ABSTRACT
Objectives
Corticosteroid is first-line therapy in immune thrombocytopenia. However, nearly 30% of patients appear in steroid-resistance. Our research analyses the relevant indicators of patients and develops a risk prediction model to predict the poor response to steroid-therapy in ITP patients.
Methods
We collected data from 111 ITP patients admitted to Xiamen University Zhongshan Hospital from 2013 to 2019 as the training cohort and 65 ITP patients during 2019–2020 as the external validation cohort. Screening significant factors(P < 0.05) in univariate analysis, and further identified to be independent variables in multivariable logistic regression analysis. Incorporated the significant risk factors in and presented them with a nomogram based on independent risk predictors. The nomogram was assessed by receiver operating characteristics curves and decision curve analysis.
Results
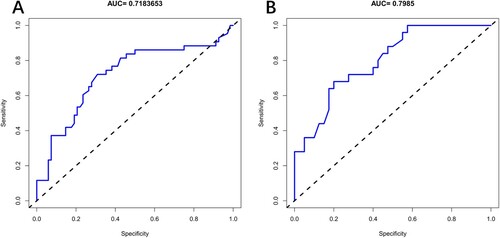
We constructed a steroid-resistance prediction model based on the potential predictors including age, serum ferritin and expression of HBsAg. As a result, based on the area under the ROC curves, the training cohort (AUC: 0.718, 95% CI: 0.615–0.821) and the external validation cohort (AUC:0.799,95%CI:0.692–0.905), which displayed good discrimination. The decision curve showed that predicting the steroid-refractory risk in ITP patients using this nomogram with a range of the threshold probability between >16% and <70%. The nomogram appears good performance in predicting steroid-refractory ITP patients.
Conclusion
Prediction model shows that elder patients with a high level of ferritin and positive expression of HBsAg may appear a high possibility of steroid-resistance. For these patients, TPO-RAs can be considered to help patients to get better treatment effects and develop a better health-related quality of life.
1. Introduction
Immune thrombocytopenia (ITP) is characterized by immune-mediated peripheral platelet destruction and impaired platelet production with a consequent increased risk of bleeding. Recommended first-line treatment of symptomatic ITP is glucocorticoids, but the majority of adult patients relapse within the first year of treatment and require additional therapy [Citation1–5]. A study shows that 98% of patients with corticosteroid exposure experienced one or more side events, and 38% of patients need to stop or reduce corticosteroid therapy [Citation6]. Biomarkers are needed to identify steroid-resistant ITP patients before prolonged courses of systemic steroids, which can lead to serious adverse events and worsen the health-related quality of life (HRQoL), and allow the early introduction of alternative therapy before bleeding symptoms occurs.
This research aims to develop a new prediction model to evaluate whether ITP patients are at high-risk of corticosteroid resistance, and help clinicians to choose better therapy.
2. Materials and methods
2.1. Patients
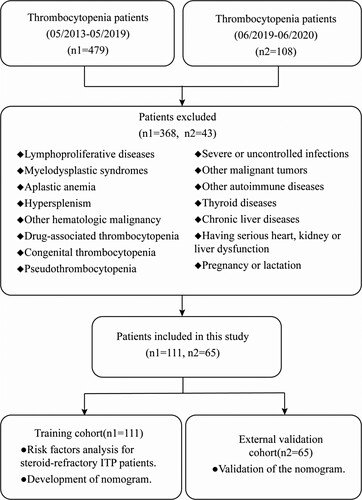
We comprehensively collected data from 111 ITP patients admitted to Xiamen University Zhongshan Hospital from May 2013 to May 2019 as a training cohort and 65 ITP patients during June 2019 to June 2020 as an external validation cohort. All patients met the Consensus of Chinese experts on the diagnosis and treatment of adult primary immune thrombocytopenia (version 2016). Baseline clinicopathologic data, including age, gender, serum ferritin(SF), C-creative protein(CRP), erythrocyte sedimentation rate(ESR), procalcitonin(PCT), albumin, globulin, immunoglobulin G(IgG), immunoglobulin M(IgM), immunoglobulin A(IgA), rheumatic related antibody, Epstein- Barr Virus deoxyribonucleic acid(EBV-DNA), Hepatitis B surface antigen(HBsAg). Patients received standard corticosteroids therapy doses (eg, prednisone1 mg/kg/d, dexamethasone 40 mg/d×4) then gradually reduced the doses. For 3–6 months treatment, the patients still needed a large dose (prednisone 15 mg/d) or more to keep platelet count >30 × 109 /L or no response were defined as the steroid-resistance group and others as the non-steroid-resistance group. We defined rheumatism related antibody such as the positive expression of anti-Sjögren’s syndrome A/B antibody (SSA/B) and so on while cannot meet the diagnostic criteria for rheumatism related diseases as the positive expression of rheumatism related antibody. Patients with positive expression of HBsAg, and the absence of HBeAg and presence of anti-HBe, undetectable or low levels of HBV DNA in PCR-based assays, with normal ALT levels called the inactive HBsAg carrier state [Citation7–9]. Only hepatitis B virus carriers are enrolled in our study, and the Hepatic dysfunction HBV patients had excluded.
This research protocol was conducted following the Declaration of Helsinki and had been approved by the Ethics Committee of Xiamen University Zhongshan Hospital. We reviewed and collected the relevant medical records and follow-up data after the patient’s informed consent.
2.2. Statistical analysis
All of the data were analyzed using R software (version 3.6.3; https://www.R-project.org). The significance level for all of the statistical tests was set at 0.05. All statistical significance levels were two-sided.
Patient characteristics and factors were analyzed using student’s t-test and chi-square tests. Ages are given as the medians with ranges, other variables are expressed as count (%), and performed univariate analysis to examine the difference between the steroid-resistance group and the non-steroid-resistance group. Apply binary logistic regression modeling technique to analyze risk factors for steroid-refractory ITP patients. Variables that had a p-value of < 0.05 in univariate analysis were selected into the multivariable logistic analysis to get further independent risk factors. In the multivariable logistic analysis, variables with a p-value of < 0.05 were identified to be the independent risk factors automatically and finally selected into the final model. Regression coefficients were used to generate prognostic nomograms. Model discrimination was measured quantitatively with the concordance index. Internal validation was performed using 1000 bootstrap resampling to quantify the overfitting of our modeling strategy and predict the future performance of the model. 65 new patients we collected were used as the external validation cohort to evaluate the model’s practicality.
3. Results
3.1. Patient characteristics
We analyzed 176 ITP patients admitted to Xiamen University Zhongshan Hospital from 2013 to 2020 and we divided the patients into a training cohort (n1 = 111) and an external validation cohort (n2 = 65) (). The characteristics of patients in the training cohort and the external validation cohort are summarized in .
Table 1. Characteristics of ITP patients in training and external validation cohort.
3.2. Predictors for steroid-refractory ITP patients
A total of 43 in 111 ITP cases of training cohort appeared steroid-resistance (38.7%). The univariate logistic regression analyses showed eight variables (P < 0.05) were significantly correlated with steroid-refractory ITP patients () including age, CRP, globulin, IgG, IgM, IgA, serum ferritin and HBsAg.
Table 2. Risk factors of steroid-refractory ITP patients in the training and external validation cohort.
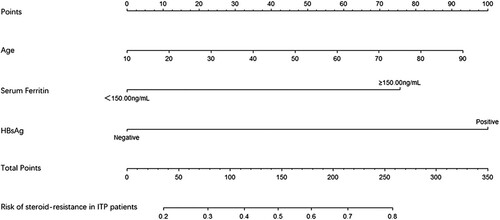
To explore the independent risk factors for steroid-refractory ITP patients, we performed a multivariable analysis based on the results of the univariate analysis. Eight risk factors aforementioned were included in the logistic regression model. Finally, age (odds ratio, OR 0.973, 95% CI, 0.951–0.995), high level of serum ferritin (OR 2.570, 95% CI,1.051–6.286), and the positive expression of HBsAg (OR 3.482, 95%CI,1.492–8.126) were identified as risk factors compared to non-steroid-refractory ITP patients ().
Table 3. Multivariate logistic regression analysis for steroid-resistance in ITP patients.
3.3. Development of the prediction model
The above three independent predictors of steroid-refractory ITP patients were integrated into a steroid-refractory ITP patients risk estimation nomogram ().
3.4. Apparent performance and validation of the nomogram
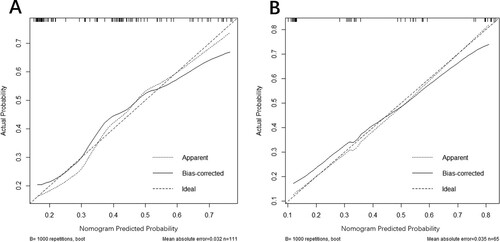
The calibration curves of the nomogram for predicting steroid-resistance in ITP patients appeared in good agreement in the training and the external validation cohort (). We constructed receiver operating characteristic curves (ROC) for the prediction model including the training and the external validation cohort (). Based on the area under ROC curves (AUC), the training cohort (AUC: 0.718, 95% CI: 0.615–0.821) and the external validation cohort (AUC:0.799, 95%CI:0.692–0.905), which suggested the model has a good discrimination and prediction capability in predicting the steroid-resistance in ITP patients.
Figure 3. Calibration plots of the nomogram for predicting steroid-resistance in ITP patients. (A) Calibration plots of the nomogram for the training cohort. (B) Calibration plots of the nomogram for the external validation cohort. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line stands for the performance of the nomogram, of which a closer fit to the diagonal dotted line represents a better prediction of the nomogram.
3.5. Clinical use
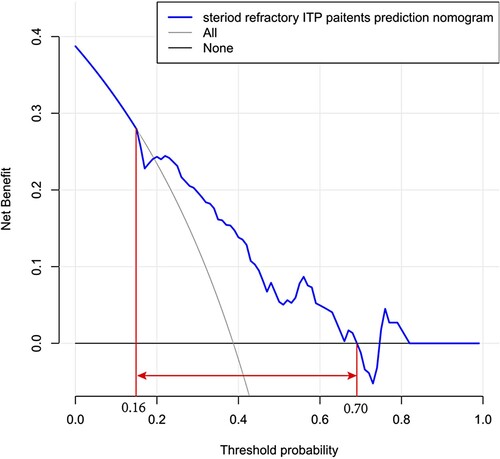
Decision Curve Analysis (DCA) was performed in , which showed that predicting the steroid-refractory risk in ITP patients using this nomogram would be better than other patients with a range of the threshold about between >16% and <70%.
Figure 5. Decision curve analysis for steroid-refractory ITP patients. The y-axis represents the net benefit, and the x-axis represents the steroid-refractory threshold probability in ITP patients. The blue line represents the prediction nomogram of steroid-refractory ITP patients. The gray line represents the assumption that all patients were steroid-resistant. The black line represents the assumption that no patients were steroid-resistant. The decision curve showed that predicting the steroid-refractory risk in ITP patients using this nomogram with a range of the threshold probability between >16% and <70%.
4. Discussion
Our research found that age, high level of serum ferritin and the positive expression of HBsAg were three risk factors of steroid-refractory ITP patients. We further constructed a steroid-resistance prediction model. The model was validated by the external validation cohort and performed by a nomogram. As a result, based on the area under the receiver operating characteristic curves (AUC), the training cohort (AUC: 0.718, 95% CI: 0.615–0.821) and the external validation cohort (AUC:0.799, 95%CI:0.692–0.905), which displayed good discrimination. Based on DCA, the nomogram displayed a comparable result to steroid-resistance rate in elder ITP patients with high level of serum ferritin level and positive expression of HBsAg. The nomogram appears to a good performance in predicting the steroid-refractory ITP patients. It can help clinicians identify steroid-refractory ITP patients early and choose a better therapy.
Besides iron overload, frequent blood transfusion, hepatocellular disease and malignant tumor, ferritin elevation can be reactive, especially in inflammatory disease, which indicates the activation of macrophages [Citation10,Citation11]. Acute myeloid leukemia (AML) patients with a high level of serum ferritin overall survival(OS) and recurrence-free survival(RFS) were significantly reduced [Citation12]. Patients with a high level of serum ferritin in nasopharyngeal carcinoma (NPC), multiple sclerosis (MS), acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF), and many liver diseases tend to suffer disease progression and develop a poor prognosis [Citation13–17]. As for treatment, series of studies show that in liver transplantation, adult-onset still’s disease (AOSD), macrophage activation syndrome (MAS) and acute lupus hemophagocytic syndrome (ALHS), patients with a high level of serum ferritin have a high mortality rate and a poor response to corticosteroid therapy [Citation16,Citation18–22]. Eltrombopag, a TPO receptor agonist, can promote the proliferation of bone marrow megakaryocytes and increase megakaryocytes to form platelets [Citation23]. Moreover, eltrombopag is also a powerful iron chelator, which decreases the total cellular iron and ferritin in different cell lines [Citation24]. A study of small samples found that a majority of children ITP patients appeared to therapy-induced iron deficiency with the treatment of eltrombopag [Citation25]. Studies pointed out that eltrombopag and dexamethasone have synergism, which reduces the level of serum ferritin while raising platelets [Citation24,Citation26,Citation27]. In our center, some ITP patients with a high level of serum ferritin get a longer period of remission and decrement of serum ferritin for the treatment of eltrombopag combined with high-dose dexamethasone. This requires further prospective study to verify.
Although most ITP patients are primary, hepatitis viruses can cause ITP [Citation28–31], including hepatitis A, hepatitis B, hepatitis C, hepatitis E, which can cause immune dysfunction and affects the function of megakaryocyte [Citation32–37]. Series of studies indicated that the sensitivity of HCV-ITP patients to corticosteroids was significantly lower than the one without HCV and was correlated with HCV level [Citation38–40]. Different from foreign countries, the most common form of viral hepatitis in China is hepatitis B. Our study found that ITP patients with positive expression of HBsAg appear poor responses to corticosteroid. There are occult hepatitis B infection(OBI) cases that occur the reactivation of hepatitis B virus and lead to hepatic dysfunction when using rituximab, corticosteroid and other immunity inhibitors [Citation28,Citation41,Citation42]. The treatment of ITP patients with hepatitis B should be specific but there is no consensus so far. Studies pointed out that eltrombopag can increase the counts of platelets and had been approved for the treatment of hepatitis C virus-associated immune thrombocytopenia(HCV-ITP) [Citation43–45]. However, there are few studies about the therapy in HBV infected ITP patients, and require further discussion.
Our research found that age was a risk factor for steroid-resistance. For elder patients, glucocorticoid therapy, rituximab and other immune suppressive drugs tend to have more side-effects [Citation46–48]. As for splenectomy therapy may grow further immunosuppressive and increases the chance of severe infection [Citation46,Citation49,Citation50]. In this case, eltrombopag may become a safe and effective choice [Citation51–53].
5. Conclusions
Our prediction model shows that elder patients with a high level of ferritin and positive expression of HBsAg may appear a high possibility of steroid-resistance. It can help clinicians identify steroid-refractory ITP patients early. For these patients, TPO-RAs combined with first-line corticosteroid therapy can be considered to help patients to get better treatment effects and develop a better health-related quality of life.
Acknowledgements
The authors thank CHUAN HU and JINSHUI PAN for their data statistics support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barsam SJ, Psaila B, Forestier M, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011 May;117(21):5723–5732.
- Audia S, Mahevas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017 Jun;16(6):620–632.
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017 May;129(21):2829–2835.
- Piel-Julian ML, Mahevas M, Germain J, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemostasis. 2018 Sep;16(9):1830–1842.
- Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019 Sep;381(10):945–955.
- Brown TM, Horblyuk RV, Grotzinger KM, et al. Patient-reported treatment burden of chronic immune thrombocytopenia therapies. BMC Blood Disord. 2012 Mar 22;12:2.
- Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2008 Aug;2(4):553–562.
- Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J. 2005 Sep 28;2:82. DOI:https://doi.org/10.1186/1743-422X-2-82.
- Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013 Jul 20;10:239.
- Adams PC, Barton JC. A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation. J Hepatol. 2011 Aug;55(2):453–458.
- Efstathiou SP, Pefanis AV, Tsiakou AG, et al. Fever of unknown origin: discrimination between infectious and non-infectious causes. Eur J Intern Med. 2010 Apr;21(2):137–143.
- Ihlow J, Gross S, Sick A, et al. AML: high serum ferritin at initial diagnosis has a negative impact on long-term survival. Leuk Lymphoma. 2019 Jan;60(1):69–77.
- Chen XQ, Long XF, Liang ZG, et al. Higher N stage and serum ferritin, but lower serum albumin levels are associated with distant metastasis and poor survival in patients with nasopharyngeal carcinoma following intensity-modulated radiotherapy. Oncotarget. 2017 Sep;8(42):73177–73186.
- Ferreira KPZ, Oliveira SR, Kallaur AP, et al. Disease progression and oxidative stress are associated with higher serum ferritin levels in patients with multiple sclerosis. J Neurol Sci. 2017 Feb;373:236–241.
- Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J. 2018 Aug;12(8):2378–2389.
- Taubert R, Hardtke-Wolenski M, Noyan F, et al. Hyperferritinemia and hypergammaglobulinemia predict the treatment response to standard therapy in autoimmune hepatitis. PLoS One. 2017 Jun;12(6):e0179074.
- Datz C, Felder TK, Niederseer D, et al. Iron homeostasis in the metabolic syndrome. Eur J Clin Invest. 2013 Feb;43(2):215–224.
- Weismüller TJ, Kirchner GI, Scherer MN, et al. Serum ferritin concentration and transferrin saturation before liver transplantation predict decreased long-term recipient survival. Hepatology. 2011 Dec;54(6):2114–2124.
- Walker NM, Stuart KA, Ryan RJ, et al. Serum ferritin concentration predicts mortality in patients awaiting liver transplantation. Hepatology. 2010 May;51(5):1683–1691.
- Okamoto O, Oishi M, Fujiwara S. Steroid-resistant adult-onset still's disease which showed a quick response to methotrexate. J Dermatol. 2008 Feb;35(2):106–110.
- Ruscitti P, Cipriani P, Di Benedetto P, et al. H-ferritin and proinflammatory cytokines are increased in the bone marrow of patients affected by macrophage activation syndrome. Clin Exp Immunol. 2018 Feb;191(2):220–228.
- Takahashi H, Tsuboi H, Kurata I, et al. Predictors of the response to treatment in acute lupus hemophagocytic syndrome. Lupus. 2015 Jun;24(7):659–668.
- Di Buduo CA, Currao M, Pecci A, et al. Revealing eltrombopag's promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica. 2016 Dec;101(12):1479–1488.
- Vlachodimitropoulou E, Chen YL, Garbowski M, et al. Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood. 2017 Oct;130(17):1923–1933.
- Lambert MP, Witmer CM, Kwiatkowski JL. Therapy induced iron deficiency in children treated with eltrombopag for immune thrombocytopenia. Am J Hematol. 2017 Jun;92(6):E88–E91.
- Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012 Jul;120(2):386–394.
- Kalota A, Selak MA, Garcia-Cid LA, et al. Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival. PLoS One. 2015 Apr;10(4):e0126691.
- Allegra A, Penna G, Alonci A, et al. Exacerbation of chronic idiopathic thrombocytopenic purpura following reactivation of an occult hepatitis B. Medical Oncology. 2010 Sep;27(3):912–914.
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009 Jun;113(26):6511–6521.
- Neunert C, Lim W, Crowther M, et al. The American society of hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011 Apr 21;117(16):4190–4207.
- Kim EB, Kim DS, Park SJ, et al. Hepatitis B virus reactivation in a surface antigen-negative and antibody-positive patient after rituximab plus CHOP chemotherapy. Cancer Res Treat. 2008 Mar;40(1):36–38.
- Michalska Z, Stalke P, Witczak-Malinowska K, et al. Autoimmune reactions in HBV and HCV. Med Sci Monit. 2001 May;7(Suppl 1):175–180.
- Weksler BB. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007 Nov;26(Suppl 1):13–19.
- Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009 Jan;46(1 Suppl 2):S2–14.
- Lebano R, Rosato V, Masarone M, et al. The effect of antiviral therapy on hepatitis C virus-related thrombocytopenia: a case report. BMC Res Notes. 2014 Jan 24;7:59.
- Hamaia S, Li C, Allain JP. The dynamics of hepatitis C virus binding to platelets and 2 mononuclear cell lines. Blood. 2001 Oct 15;98(8):2293–2300.
- Chiao EY, Engels EA, Kramer JR, et al. Risk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infection. Arch Intern Med. 2009 Feb 23;169(4):357–363.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005 Jun;38(4):295–301.
- Geier MR, Geier DA. A case-series of adverse events, positive re-challenge of symptoms, and events in identical twins following hepatitis B vaccination: analysis of the vaccine adverse event reporting system (VAERS) database and literature review. Clin Exp Rheumatol. 2004 Nov–Dec;22(6):749–755.
- He Q, Song XY, Huang YC, et al. Dexamethasone stimulates hepatitis B virus (HBV) replication through autophagy. Med Sci Monit. 2018 Jul;24:4617–4624.
- Lubel JS, Testro AG, Angus PW. Hepatitis B virus reactivation following immunosuppressive therapy: guidelines for prevention and management. Intern Med J. 2007 Oct;37(10):705–712.
- Lindh M, Uhnoo I, Blackberg J, et al. Treatment of chronic hepatitis B infection: an update of Swedish recommendations. Scand J Infect Dis. 2008;40(6–7):436–450.
- Maan R, Veldt BJ, Janssen HL. Eltrombopag for thrombocytopenic patients with chronic HCV infection. Gastroenterology. 2014 Jul;147(1):254–255..
- Kayar Y, Danalioglu A, Kocaman O, et al. Eltrombopag: how secure in triple therapy of HCV? Acta Gastroenterol Belg. 2015 Dec;78(4):445–446.
- Bussel JB, Mahmud SN, Brigstocke SL, et al. Tapering eltrombopag in patients with chronic ITP: How successful is this and in whom does it work? Blood. 2015;126(23):1054.
- Chang H, Tang TC, Hung YS, et al. Immune thrombocytopenia: effectiveness of frontline steroids and comparison of azathioprine, splenectomy, and rituximab as second-line treatment. Eur J Haematol. 2018 Oct;101(4):549–555.
- Dou X, Yang R. Current and emerging treatments for immune thrombocytopenia. Expert Rev Hematol. 2019 Sep;12(9):723–732.
- Mithoowani S, Arnold DM. First-line therapy for immune thrombocytopenia. Hamostaseologie. 2019 Aug;39(3):259–265.
- Ahmed R, Devasia AJ, Viswabandya A, et al. Long-term outcome following splenectomy for chronic and persistent immune thrombocytopenia (ITP) in adults and children: splenectomy in ITP. Ann Hematol. 2016 Sep;95(9):1429–1434.
- Kado R, McCune WJ. Treatment of primary and secondary immune thrombocytopenia. Curr Opin Rheumatol. 2019 May;31(3):213–222.
- Lucchini E, Fanin R, Cooper N, et al. Management of immune thrombocytopenia in elderly patients. Eur J Intern Med. 2018 Dec;58:70–76.
- González-López TJ, Sánchez-González B, Jarque I, et al. Use of eltrombopag for patients 65 years old or older with immune thrombocytopenia. Eur J Haematol. 2020 Mar;104(3):259–270.
- Uto Y, Fujiwara S, Arai N, et al. Age and bone marrow cellularity are associated with response to eltrombopag in Japanese adult immune thrombocytopenia patients: a retrospective single-center study. Rinsho Byori. 2015 May;63(5):548–556.