ABSTRACT
Objective:
The incidence of MM in most registries remains stable or showing only a slightly increase. However, prevalence of MM is increasing due to the increase in overall survival in the last two decades. The aim of this study was to observe changes in biochemical parameters during the diagnosis and treatment of MM.
Methods:
A retrospective analysis was made of the biochemical indicators, survival time, and related adverse events of 196 patients with MM.
Results:
Of the 196 patients with MM, 26 were diagnosed with DM (DM-MM group) at the first diagnosis, 31 with steroid-induced diabetes mellitus (SID-MM group) during treatment, and 139 without DM (MM group). There was no significant difference between the three groups in the mean age of onset, sex ratio, incidence of hypercalcemia, renal dysfunction, anemia, abnormal lactate dehydrogenase, and median value of D-dimer and fibrinogen during diagnosis and treatment. There was no significant difference in survival time between the SID-MM and MM groups, but there was a significant difference between the DM-MM and MM groups.
Conclusion:
There was no significant difference between the three groups in the incidence of hypercalcemia, anemia, and renal function impairment. The survival time of patients with DM was shorter than that of patients without DM.
Introduction
Multiple myeloma (MM) is a malignant neoplasm of plasma cells that accumulate in bone marrow, leading to bone destruction and marrow failure, and 6–24% of these cases are complicated with diabetes mellitus (DM). [Citation1–3] Other MM-related complications include hypercalcemia, renal insufficiency, anemia, and infections [Citation4]. But smoldering multiple myeloma absence of end-organ damage such as lytic bone lesions, anemia, hypercalcemia, or renal failure that can be attributed to a plasma cell proliferative disorder [Citation5]. Most patients have serum M-protein with or without associated urinary M-protein. However, some have neither serum nor urine M-protein and therefore had nonsecretory myeloma [Citation4]. Data from randomized clinical trials revealed overall 24months survival rate 66.75-95.6% [Citation6], an overall five-year survival rate of about 54% and a median overall survival of approximately six years [Citation7]. A high ISS stage correlates with a poor prognosis [Citation8]. Age also plays a critical role in survival, with impressive improvement in patients younger than 70 years [Citation9]. MM accounts for 1.3% of all malignancies and 15% of all hematological neoplasms. Over 140 000 cases of MM are diagnosed worldwide per year with a lifetime risk of MM in economically developed countries of 0.6% to 1%.The incidence of MM varies by sociodemographic status with the highest rate in high-income countries (4–6 per 100 000) and a 10-fold difference between countries with the lowest and the highest rates [Citation10]. The number of diabetic patients in developing countries is estimated to increase by 69% in 2010–2030 and by 20% in developed countries [Citation11]. The relationship between diabetes mellitus (DM) and MM has attracted much attention. There was a trend toward significantly increased odds of MM in patients with T2DM in a meta-analysis of 10 studies (OR = 1.22; 95% CI 0.98–1.53; p = 0.08) [Citation12]. But another meta-analysis showed that T2DM is not a risk factor of MM [Citation13]. The coexistence of the two conditions in a patient forms a major challenge for physicians. Dexamethasone- and prednisone-based regimens are part of the conventional and new methods to treat newly diagnosed or recurrent/multiple myeloma [Citation14].Glucocorticoids are associated with a number of side effects including the development of new onset hyperglycemia or diabetes. Meta-Analysis revealed that the rate at which patients developed diabetes was 18.6% [Citation15].We want to know whether steroid-induced DM(SID) and diabetes can exacerbate changes in biochemical parameters and survival. Further studies are needed to assess the impact of T2DM and SID on clinical indicators and survival in patients with multiple myeloma.
The purpose of this study was to compare various biochemical parameters and survival time after diagnosis in patients with MM with DM at baseline, MM with steroid-induced DM, and those without DM.
Materials and methods
General information
We retrospectively analyzed 196 MM patients who were hospitalized in the Department of Hematology, Tumor Hospital, Tianjin Medical University between June 2006 and August 2016 and followed up till August 2017. The patients, some with type 2 DM occurring at different stages of MM, were divided into three groups: (1) 26 MM patients with DM (the DM-MM group) at the first diagnosis; (2) 31 patients with SID (the SID-MM group) developing during treatment; and (3) 139 patients without DM (the MM group). There were 115 men and 81 women, with a median age of 60 years (33–83 years). The exclusion criteria were as follows: (1) patients who were hospitalized only once and whose changes in blood sugar could not be observed; (2) patients with a lack of important indicators, such as blood sugar, which could not be compared before and after treatment; (3) patients who were not treated in our hospital for the first time in the course of their diagnosis and treatment; and (4) patients whose survival time after diagnosis was less than 4 weeks. All the patients underwent bone marrow puncture, blood/urine immunofixation electrophoresis, immunoglobulin assay, blood biochemistry, imaging (traditional radiology, CT and MRI, few people have PET-CT), and other examinations for diagnosis and clinical staging. The basic clinical data are shown in and .
Table 1. Clinical characteristics.
Table 2. Comparison of biochemical parameters in three groups.
The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (NO.bc2021117). Due to the retrospective nature of the study, informed consent was waived.
Diagnostic criteria and detection methods
International Myeloma Working Group criteria were used as the diagnostic criteria [Citation16]. The diagnosis of type 2 DM was based on its previous diagnostic record and blood glucose level at the time of first admission (Diabetes Mellitus World Health Organization Diagnostic Criteria were used as the diagnostic criteria. [Citation17] fasting plasma glucose ≥ 7.0mmol/l (126mg/dl) or 2–h plasma glucose ≥ 11.1mmol/l (200mg/dl))or glycosylated hemoglobin > 6.5%. The diagnostic criteria for SID were: normal fasting blood glucose level before treatment, > 7.0 mmol/L fasting blood glucose after hormone treatment, and >11.11 mmol/L fasting blood glucose 2 hours after a meal, at least twice [Citation18]. The subjects fasted for 8 hours and blood was collected to detect fasting plasma glucose, liver and kidney function, blood coagulation, fibrin degradation products, D-dimer, electrolytes, and blood calcium. Plasma total calcium content > 2.8 mmol/L was diagnosed as hypercalcemia.
Treatment strategies
Treatment strategies were divided into bortezomib-containing regimens (bortezomib + dexamethasone; bortezomib + dexamethasone + cyclophosphamide; bortezomib + dexamethasone + doxorubicin liposomes), and bortezomib-free regimens (VAD, TD, DT-PACE). The efficacy was evaluated according to the modified evaluation criteria of MM by the Chinese Working Group on Myeloma in 2016. Patients with elevated plasma glucose were treated with diet control, oral hypoglycemic drugs, including metformin, and insulin. The specific treatment plan was based on consultation with DM experts.
Statistical analysis
SPSS 22.0 software was used for statistical analysis. The descriptive analysis of each variable was carried out first, and the data that did not conform to the normal distribution were described by the median. A Rank sum test, Mann–Whitney U test or Kruskal–Wallis H test was used for univariate analysis and data comparison between the groups. Survival analysis was performed by the Kaplan-Meier method, and survival rates were compared by the Log-rank method. The difference was statistically significant at P < 0.05.
Results
General data
The overall incidence was higher in male than in female patients, with a ratio of 1.42:1, but there was no difference between the three groups in the proportion of male and female patients (p > 0.05). The median age at initial diagnosis was 60 years: 60 years in the MM group, 59 years in the SID-MM group and 61 years in the DM-MM group. There was no difference in age at onset among the three groups. Patients aged > 65 years accounted for 21.58% in the MM group and 3.2% in the SID-MM group, meaning a significant difference between the two groups (p = 0.0183), but there was no significant difference between the MM and DM-MM groups (p > 0.9999). The proportion of elderly patients was lower in the SID-MM than the MM group. The proportions of immunoglobulin isotypes in the three groups were similar. IgG was the most common type followed by IgA, and the incidence of non-secretory type and IgD was the lowest, and there was no difference between the three groups. There was no difference between the three groups in the proportion of patients with hemoglobin < 100 g/L at the first diagnosis. The plasma calcium concentration was the highest serum calcium parameter detected during diagnosis and treatment. There was no difference between the three groups in median serum calcium level or the incidence of hypercalcemia. Plasma creatinine was the highest in the three groups. The incidence of renal insufficiency in the MM group was 23.74%, SID-MM was 6.45%, and DM-MM groups was 11.54%, The incidence of renal insufficiency in the MM group was higher than in the SID-MM and DM-MM groups. Blood urea nitrogen was high during diagnosis and treatment, and the median level was higher than the normal level, but there was no significant difference between the three groups. Beta-2 microglobulin was high but there was no significant difference between the three groups. Plasma lactate dehydrogenase (LDH) was high; the median level was higher than the upper limit of normal, but there was no significant difference between the three groups. The median value of D-dimer was significantly higher than the reference value, but there was no significant difference between the three groups. There was no significant difference in the rate of stem cell transplantation between the three groups. There was no significant difference between the three groups in the proportion of patients who had used bortezomib-containing regimens for their initial treatment. The median level of fibrinogen was higher than the normal level, but there was no difference between the three groups.
Data concerning the DM-MM group
The hypoglycemic treatment methods used for the 26 patients in the DM-MM group are shown in . Dietary control was used for 3 patients, insulin for 8 of them, a metformin-containing regimen for 4 others, and the remaining 11 patients were treated with other oral hypoglycemic agents. There was no significant difference in survival time between the metformin and insulin groups (p = 0.7834).
Table 3. The hypoglycemic treatment methods used in the DM-MM group.
A comparison of survival time
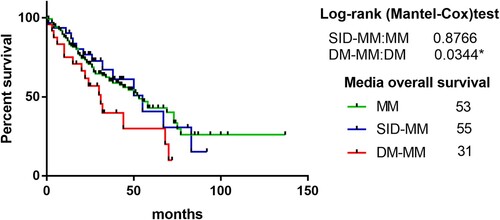
Of the 139 patients in the MM group, 15 were lost to follow-up, of the 31 patients in the SID-MM group, no loss to follow-up occurred, and of the 26 patients in the DM-MM group, 2 were lost to follow-up. The median survival time was 53 months in the MM group, 55 months in the SID-MM group, and 31 months in the DM-MM group. There was no significant difference between the MM and SID-MM groups (p = 0.8766) (), but there was a significant difference between the MM and DM-MM groups (p = 0.0344) (). Overall, the survival time of MM patients complicated with DM was shorter than that of patients without DM.
Figure 1. A comparison of survival time between the MM group and the DM-MM group, MM group and the MM-SID group. There was no significant difference in between the MM group and the MM-SID. The difference between MM group and the DM-MM group was significant. The survival time of the MM group was significantly longer than that of the DM-MM group.
Discussion
Multiple myeloma (MM) is a disease in which there is a clonal expansion of plasma cells in the bone marrow and that results in the production of a monoclonal protein and end-organ damage [Citation19]. About 8–18% of cancer patients have DM [Citation20]. After a diagnosis of DM, both men and women have an increased risk of liver, pancreas, gallbladder, endometrium, stomach, and kidney cancers, as well as a risk of benign and malignant brain tumors, colorectal cancer, lung cancer, leukemia, and non-Hodgkin’s lymphoma [Citation21]. Large scale statistical research studies in Asia suggest that type 2 DM increases the risk of death from a number of cancers by 26% [Citation22]. However, specific data on the impact of DM on the course of MM are rarely reported on in mainland China.
In this study, we retrospectively analyzed 196 patients with MM, including 139 without DM, 26 patients with DM at the time of diagnosis of MM, and 31 patients who developed SID during treatment. In the three cohorts, there were no significant differences in the median age of onset (60, 59, and 61 years), sex ratio, median age of onset, or MM immunotype.
However, MM patients are clearly heterogenous, and survival time can vary from months to several years. The total survival time of patients with high LDH is significantly shortened, making it a poor prognostic parameter of overall survival (OS) and progression-free survival (PFS) [Citation23]. Other studies have shown that infection is the main cause of early mortality in patients with MM, while high beta-2 microglobulin and LDH and low serum albumin levels are the poor prognostic factors of early mortality [Citation24]. Compared with healthy people, LDH-A is overexpressed in pancreatic islet secretory cells, which affects insulin secretion [Citation25]. We found that the median LDH level of the three groups was higher than the norm, but there was no difference between the three groups, and the increased blood glucose had no effect on LDH, regardless of blood sugar status and staging.
Calcium ions are important in the process of cell metabolism. Their regulation includes three main mechanisms, namely kidney filtration and absorption, bone renewal, and intestinal dietary absorption [Citation26]. However, there were no significant differences in the median serum calcium and the incidence of hypercalcemia in the three groups in our study. The relationship between serum calcium and DM remains unclear. Earlier experimental studies have shown that intracellular hypercalcemia can attenuate the effects of insulin in cells, reduce sugar transport, especially glucose transporter 4, and extend the activation of insulin receptors [Citation27]. Albumin and serum calcium levels are positively correlated with the risk of DM [Citation27]. Our study showed that there was no significant difference between the three groups in the incidence of hypercalcemia. It would appear that increased blood sugar does not increase the incidence of hypercalcemia.
Renal function impairment is a common complication of DM and MM. The survival time of patients with renal function impairment is usually lower than that of patients without renal function impairment [Citation28, Citation29]. Most studies indicate that serum creatinine at presentation is only predictive of a worse renal outcome, and myeloma patients with renal insufficiency, whether dependent on dialysis or not, can benefit from novel therapeutic agents [Citation30]. Kidney damage caused by DM is related to old age but not to gender and the length of illness [Citation31]. The response rate to chemotherapy in patients with renal failure is lower than that in patients with normal renal function. The serum creatinine level and the response to chemotherapy are clearly associated with the survival rate [Citation28].
MM patients have abnormal coagulation and bleeding. D-dimer is associated with poor prognosis in a variety of tumors [Citation32, Citation33], and it is also associated with OS but not PFS [Citation32]. Tissue factor (TF) activates factor VII, and TF–VIIa complex activates factor X, thus producing D-dimer, which is itself a degradation product. TF up-regulates vascular endothelial growth factor and down-regulates thrombin-sensitive protein, thus promoting angiogenesis, which is related to tumor progression [Citation33]. In this study, the median levels of D-dimer in the three groups were higher than the upper limit of normal, but there was no difference between the three groups. Fibrinogen, a glycoprotein synthesized by the liver, can initiate platelet adhesion and aggregation and act as a substrate for factor VIII and fibrinolytic enzymes [Citation34]. Tumor growth produces a large number of inflammatory chemokines, which can increase the level of fibrinogen [Citation35], and the increase of fibrinogen is related to tumor progression [Citation36]. Fibrinogen production is increased significantly in type 2 DM, and glucagon may contribute to fibrinogen production [Citation37]. Fibrinogen levels were higher than normal in our three groups. There was a gradient change between the three groups, but the difference was not of statistical significance.
Anemia is one of the main clinical manifestations of MM, with positive cytochrome anemia being predominant. Its mechanism includes abnormal up-regulation of apoptotic receptors by myeloma cells, Fas ligand, and tumor necrosis factor-related apoptosis inducing ligand. Highly malignant myeloma cells also participate in ineffective hematopoiesis and the chronic exhaustion of erythrocytes [Citation38]. Most studies suggest that anemia occurs in patients with DM complicated with kidney damage, but other studies suggest that anemia occurs before kidney damage [Citation39]. The serum erythropoetin (EPO) level in patients with DM is lower than that of normal individuals. The sensitivity of erythrocytes to lipid peroxide is negatively correlated with EPO, while impairment of glucose metabolism is correlated with oxidative stress [Citation40]. Hyperglycemia can also lead to anemia, and chronic hyperglycemia can destroy the renal interstitium. Androgen levels are decreased by systemic inflammation induced by DM [Citation39]. Many mechanisms can cause anemia. We found that the median hemoglobin level in the three groups was lower than normal, but there was no difference in the degree of anemia between patients with high and normal blood sugar. Increased blood sugar did not aggravate the severity of anemia in patients with MM.
The International Staging System (ISS) is an important system for predicting the prognosis of MM. Since 2005, plasma beta-2 microglobulin combined with plasma albumin concentration have been recognized as simple and powerful prognostic factors for MM patients, but the reason for the combination is still not clear. In 2007, Fonseca and San Miguel stressed that cells should also be included in evaluating the prognosis of MM patients [Citation41]. Most ISS data were derived from data for MM patients from 1981 to 2002. All patients were treated with traditional therapy. To determine whether new drugs and strategies have an impact on ISS staging, Lu et al. found that the predictive value of ISS staging for Chinese patients with MM using the new treatment methods is still applicable [Citation42]. It is suggested that ISS staging has little predictive effect on new therapies, while the combination of hemoglobin and plasmacytoma staging plays a predictive role in the era of new therapies [Citation43].
Of the initial 196 patients, 15 were lost to follow-up in the MM group, none in the SID-MM group, and 2 in the DM-MM group. The survival time of DM-MM patients was significantly shorter than that of MM patients without DM and no different than that of MM patients with DM induced during treatment. Many MM risk loci were found in several study. Integration of gene expression, epigenetic profiling and in situ Hi-C data suggested altered B-cell differentiation, dysregulation of autophagy/apoptosis, and cell cycle signaling as shared perturbed mechanistic pathways [Citation44]. Patients with DM have an increase in insulin-like growth factor (IGF)-I [Citation45]. IGF-I has been shown to stimulate the proliferation of MM cells. IGF-I can stimulate the cascade signal amplification of interleukin (IL)−6 to trigger the differentiation and proliferation of MM cells. IL-6 and IGF-I have different downstream signaling molecules. IL-6 activates the signal transducer and activator of transcription 3 phosphorylation, and IGF-I leads to insulin receptor substrate phosphorylation. These signaling molecules finally converge to activate RAS/MAPK pathways [Citation46]. Cell experiments have shown that a hyperglycemic environment can reduce the effectiveness of chemotherapeutic drugs [Citation47]. Hyperglycemia and chemotherapeutic agents can induce the high expression of peroxisome proliferator-activated receptor γ co-activator (PGC)−1α at the mRNA and protein levels. PGC-1α is related to the antioxidant system, while superoxide dismutase-2 and catalase are also up-regulated. PGC-1α inhibitors can enhance the efficacy of anti-tumor drugs and reduce the expression of antioxidant factors [Citation47]. Single nucleotide polymorphisms (SNPs) causing DM are considered to be a risk factor for MM and affect the survival of MM patients. Meta-analysis shows that rs7501939 located in the HNF1B gene can negatively affect OS. The polymorphism of the rs13266634 allele is associated with poor survival in men but not women [Citation48]. In this study, the survival time of SID-MM patients was not significantly different from that of MM patients. The reasons may be as follows: (1) The blood sugar of SID-MM patients increased after treatment. The overall time of blood sugar increase was shorter than that of patients with previous DM, and the effect of blood sugar increase on the organs was relatively minor. (2) The number of patients aged > 65 years was lower than in the MM group. (3) The damage to renal function was less than in the MM group. (4) There were more patients with transplants in this group. Metformin can inhibit MM cell proliferation, and induce cell apoptosis and cell cycle arrest in the G0/G1 phase. Metformin can activate caspase 3, caspase 9, Poly [ADP-ribose] polymerase 1(PARP-1), Bak, and p21; it can inactivate Mcl-1, hiap-1, cyclin D1, cyclin-dependent kinase (CDK) 4, and CDK6; and it can inhibit expression of IGF-I receptor, phosphatidylinositol 3 kinase (PI3K), protein kinase B (Akt), and downstream mammalian rapamycin target protein (mTOR). IGF-I blocks metformin-induced MM cell apoptosis and PI3K/Akt signaling pathway reactivation. Metformin can inhibit MM cell proliferation through the IGF-1R/PI3K/Akt/mTOR signaling pathway [Citation49]. In this study, the survival time of patients treated with metformin was not superior to that in those treated with insulin, mainly due to the fact that fewer patients took metformin in this group. The final outcome remains to be determined.
Conclusion
This study showed that there were no significant differences between the MM, DM-MM, and SID-MM groups in terms of the incidence and degree of anemia, renal dysfunction, changes in LDH, and changes in coagulation mechanism. In terms of survival, there is a significant difference between MM and DM-MM patients.
Ethics approval and consent to participate
This study was approved by ethics committee of Tumor Hospital, Tianjin Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Authors’ contributions
Conception and design of the research: Zheng-Yue Dou. Acquisition of data: Bing Xia. Writing of the manuscript: Chao-Yu Wang. Critical revision of the manuscript for intellectual content: Yan-Jie Xu, Yi-Zhuo Zhang. All authors read and approved the final draft.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
- Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–53.
- Richardson PG, Kumar SK, Masszi T, et al. Final overall survival analysis of the TOURMALINE-MM1 phase III trial of ixazomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2021;39(22):2430–2442.
- Avivi I, Yekutiel N, Cohen I, et al. Diabetes, but not pre-diabetes, is associated with shorter time to second-line therapy and worse outcomes in patients with multiple myeloma. Leuk Lymphoma. 2021;62(11):2785–2792.
- Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(2):230–269.
- Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–7.
- Li J, Bao L, Xia Z, et al. Ixazomib-based frontline therapy in patients with newly diagnosed multiple myeloma in real-life practice showed comparable efficacy and safety profile with those reported in clinical trial: a multi-center study. Ann Hematol. 2020;99(11):2589–2598.
- Awada H, Thapa B, Awada H, et al. A comprehensive review of the genomics of multiple myeloma: evolutionary trajectories, gene expression profiling, and emerging therapeutics. Cells. 2021;10(8):1961. doi:10.3390/cells10081961. PMID: 34440730; PMCID: PMC8391934.
- Firth J. Haematology: multiple myeloma. Clin Med (Lond). 2019;19(1):58–60.
- Chang-Chan DY, Ríos-Tamayo R, Rodríguez Barranco M, et al. Trends of incidence, mortality and survival of multiple myeloma in Spain. A twenty-three-year population-based study. Clin Transl Oncol. 2021;23(7):1429–1439.
- Hemminki K, Försti A, Houlston R, et al. Epidemiology, genetics and treatment of multiple myeloma and precursor diseases. Int J Cancer. 2021;149(12):1980–1996.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14.
- Castillo JJ, Mull N, Reagan JL, et al. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood. 2012;119(21):4845–50.
- Zhang C, Sha Y, Liu H, et al. Type 2 diabetes mellitus does not increase the risk of multiple myeloma: a systematic review and meta-analysis. Transl Cancer Res. 2020;9(4):2884–2894.
- Ali MA, Ahmed YA, Ibrahim A. Clinical challenges: myeloma and concomitant type 2 diabetes. South Asian J Cancer. 2013;02(4):290–5.
- Liu XX, Zhu XM, Miao Q, et al. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65(4):324–32.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48.
- Organization WH. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva World Health Organization; 2006.
- Kim SY, Yoo CG, Lee CT, et al. Incidence and risk factors of steroid-induced diabetes in patients with respiratory disease. J Korean Med Sci. 2011;26(2):264–7.
- Das S, Yazit JN, Azmani N, et al. Multiple myeloma: challenges encountered and future options for better treatment. Int J Mol Sci. 2022;23(3):1649. doi:10.3390/ijms23031649. PMID: 35163567; PMCID: PMC8836148.
- Ahmed YA, Eltayeb A. Clinical challenges: myeloma and concomitant type 2 diabetes. Int J Hematol Oncol Stem Cell Res. 2013;7(1):34–41.
- Dankner R, Boffetta P, Balicer RD, et al. Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2.3 million adults. Am J Epidemiol. 2016;183(12):1098–106.
- Chen Y, Wu F, Saito E, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60(6):1022–1032.
- Gu Y, Yuan YH, Xu J, et al. High serum lactate dehydrogenase predicts an unfavorable outcome in Chinese elderly patients with multiple myeloma. Oncotarget. 2017;8(29):48350–48361.
- Chen YK, Han SM, Yang Y, et al. Early mortality in multiple myeloma: experiences from a single institution. Hematology. 2016;21(7):392–8.
- Chen Y, Wang X, Shao X. A combination of human embryonic stem cell-derived pancreatic endoderm transplant with LDHA-repressing miRNA Can attenuate high-Fat diet induced type II diabetes in mice. J Diabetes Res. 2015;2015:796912.
- Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 5):S23–30.
- Suh S, Bae JC, Jin SM, et al. Serum calcium changes and risk of type 2 diabetes mellitus in Asian population. Diabetes Res Clin Pract. 2017;133:109–114.
- Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158(17):1889–93.
- Griniūte R, Bumblyte IA. Clinical and laboratory features and prognostic implications in myeloma with and without renal impairment. Medicina (Kaunas). 2003;39(Suppl 1):41–7.
- Soleymanian T, Soleimani A, Musavi A, et al. Outcome of patients with multiple myeloma and renal failure on novel regimens. Saudi J Kidney Dis Transpl. 2016;27(2):335–40.
- Martínez Candela J, Sangrós González J, García Soidán FJ, et al. Chronic renal disease in spain: prevalence and related factors in persons with diabetes mellitus older than 64 years. Nefrologia. 2018;38(4):401–413.
- Nakamura K, Nakayama K, Ishikawa M, et al. High pretreatment plasma D-dimer levels are related to shorter overall survival in endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2016;201:89–93.
- Nakamura K, Nakayama K, Ishikawa M, et al. High pre-treatment plasma D-dimer level as a potential prognostic biomarker for cervical carcinoma. Anticancer Res. 2016;36(6):2933–8.
- Wang R, Liu R, Zhao L, et al. Influence of type 2 diabetes mellitus on Khorana venous thromboembolism risk in colorectal cancer patients. Rev Esp Enferm Dig. 2017;109(7):503–509.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
- Seebacher V, Polterauer S, Grimm C, et al. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 2010;102(6):952–6.
- Barazzoni R, Zanetti M, Davanzo G, et al. Increased fibrinogen production in type 2 diabetic patients without detectable vascular complications: correlation with plasma glucagon concentrations. J Clin Endocrinol Metab. 2000;85(9):3121–5.
- Silvestris F, Cafforio P, Tucci M, et al. Negative regulation of erythroblast maturation by Fas-L(+)/TRAIL(+) highly malignant plasma cells: a major pathogenetic mechanism of anemia in multiple myeloma. Blood. 2002;99(4):1305–13.
- Antwi-Bafour S, Hammond S, Adjei JK, et al. A case-control study of prevalence of anemia among patients with type 2 diabetes. J Med Case Rep. 2016;10(1):110.
- Gradinaru D, Margina D, Ilie M, et al. Correlation between erythropoietin serum levels and erythrocyte susceptibility to lipid peroxidation in elderly with type 2 diabetes. Acta Physiol Hung. 2015;102(4):400–8.
- Bataille R, Annweiler C, Beauchet O. Multiple myeloma international staging system: “staging” or simply “aging” system. Clin Lymphoma Myeloma Leuk. 2013;13(6):635–7.
- Lu J, Lu J, Liu A, et al. The applicability of the international staging system in Chinese patients with multiple myeloma receiving bortezomib or thalidomide-based regimens as induction therapy: a multicenter analysis. Biomed Res Int. 2015;2015:856704.
- Iriuchishima H, Saitoh T, Handa H, et al. A new staging system to predict prognosis of patients with multiple myeloma in an era of novel therapeutic agents. Eur J Haematol. 2015;94(2):145–51.
- Chattopadhyay S, Thomsen H, Weinhold N, et al. Eight novel loci implicate shared genetic etiology in multiple myeloma, AL amyloidosis, and monoclonal gammopathy of unknown significance. Leukemia. 2020;34(4):1187–1191.
- NeamŢu MC, Avramescu ET, Marcu IR, et al. The correlation between insulin-like growth factor with glycemic control, glomerular filtration rate, blood pressure, hematological changes or body mass index in patients with type 2 diabetes mellitus. Rom J Morphol Embryol. 2017;58(3):857–861.
- Ferlin M, Noraz N, Hertogh C, et al. Insulin-like growth factor induces the survival and proliferation of myeloma cells through an interleukin-6-independent transduction pathway. Br J Haematol. 2000;111(2):626–34.
- Yu W, Cao D, Zhou H, et al. PGC-1α is responsible for survival of multiple myeloma cells under hyperglycemia and chemotherapy. Oncol Rep. 2015;33(4):2086–92.
- Ríos-Tamayo R, Lupiañez CB, Campa D, et al. A common variant within the HNF1B gene is associated with overall survival of multiple myeloma patients: results from the IMMEnSE consortium and meta-analysis. Oncotarget. 2016;7(37):59029–59048.
- Zi FM, He JS, Li Y, et al. Metformin displays anti-myeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015;356(2 Pt B):443–53.
