
- Feys J, Nodarian M, Aygalenq P, et al. Hereditary hyperferritinemia syndrome and cataract. J Fr Ophtalmol. 2001;24:847–850.
- Fritsche-Polanz R, Wallner M, Cohen G, et al. Granulocyte function in patients with L-ferritin iron-responsive element (IRE) 39C -> T-positive hereditary hyperferritinaemia-cataract syndrome. Eur J Clin Investig. 2004;34:701–708. DOI: https://doi.org/10.1111/j.1365-2362.2004.01408.x.
- Garderet L, Hermelin B, Gorin N, et al. Hereditary hyperferritinemia-cataract syndrome: a novel mutation in the iron-responsive element of the L-ferritin gene in a French family. Am J Med. 2004;117:138–139. DOI: https://doi.org/10.1016/j.amjmed.2004.02.033.
- Giansily M, Beaumont C, Desveaux C, et al. Denaturing gradient gel electrophoresis screening for mutations in the hereditary hyperferritinaemia cataract syndrome. Br J Haematol. 2001;112:51–54. DOI: https://doi.org/10.1046/j.1365-2141.2001.02513.x.
- Girelli D, Corrocher R, Bisceglia L, et al. Hereditary hyperferritinemia-cataract syndrome caused by a 29-base pair deletion in the iron responsive element of ferritin L-subunit gene. Blood. 1997;90:2084–2088.
- Gonzalez-Huerta L, Ramirez-Sanchez V, Rivera-Vega M, et al. A family with hereditary hyperferritinaemia cataract syndrome: evidence of incomplete penetrance and clinical heterogeneity. Br J Haematol. 2008;143:596–598. DOI: https://doi.org/10.1111/j.1365-2141.2008.07345.x.
- Hetet G, Devaux I, Soufir N, et al. Molecular analyses of patients with hyperferritinemia and normal serum iron values reveal both L ferritin IRE and 3 new ferroportin (SLC11A3) mutations. Blood. 2003;102:1904–1910. DOI: https://doi.org/10.1182/blood-2003-02-0439.
- Ladero J, Balas A, García-Sánchez F, et al. Hereditary hyperferritinemia-cataract syndrome. Study of a new family in Spain. Rev Esp Enferm Dig. 2004;96:507-9–510-1. DOI: https://doi.org/10.4321/S1130-01082004000700008.
- Lenzhofer M, Schroedl F, Trost A, et al. Aqueous humor ferritin in hereditary hyperferritinemia cataract syndrome. Optom Vis Sci. 2015;92. DOI: https://doi.org/10.1097/OPX.0000000000000544.
- Maccora K, Souzeau E, Ruddle J, et al. Simultaneous presentation of hereditary hyperferritinaemia cataract syndrome and hereditary haemochromatosis. Clin Exp Ophthalmol. 2018;46. DOI: https://doi.org/10.1111/ceo.13318.
- Martín D, Bravo A, Balas A. Hereditary hyperferritinemia and cataract syndrome: a de novo mutation. An Pediatr. 2008;68:408–410.
- Martin M, Fargion S, Brissot P, et al. A point mutation in the bulge of the iron-responsive element of the L ferritin gene in two families with hereditary hyperferritinaemia–cataract syndrome. Blood. 1998;91:319–323. DOI: https://doi.org/10.1182/blood.V91.1.319.
- Mattila R-M, Sainio A, Järveläinen M, et al. A novel double nucleotide variant in the ferritin-L iron-responsive element in a Finnish patient with hereditary hyperferritinaemia-cataract syndrome. Acta Ophthalmol. 2017;96. DOI: https://doi.org/10.1111/aos.13492.
- Meneses F, Schnabel B, Silva I, et al. Identification of the mutations associated with hereditary hyperferritinemia cataract syndrome and hemochromatosis in a Brazilian family. Clin Genet. 2011;79:189–192. DOI: https://doi.org/10.1111/j.1399-0004.2010.01517.x.
- Mumford AD, Cree I, Arnold J, et al. The lens in hereditary hyperferritinaemia cataract syndrome contains crystalline deposits of L-ferritin. Br J Ophthalmol. 2000;84:697–700. DOI: https://doi.org/10.1136/bjo.84.7.697.
- Papanikolaou G, Chandrinou H, Bouzas E, et al. Hereditary hyperferritinemia cataract syndrome in three unrelated families of western Greek origin caused by the C39 > G mutation of L-ferritin IRE. Blood Cells Mol Dis. 2006;36:33–40. DOI: https://doi.org/10.1016/j.bcmd.2005.10.003.
- Perez de Nanclares G, Castaño L, Martul P, et al. Molecular analysis of hereditary hyperferritinemia-cataract syndrome in a large Basque family. J Pediatr Endocrinol Metab. 2001;14:295–300. DOI: https://doi.org/10.1515/JPEM.2001.14.3.295.
- Perruccio K, Arcioni F, Cerri C, et al. The hereditary hyperferritinemia-cataract syndrome in 2 Italian families. Case Rep Pediatr. 2013;2013:806034. DOI: https://doi.org/10.1155/2013/806034.
- Phillips J, Warby C, Kushner J. Identification of a novel mutation in the L-ferritin IRE leading to hereditary hyperferritinemia-cataract syndrome. Am J Med Genet Part A. 2005;134A:77–79. DOI: https://doi.org/10.1002/ajmg.a.30425.
- Rochow N, Bachmaier N, Tost F, et al. The case of a 1-year -old girl with hereditary hyperferritinemia cataract syndrome. Pediatr Hematol Oncol. 2009;26:136–141. DOI: https://doi.org/10.1080/08880010902754842.
- Rüfer A, Howell J, Lange A, et al. Hereditary hyperferritinemia-cataract syndrome (HHCS) presenting with iron deficiency anemia associated with a new mutation in the iron responsive element of the L ferritin gene in a Swiss family. Eur J Haematol. 2011;87:274–278. DOI: https://doi.org/10.1111/j.1600-0609.2011.01607.x.
- Serra M, Longo F, Roetto A, et al. A child with hyperferritinemia: case report. Ital J Pediatr. 2011;37:20. DOI: https://doi.org/10.1186/1824-7288-37-20.
- Shekunov J, de Groen PC, Lindor NM, et al. Hereditary hyperferritinemia-cataract syndrome in two large multigenerational American families. J Am Assoc Pediatr Ophthalmol Strabismus. 2011;15:356–361. DOI: https://doi.org/10.1016/j.jaapos.2011.03.020.
- Simsek S, Nanayakkara P, Keek J, et al. Two Dutch families with hereditary hyperferritinaemia-cataract syndrome and heterozygosity for an HFE-related haemochromatosis gene mutation. Neth J Med. 2003;61:291–295.
- Vanita V, Hejtmancik J, Hennies H, et al. Sutural cataract associated with a mutation in the ferritin light chain gene (FTL) in a family of Indian origin. Mol Vis. 2006;12:93–99.
- Volkmann M, Richter R, Herrmann T, et al. Hereditary hyperferritinaemia-cataract syndrome (HHCS) – an underestimated condition: ferritin light chain variant spectrum in German families. Clin Chem Lab Med. 2019;57; DOI: https://doi.org/10.1515/cclm-2018-1354.
- Volkmann M, Schiff J, Hör M, et al. Pair of siblings of Italian ethnicity with hyperferritinemia and cataract. Internist (Berl). 2000;41:381–384.
- Xu M, Zhao X, Sun F, et al. A case of iron deficiency anemia with extremely hyperferritinemia responds well to oral iron: the first identified hereditary hyperferritinemia cataract syndrome in China. Ann Hematol. 2020. DOI: https://doi.org/10.1007/s00277-020-04085-4.
- Yazar S, Franchina M, Craig J, et al. Ferritin light chain gene mutation in a large Australian family with hereditary hyperferritinemia-cataract syndrome. Ophthalmic Genet. 2016;38:1–4. DOI: https://doi.org/10.3109/13816810.2016.1164195.
- Yin D, Kulhalli V, Walker A. Raised serum ferritin concentration in hereditary hyperferritinemia cataract syndrome is not a marker for iron overload. Hepatology. 2014;59. DOI: https://doi.org/10.1002/hep.26681.
- Brooks D, Manova-Todorova K, Farmer J, et al. Ferritin crystal cataracts in hereditary hyperferritinemia cataract syndrome. Investig Ophthalmol Vis Sci. 2002;43:1121–1126.
- Nos F, Hernandez G, Ferrer-Cortès X, et al. Hereditary hyperferritinemia cataract syndrome: ferritin L gene and physiopathology behind the disease—report of new cases. Int J Mol Sci. 2021;22:5451. DOI: https://doi.org/10.3390/ijms22115451.
- Van de Sompele S, Pécheux L, Couso J, et al. Functional characterization of a novel non-coding mutation “ghent + 49A > G” in the iron-responsive element of L-ferritin causing hereditary hyperferritinaemia-cataract syndrome. Sci Rep. 2017;7(1):18025. DOI: https://doi.org/10.1038/s41598-017-18326-6.
- Ferrari F, Foglieni B, Arosio P, et al. Microelectronic DNA chip for hereditary hyperferritinemia cataract syndrome, a model for large-scale analysis of disorders of iron metabolism. Hum Mutat. 2006;27:201–208. DOI: https://doi.org/10.1002/humu.20294.
- Faniello MC, Di Sanzo M, Quaresima B, et al. Bilateral cataract in a subject carrying a C to A transition in the L ferritin promoter region. Clin Biochem. 2009;42:911–914.
- Durupt S, Durieu I, Salles B, et al. A potential etiology of elevated ferritin: hyperferritinemia-cataract syndrome. Rev Med Interne. 2001;22:83–85.
- García Erce JA, Cortés T, Cremonesi L, et al. Hyperferritinemia-cataract syndrome associated to the HFE gene mutation. two new Spanish families and a new mutation (A37T: “zaragoza”). Med Clin. 2006;127:55–58.
- Garber I, Pudek M. A novel deletion in the iron-response element of the L-ferritin gene, causing hyperferritinaemia cataract syndrome. Ann Clin Biochem. 2014;51:710–713.
- Cadenas B, Fita-Torró J, Bermúdez-Cortés M, et al. L-Ferritin: One gene, five diseases; from hereditary hyperferritinemia to hypoferritinemia-report of New cases. Pharmaceuticals. 2019;12:17.
- Muñoz-Muñoz J, Cuadrado-Grande N, Moreno-Carralero MI, et al. Hereditary hyperferritinemia cataract syndrome in four patients with mutations in the IRE of the FTL gene. Clin Genet. 2013;83:491–493.