ABSTRACT
Objectives
In the absence of head-to-head comparisons across relapsed/refractory multiple myeloma (RRMM) treatments following the approval of the oral proteasome inhibitor ixazomib, in combination with lenalidomide and dexamethasone (IRd), we conducted an indirect comparison of the efficacy of IRd relative to several RRMM therapies using Bayesian fixed-effects network meta-analysis (NMA) models.
Methods
Data for the NMA were obtained through a systematic literature review (conducted in June 2020), which identified randomized controlled trials (base case) and observational studies (extended network analysis) reporting overall survival (OS), progression-free survival (PFS), and overall response rate (ORR).
Results
In the base case, IRd was associated with a significantly longer PFS than lenalidomide and dexamethasone (Rd), bortezomib monotherapy (V), dexamethasone (Dex), and pomalidomide and dexamethasone (Pom-dex), a significantly shorter PFS than daratumumab, lenalidomide, and dexamethasone (DRd), and a PFS comparable to elotuzumab, lenalidomide, and dexamethasone (ERd) and carfilzomib, lenalidomide, and dexamethasone (KRd). IRd was associated with a significantly longer OS than V, Dex, and Pom-dex, and an OS comparable to Rd, ERd, KRd, and DRd. The ORR of IRd was significantly higher than Rd, V, and Dex, significantly lower than KRd and DRd, and comparable to Pom-dex and ERd. The extended network analyses and sensitivity analyses were consistent with the base case.
Discussion
This NMA shows that IRd is relatively efficacious among RRMM treatments. Being an oral regimen, IRd is also convenient to manage.
Conclusion
IRd could be a preferable treatment option for many patients with RRMM, particularly those seeking an efficacious and convenient therapeutic option.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy associated with significant morbidity and mortality [Citation1–3]. The global incidence of MM has increased by 126% from 1990 to 2016 [Citation3]. Advancements in novel targeted therapies (e.g. proteasome inhibitors and immunomodulatory agents) have transformed MM from an untreatable condition to a chronic illness managed in an outpatient setting [Citation4]. To address the unmet clinical needs of patients with relapsed/refractory (RR) MM, the spectrum of novel agents has expanded considerably in the past decade [Citation5,Citation6].
Given the rapidly expanding treatment landscape for RRMM, there is a need for healthcare stakeholders to compare the relative efficacy of therapies to inform treatment decisions. In the absence of head-to-head comparisons among available therapies for RRMM, indirect treatment comparisons can offer decision makers valuable insight. Although prior network meta-analysis (NMA) research in the RRMM setting has been conducted [Citation7–11], newer agents have since been approved; thus, assessment of the more contemporary treatment landscape is warranted. Furthermore, no prior NMAs in this setting have incorporated observational studies, which allows the comparisons of a broader spectrum of treatments and may provide supportive evidence to NMAs generated based on randomized controlled trials (RCTs) alone. This study aimed to assess the relative efficacy of the first and only oral proteasome inhibitor-based lenalidomide and dexamethasone (Rd) backbone triplet regimen, IRd, wherein ixazomib is the only oral proteasome inhibitor, with several combination and monotherapies approved for RRMM. A systematic literature review (SLR) was first conducted to identify clinical data to inform the indirect treatment comparisons. Bayesian NMAs were then conducted to estimate the relative efficacy of IRd versus other therapies in RCTs; extended network analyses including observational studies were also conducted.
Methods
Search strategy
Clinical trials and observational studies reporting on treatments for RRMM were identified following a systematic search of EMBASE, PubMed/Medline, and Ovid conducted on June 26, 2020. The search terms ‘multiple myeloma’, ‘relapse’, and ‘refractory’ were used in addition to keywords for specific therapies of interest (e.g. ‘bortezomib’, ‘lenalidomide’, ‘ixazomib’). The SLR followed the guidelines from the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [Citation12].
To supplement the SLR, a targeted review of relevant websites (the US Food and Drug Administration) and conferences (2020 American Society of Clinical Oncology [ASCO], 2020 European Hematology Association [EHA]), which were hosted after the June 2020 SLR search date, was conducted to ensure the most up-to-date and relevant studies were included.
Eligibility criteria
To be included in the present analysis, all studies were required to be peer-reviewed manuscripts or conference abstracts of multi-arm Phase II/III RCTs or observational studies that included patients ≥18 years with a diagnosis of RRMM with ≥1 prior line of therapy who received one of the following interventions: bortezomib, lenalidomide, and dexamethasone (VRd); elotuzumab, lenalidomide, and dexamethasone (ERd); carfilzomib, lenalidomide, and dexamethasone (KRd); daratumumab, lenalidomide, and dexamethasone (DRd); lenalidomide and dexamethasone (Rd); dexamethasone (Dex); pomalidomide and dexamethasone (Pom-dex); bortezomib monotherapy (V); bortezomib and dexamethasone (Vd); panobinostat, bortezomib, and dexamethasone (PVd); pomalidomide, bortezomib, and dexamethasone (PomVd); selinexor, bortezomib, and dexamethasone (SVd); daratumumab, bortezomib, and dexamethasone (DVd); carfilzomib and dexamethasone (Kd); carfilzomib, dexamethasone, and daratumumab (KdD); isatuximab, carfilzomib, and dexamethasone (IsaKd); ide-cel; bb2121; and BM. In addition, studies were required to have results on ≥1 of the following efficacy outcomes: overall survival (OS), progression-free survival (PFS), or overall response rate (ORR).
Statistical analysis
Network meta-analysis
In the NMA, the latest results of the TOURMALINE-MM1 trial (NCT01564537; final data cut-off for OS [median follow-up 85 months] and second interim analyses for PFS and ORR [median follow-up 23 months]) and the final results of the TOURMALINE-MM1 China Continuation study (median follow-up: OS, 20 months; PFS, 7 months) comparing IRd vs. Rd were used to perform an indirect comparison of IRd against other treatments of interest. All outcomes were analyzed with a fixed-effects Bayesian NMA using Markov Chain Monte Carlo simulation technique with up to 20,000 iterations. A fixed-effects NMA model was used due to sparse networks (few edges in the networks were supported by evidence from >1 study). Hazard ratios (HRs) for PFS and OS and odds ratios (ORs) for ORRs from included studies were used as inputs for the NMA. When PFS was not reported, time to progression (TTP) was used as a proxy. The comparative estimates were summarized using the posterior median HRs for PFS and OS, or ORs for ORR between IRd and other treatments, as well as the associated 95% credible intervals (CrIs).
All analyses were implemented using the statistical software R 3.0 and JAGS and OpenBUGS software packages. All codes were based on the 2011 NICE Decision Support Unit Technical Support Document 2 by Dias et al. [Citation13].
Analyses in the extended network and subgroups
The NMA base case analysis only included peer-reviewed RCTs. Additional analyses, based on an extended network including both RCTs and observational studies, were conducted to assess the applicability of the base case findings beyond the clinical trial setting.
Subgroup analyses were conducted using the extended network among patients with the high-risk cytogenetic subtype of MM (t(4;14) or t(14;16) or with del(17p)), patients with one prior therapy, and patients with 2+ prior therapies. PFS was evaluated in all subgroups and OS was evaluated in subgroups based on prior therapies only, since no OS data were available for the high-risk cytogenetics subgroup. Subgroup analyses were not conducted for ORR. In cases where studies presenting data for a specific subgroup could not be linked to the network, results from the overall population were used to allow for a comparison between treatment arms of interest.
Sensitivity analyses
As OS data from clinical trials often do not reflect the treatment effect of the intervention or control in isolation but include the treatment effect of subsequent therapies, they can be difficult to interpret without information on the subsequent therapy profile. Since patient-level data were available for the TOURMALINE-MM1 trial, we estimated the relative OS for IRd vs. Rd following adjustments for the effect of any subsequent therapy. Adjustments were made based on rank-preserving structural failure time models (RPSFTM) and inverse probability of censoring weighting (IPCW). The resulting adjusted OS HRs for IRd vs. Rd were then explored in sensitivity analyses within the NMA framework.
Results
Literature search
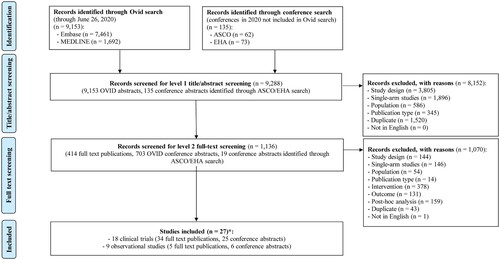
The PRISMA flow diagram summarizing the study selection process is presented in . Thirty-nine full-text articles and 31 conference abstracts were identified, including four manuscripts published after the completion of the SLR search [Citation14–17]. These publications spanned 27 studies − including 18 RCTs and nine observational studies. Multiple publications of the same RCT or observational study presenting on different data cut-offs or different subgroups were identified. Characteristics of each study included in the NMA are summarized in Supplemental Table 1.
Figure 1. PRISMA flowchart for study selection. ASCO, American Society of Clinical Oncology; EHA, European Hematology Association; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis. *Four full text publications for included studies were identified manually at a later date, after the completion of the Ovid search, and were incorporated into the SLR results.
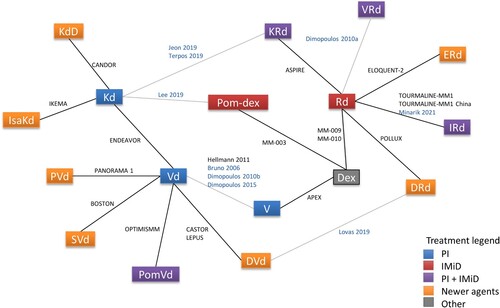
Comparator regimens included in the NMA were: VRd, ERd, KRd, DRd, Rd, Dex, Pom-dex, V, Vd, PVd, PomVd, SVd, DVd, Kd, KdD, and IsaKd. The network diagram of comparators and studies included in the NMA is illustrated in . Although ide-cel (bb2121) and BM were included in the search, no studies were identified as the trials are ongoing and data were not published at the time of the search.
Figure 2. Network diagram for all studies included in the NMA. IMiD, immunomodulatory drugs; NMA, network meta-analysis; PI, proteasome inhibitor.
Notes: Studies listed in blue font are observational and those listed in black font are clinical trials. Gray connecting lines indicate links dependent on observational studies (i.e. these links and network branches dependent on them only appear in the extended network analyses and not in the base case analyses).
PFS analyses
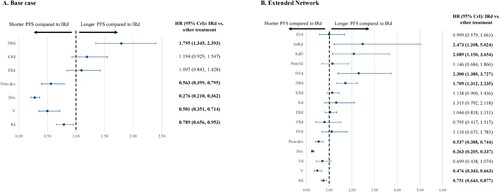
Base case NMA
Nine RCTs evaluating eight treatments were included in the base case NMA (Supplemental Figure 1A). IRd was associated with a significantly longer PFS compared to Rd (HR 0.789; 95% CrI [0.656-0.953]), V (0.501 [0.351-0.714]), Dex (0.276 [0.210-0.362]), and Pom-dex (0.563 [0.399-0.795]), but a significantly shorter PFS compared to DRd (1.795 [1.345-2.393]). IRd was not associated with any significant differences in PFS compared to ERd (1.097 [0.843-1.428]) or KRd (1.194 [0.929-1.547]) (A).
Extended NMA
Results from the extended network analysis for PFS included 21 studies (17 RCTs, four observational studies) that evaluated 17 treatments (Supplemental Figure 1A). The relative efficacy estimates when including observational data were similar to the RCT network. However, inclusion of observational studies also suggested statistically significantly longer PFS in DVd (2.300 [1.380-3.727]), KdD (2.089 [1.150-3.654]), and IsaKd (2.473 [1.208-5.024)]) compared to IRd (B).
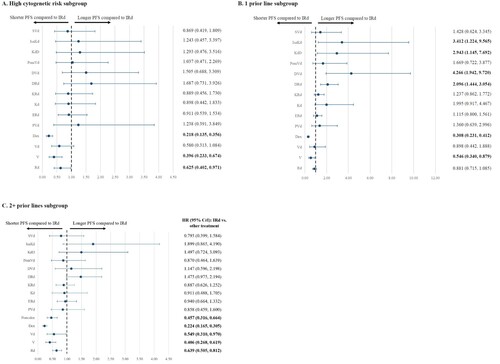
Subgroup analyses
The network diagrams for studies included in the subgroup analyses for PFS are illustrated in Supplemental Figures 1B-1D. Results from the subgroup analyses generally confirmed findings from the base case and extended NMA. For patients in the high-risk cytogenetics subgroup, a significant PFS benefit was found in IRd compared to Rd, V, and Dex. For the subgroup of patients with one prior line of therapy, IRd was associated with a significantly longer PFS when compared to Dex and V, but a significantly shorter PFS when compared to DRd, DVd, KdD, and IsaKd. For the subgroup of patients with 2+ prior lines of therapy, IRd was found to statistically significantly prolong PFS compared to Rd, V, Vd, Dex, and Pom-dex (A-4C).
OS analyses
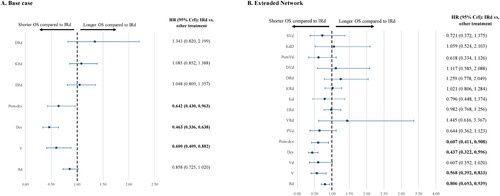
Base case NMA
Nine RCTs evaluating eight treatments were included in the base case NMA (Supplemental Figure 2A). Among the studies with OS data, IRd was associated with a significantly longer OS compared to V (HR 0.600; 95% CrI [0.409-0.882]), Dex (0.463 [0.336-0.638]), and Pom-dex (0.642 [0.430-0.963]) (A). No significant difference was found when comparing IRd vs. Rd (0.858 [0.725-1.020]), ERd (1.048 [0.809-1.357]), KRd (1.085 [0.852-1.388]), or DRd (1.343 [0.820-2.199]).
Extended NMA
OS results from the extended NMA included 20 studies (16 RCTs, four observational studies) comparing 16 treatments (Supplemental Figure 2A) and were found to be generally consistent with base case analysis. However, the treatment effect for IRd vs. Rd showed statistical significance (0.806 [0.693-0.939]). Additional comparisons were enabled for IRd vs. Vd (0.607 [0.352-1.020]), PVd (0.644 [0.362-1.123]), VRd (1.445 [0.616-3.367]), Kd (0.796 [0.448-1.374]), DVd (1.117 [0.585-2.088]), PomVd (0.618 [0.334-1.126]), KdD (1.059 [0.524-2.103]), and SVd (0.721 [0.372-1.375]) (B).
Subgroup analyses
The network diagrams for studies included in the subgroup analyses for OS are illustrated in Supplemental Figure 2B-2C. Among patients with one prior line of therapy, IRd was associated with a significantly longer OS compared to Dex. Among patients with 2+ prior lines of therapy, IRd was associated with a significantly longer OS compared to Rd, Dex, and Pom-dex (Supplemental Figure 3A-3B).
Sensitivity analyses
Following adjustment for receipt of subsequent therapies using the IPCW method on the TOURMALINE-MM1 IRd vs. Rd data, the HR for OS improved from 0.94 to 0.70 in favor of IRd. When using the IPCW-adjusted HR in the base case network, improvements in OS were observed in the sensitivity analyses (A), where significant OS benefit was found in IRd vs. V (0.490 [0.295-0.815]), Dex (0.377 [0.238-0.601]), and Pom-dex (0.523 [0.309-0.886]). In the analysis of the extended network, the above comparisons remained similar to base case and the OS statistically favored IRd over Rd (0.660 [0.514-0.849]), Vd (0.496 [0.278-0.866]), PVd (0.527 [0.286-0.953]), and PomVd (0.505 [0.267-0.953]) (B). Among patients with one prior line of therapy in the TOURMALINE-MM1 trial, the IPCW-adjusted HR reduced from 1.02 to 0.95, which translated to a significantly longer OS compared to Dex (0.513 [0.296-0.893]) in the NMA. Finally, when the IPCW-adjusted HR of 0.53 was used in place of the original estimate of 0.85 among patients with 2+ prior lines of therapy, IRd was associated with a significantly longer OS compared to V (0.443 [0.214-0.914]) in addition to Rd (0.530 [0.313-0.905]), Dex (0.288 [0.158-0.520]), and Pom-dex (0.399 [0.208-0.764]) (Supplemental Figure 4A-4B).
Figure 6. NMAs of OS (sensitivity analyses). CrI, credible interval; HR, hazard ratio; IPCW, inverse probability of censoring weighting; NMA, network meta-analysis; OS, overall survivial; RPSFTM, rank-preserving structural failure time models.
Note: Bolded font indicates that the results are statistically significant. IPCW- or RPSFTM-adjusted HR for OS from the TOURMALINE-MM1 trial was used in these sensitivity analyses.
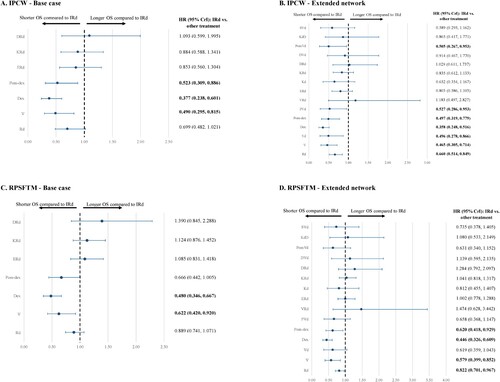
Similarly, the HRs for IRd vs. Rd reduced or remained comparable to the original estimates when the RPSFTM method was used to adjust for receipt of subsequent therapies in TOURMALINE-MM1 (RPSFTM-adjusted HR: overall: 0.89, one prior line: 1.03, 2+ prior lines: 0.68). When the RPSFTM-adjusted HRs were used in the NMA, results were similar to the main analyses. In the base case analysis, a significantly longer OS was observed for IRd compared to V (0.622 [0.420-0.920]) and Dex (0.480 [0.346-0.667]) (C). In the extended network analysis, these results remained consistent and IRd also demonstrated a statistical advantage in OS compared to Rd (0.822 [0.701-0.967]) and Pom-dex (0.620 [0.418-0.929]) (D). Among patients with one prior line of therapy, OS was significantly longer for IRd when compared to Dex (0.556 [0.388-0.794]), but significantly shorter when compared to DVd (3.461 [1.014-12.050]). Among patients with 2+ prior lines of therapy, IRd was associated with a significantly longer OS compared to Rd (0.680 [0.512-0.907]), Dex (0.369 [0.248-0.545]), and Pom-dex (0.512 [0.320-0.814]) (Supplemental Figure 4C-4D).
ORR analyses
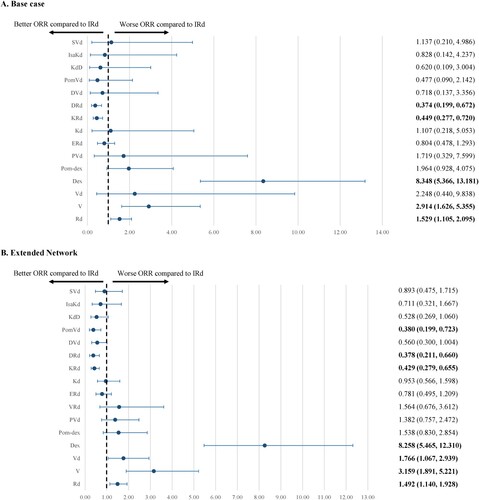
Base case NMA
Eighteen RCTs evaluating 16 treatments were included in the base case NMA (Supplemental Figure 5). IRd was associated with a significantly better ORR compared to Rd (OR 1.529; 95% CrI [1.105-2.095]), V (2.914 [1.626-5.355]), and Dex (8.348 [5.366-13.181]), but a significantly less favorable ORR compared to KRd (0.449 [0.277-0.720]) and DRd (0.374 [0.199-0.672]). IRd was not associated with any significant difference in ORR compared to Vd (2.248 [0.440-9.838]), Pom-Dex (1.964 [0.928-4.075]), PVd (1.719 [0.329-7.599]), ERd (0.804 [0.478-1.293]), Kd (1.107 [0.218-5.053]), DVd (0.718 [0.137-3.356]), PomVd (0.477 [0.090-2.142]), KdD (0.620 [0.109-3.004]), IsaKd (0.828 [0.142-4.237]), and SVd (1.137 [0.210-4.986]) ().
Extended NMA
The extended NMA of 27 studies (18 RCTs, nine observational studies) comparing 17 treatments (Supplemental Figure 5) confirmed the results from the base case NMA. IRd was associated with a significantly better ORR compared to Rd (1.492 [1.140-1.928]), V (3.159 [1.891-5.221]), Vd (1.766 [1.067-2.939]), and Dex (8.258 [5.465-12.310]), but a significantly less favorable ORR compared to KRd (0.429 [0.279-0.655]), DRd (0.378 [0.211-0.660]), and PomVd (0.380 [0.199-0.723]) ().
Discussion
In the absence of head-to-head trials, an NMA provides useful estimates of the relative efficacy of IRd and other therapies for RRMM. Networks based on RCT data estimated a significant benefit in PFS for IRd vs. Rd, V, Dex, and Pom-dex. However, PFS outcomes with IRd were less favorable than DRd. IRd was found to have similar PFS outcomes to ERd and KRd. Although IRd significantly prolonged OS when compared to V, Dex, and Pom-dex in the network based on RCT data, it did not differ significantly from Rd, ERd, KRd, and DRd when using the unadjusted data from TOURMALINE-MM1. For ORR, IRd was associated with a significantly better response rate relative to Rd, V, and Dex. However, KRd and DRd had significantly better ORR compared to IRd. ORR comparisons of IRd to Vd, Pom-dex, PVd, ERd, Kd, DVd, PomVd, KdD, IsaKd, and SVd did not significantly differ in the base case. Although K-based triplets had statistically better ORR, this did not translate to OS or PFS. In general, results from the extended network analyses confirmed the efficacy of IRd relative to additional therapies across a broader patient population than in clinical trials and allowed for inclusion of additional therapies. Additionally, subgroup analyses demonstrated IRd to be particularly efficacious among patients with high-risk cytogenic subtype and those with at least two prior therapies.
Prior NMA research has shown that in the RRMM setting, combination regimens that include an immunomodulatory backbone (e.g. lenalidomide) and anti-MM monoclonal antibodies (e.g. daratumumab) or proteasome inhibitors (e.g. ixazomib) may be an effective therapy [Citation7]. Additional studies have confirmed that DRd is a particularly effective regimen [Citation7–11]. The present analysis expands the literature by examining key areas that have yet to be evaluated. For example, by evaluating the base case networks of RCTs and incorporating observational studies in the extended networks, our data resulted in more complete and larger networks, which are vital to the precision of NMAs. Importantly, they allowed comparisons of a broader spectrum of treatments that can more appropriately reflect the heterogeneity of the MM patient population than clinical trials alone. Moreover, integration of observational studies provides real-world evidence regarding the risks and benefits of medical treatments [Citation18].
The SLR that informed the present analysis has a more recent cut-off point (June 26, 2020) relative to the other SLRs; the cut-off of the most recent SLR was only up to July 1, 2018 [Citation8]. Given the inclusion of observational studies in our networks and a more recent cut-off point for literature search, we were able to incorporate into our networks the following newer agents absent from prior NMAs: KdD, IsaKd, SVd, PomVd, and VRd. Our study did not include certain older regimens, such as thalidomide, thalidomide and dexamethasone, and bortezomib in combination with pegylated liposomal doxorubicin, which can be found in prior NMAs [Citation7,Citation8,Citation11]. A recently published critical appraisal of indirect comparisons and NMAs for MM criticized the lack of consideration of prior therapies or treatment duration across RCTs [Citation19]. The present NMA assessed subgroups based on the number of prior therapies as well as previously under-evaluated features such as high cytogenetic risk. Additionally, the present analysis included a wider range of efficacy outcomes than prior studies. For example, van Beurden-Tan et al. [Citation11] and Dhakal et al. [Citation8] focused on PFS only, whereas the present analysis encompassed networks for OS and ORR. Consequently, the present analysis provides greater insight regarding the comparative efficacy of various regimens for RRMM.
Although this study addresses an important knowledge gap regarding the comparative efficacy of RRMM therapies, the OS results may have been affected by the possibility of patient crossover. Interpretation of OS outcomes can be further complicated by immature data from trials wherein primary endpoints may include shorter-term outcomes (e.g. PFS) and receipt of subsequent therapies [Citation20]. If subsequent therapies are received, the OS data reflect a pathway of treatments rather than the isolated effect of the intervention vs. control arm [Citation21,Citation22]. Quantifying the isolated treatment effect of the intervention vs. control is further complicated when different types of subsequent therapy are used [Citation20,Citation23,Citation24].
To mitigate complications, OS should be interpreted with subsequent therapy data. We explored the effects of subsequent therapies using patient-level data from TOURMALINE-MM1. To attempt to isolate the impact of IRd vs. Rd, treatment-switching analyses were conducted to remove the effect of subsequent therapies. Two methods (IPCW and RPSFTM) were used on the TOURMALINE-MM1 trial data; these methods improved the OS HR of IRd vs. Rd from 0.94 to 0.70 and 0.89, respectively. After applying these adjusted results within the NMAs, the relative efficacy estimates improved in favor of IRd. However, it is important to note that similar adjustments were not possible for other trial data, so relative OS could not be estimated in isolation of subsequent therapies. Additional research into the sequencing of alternative therapies and the impact of these sequences on patient outcomes is warranted.
As an oral regimen, IRd can be convenient and self-manageable. The ability to self-manage treatment could be particularly useful during the current COVID-19 pandemic when interaction with the healthcare system may be challenging. Additionally, patients’ receiving IRd in TOURMALINE-MM1 have been shown to maintain their health-related quality of life compared to those receiving placebo [Citation25]. Coupled with the efficacy findings in this NMA, IRd can be a preferable therapeutic option for RRMM.
Limitations
This study was subject to specific limitations. First, several data points were approximated. For example, TTP was used as a proxy for PFS when studies lacked PFS data. This is an approach that has been used previously and verified [Citation11]. For APEX (OS) and the Dimopoulos 2010 study [Citation26] (PFS and OS), the 95% CIs were estimated by digitizing the published Kaplan-Meier curve using Engauge digitization software [Citation27] This is consistent with guidance from the National Institute for Health and Care Excellence [Citation27,Citation28] In the same vein, results from the overall population were used to allow for comparisons between treatment arms of interest for certain subgroups which may have contributed to imprecise estimates.
Second, the study design and methodological quality of the included studies may have affected the results. For example, the comparison of IRd to Vd via V for PFS and OS outcomes was based on observational studies; no RCT compared Vd to V in the RRMM population. Networks including observational studies should be interpreted with caution, as these studies comprised patients with greater variation and probability of unobserved confounders in their baseline characteristics than RCTs, contributing to increased between-study heterogeneity. Meanwhile, Vd and V were compared in Dimopoulos 2010 [Citation26] and Dimopoulos 2015, [Citation29] a matched-pairs analysis of patients treated in MM-2045, APEX, and DOXIL-MMY-3001 trials. Due to the differences in data reported across studies, attempts to balance patient characteristics via multivariate analysis were not feasible. Additionally, data on time on treatment or duration of therapy are scarcely reported; thus, bias that may have been attributable to these measures could not be determined.
Conclusions
This NMA indicates that IRd is a reasonable treatment choice for patients with RRMM – particularly for patients seeking an efficacious and convenient therapeutic option. Extended network analyses including observational studies generally confirmed the efficacy of IRd across a broader patient population than in the clinical trials. Additionally, subgroup analyses indicated improved outcomes in specific patient populations (high-risk cytogenetics and 2+ prior lines of therapy).
Authorship contributions
Study design: All authors.
Formal analysis: RHB, IB, MC, MD, and MSD.
Data interpretation: All authors
Manuscript writing and editing: All authors.
All authors read and approved the final manuscript.
Disclosure of interest
MC, MD, and MSD are employees of Analysis Group, a consulting firm that received funding from Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, to conduct this study.
RHB and IB were employees of Analysis Group, Inc., during the conduct of this study.
HC was an employee of Takeda Pharmaceuticals International AG during the conduct of this study.
JD is an employee of Takeda Pharmaceuticals America, Inc.
DC is an employee of Takeda Development Center Americas, Inc. (TDCA).
Supplemental Material
Download MS Word (437.1 KB)Acknowledgements
Medical writing and editorial assistance was provided by Gloria DeWalt and Flora Chik, employees of Analysis Group, financial support for this assistance was provided by the study's sponsor.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data analyzed in this study were a re-analysis of existing data, which are publicly available as cited in the reference section.
Additional information
Funding
References
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv52–iv61.
- Rajkumar SV. Multiple myeloma: every year a new standard? Hematol Oncol. 2019;37(Suppl 1):62–65.
- Cowan AJ, Allen C, Barac A, et al. Global Burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4(9):1221–1227.
- Larsen JT, Kumar S. Evolving paradigms in the management of multiple myeloma: novel agents and targeted therapies. Rare Cancers Ther. 2015;3:47–68.
- Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol. 2012;49(Suppl 1):S16–S32.
- Moreau P. The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol. 2012;49(Suppl 1):S33–S46.
- Botta C, Ciliberto D, Rossi M, et al. Network meta-analysis of randomized trials in multiple myeloma: efficacy and safety in relapsed/refractory patients. Blood Adv. 2017;1(7):455–466.
- Dhakal B, Narra RK, Giri S, et al. Association of adverse events and associated cost with efficacy for approved relapsed and/or refractory multiple myeloma regimens: a Bayesian network meta-analysis of phase 3 randomized controlled trials. Cancer. 2020;126(12):2791–2801.
- Dimopoulos MA, Kaufman JL, White D, et al. A comparison of the efficacy of immunomodulatory-containing regimens in relapsed/refractory multiple myeloma: a network meta-analysis. Clin Lymphoma Myeloma Leuk. 2018;18(3):163–173 e166.
- Maiese EM, Ainsworth C, Le Moine JG, et al. Comparative efficacy of treatments for previously treated multiple myeloma: a systematic literature review and network meta-analysis. Clin Ther. 2018;40(3):480–494 e423.
- van Beurden-Tan CHY, Franken MG, Blommestein HM, et al. Systematic literature review and network meta-analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35(12):1312–1319.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269.
- Dias S, et al. NICE DSU technical support document 2: a generalised linear modeling framework for pairwise and network meta-analysis of randomised controlled trials.
- Weisel K, Spencer A, Lentzsch S, et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk. J Hematol Oncol. 2020;13(1):115.
- Dimopoulos MA, Lonial S, White D, et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020;10(9):91.
- Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186–197.
- Minarik J, Pika T, Radocha J, et al. Survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (Rd) in relapsed and refractory multiple myeloma patients in routine clinical practice. BMC Cancer. 2021;21(1):73.
- Cameron C, Fireman B, Hutton B, et al. Network meta-analysis incorporating randomized controlled trials and non-randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev. 2015;4:147.
- Cope S, Toor K, Popoff E, et al. Critical appraisal of published indirect comparisons and network meta-analyses of competing interventions for multiple myeloma. Value Health. 2020;23(4):441–450.
- Holstein SA, Suman VJ, McCarthy PL. Should overall survival remain an endpoint for multiple myeloma trials? Curr Hematol Malig Rep. 2019;14(1):31–38.
- Kogan AJ, Haren M. Translating cancer trial endpoints into the language of managed care. Biotechnol Healthc. 2008;5(1):22–35.
- Saad ED, Buyse M. Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol. 2016;27(3):373–378.
- Richardson PG, Kumar SK, Masszi T, et al. Final overall survival analysis of the TOURMALINE-MM1 phase III trial of ixazomib, lenalidomide, and dexamethasone in patients With relapsed or refractory multiple myeloma. J Clin Oncol. 2021;39(22):2430–2442.
- D'Souza A, Lonial S. What The princess bride teaches Us about outcomes in multiple myeloma. J Clin Oncol. 2021;39(22):2423–2425.
- Leleu X, Masszi T, Bahlis NJ, et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am J Hematol. 2018;93(8):985–993.
- Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116(16):3807–3814.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- Latimer NR. 2013. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. NICE Decision Support Unit. Available at: https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf.
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38.