ABSTRACT
Objectives:
There may be a shift in risk stratification at progression compared to that at diagnosis in patients with multiple myeloma (MM). We aimed to evaluate whether re-staging and stage migration is of prognostic impact.
Methods:
Real-world data from the National Longitudinal Cohort of Hematologic Diseases-multiple myeloma were collected; 263 consecutive patients demonstrating disease progression were finally included. Staging at diagnosis and re-staging at progression were performed using the International Staging System (ISS) and Revised International Staging System (RISS).
Results:
Based on ISS re-staging, the median post-progression survival (mPPS) of patients with stage I, II, and III was 44.2, 21.7, and 11.6 months, respectively (P < 0.0001). Based on RISS re-staging, the mPPS of patients with stage I, II, and III was 50.3, 22.2, and 11.4 months, respectively (P < 0.0001). The mPPS in patients with improved, maintained, and deteriorated ISS stage migration from diagnosis was 33.6, 20.9, and 16 months, respectively (P = 0.0051) and that with improved, maintained, and deteriorated RISS stage migration was 48.4, 23.1, and 13.9 months, respectively (P < 0.001). Compared to patients with maintained or improved disease stage, those with deteriorated ISS/RISS migration showed significantly higher incidence of Del(17P) at progression and worse PPS. Multivariate analyses indicated both re-staging and stage migration by ISS/RISS at progression were independent predictors for PPS.
Conclusions:
We demonstrated that ISS/RISS re-staging showed superior prognostic utility over ISS/RISS staging in predicting PPS. Patients with deteriorated stage migration or maintained advanced stage at progression may need more individualized treatment.
Introduction
Despite significant improvement in the prognosis of patients with newly diagnosed multiple myeloma (NDMM), the recurrence rate after remission remains high [Citation1,Citation2]. Previous studies on relapse kinetics have shown that early relapse in patients with multiple myeloma(MM) often indicates poor prognosis [Citation3–7]. Moreover, MM progression is a risk-accumulating process [Citation8–17], with possible differences in the risk stratification at progression compared to that at diagnosis. Thus, in addition to focusing on recurrence kinetics in patients with relapsed/progressive disease, it is also critical to perform dynamic risk re-stratification by re-staging the disease before second-line therapy initiation [Citation18,Citation19]. However, a uniform prognostic staging system for assessing post-progression survival (PPS) in patients with relapsed MM has not been established.
Given that clonal evolution and changes in aggressiveness may occur with MM progression due to treatment-induced selection pressure, the risk stratification [Citation20–25] is likely to alter from that at initial diagnosis, altering the prognostic trajectory. Therefore, a re-staging strategy at progression may help to indicate the prognostic significance of dynamic risk stratification; it can also help to identify unified prognostic stratification systems for patients with MM in relapsed/progression population. Previous studies [Citation26–28] have used International Staging System (ISS) or Revised International Staging System (RISS) to stratify patients with MM at relapse in clinical trials [Citation29]. However, given that the patients in the above study were not part of the same cohort that experienced relapse after receiving first-line treatment, it is still unknown whether stage migration (change of the patient's clinical stage at progression compared with the initial stage) will affect post-progression survival (PPS).
In a consecutively treated cohort of NDMM showing disease progression, we performed this real-world retrospective study to evaluate whether re-staging or stage migration using ISS/RISS is of prognostic significance.
Methods and materials
Patients and study design
Real-world data were collected through the National Longitudinal Cohort of Hematologic Diseases-multiple myeloma (NICHE-MM) (NCT04645199), which contains historical, anonymised electronic and paper medical records, and follow-up data.
After reviewing data of 912 patients with NDMM diagnosed between March 24, 2013, and December 2, 2019, at our hospital, 265 patients who were referred to a local hospital or discontinued induction treatment were excluded. Of the remaining 647 consecutive patients, 384 were further excluded owing to continuous remission. A total of 263 consecutive patients showing disease progression were finally included in the study. Briefly, patients received induction therapy mainly with proteasome inhibitor (PI)-based (such as bortezomib plus cyclophosphamide and dexamethasone) or immunomodulatory drug (IMiD)-based(such as lenalidomide, cyclophosphamide, and dexamethasone) triplet regimen. After at least four cycles of induction treatment for partial remission (PR), patients underwent either autologous stem cell transplantation (ASCT) or consolidation therapy. Subsequently, patients were treated with lenalidomide or bortezomib maintenance for 1 year, unless intolerant or continual progression was observed. Routine monitoring of treatment response was carried out every two courses during the induction treatment, as well as before and 3 months after transplantation, or post consolidation treatment, and approximately every 3 months during maintenance, and when patients started exhibiting signs of recurrence, follow-up monitoring was performed approximately every month. Renal insufficiency was defined as creatinine > 2 mg/dL. Response assessment was conducted as per the International Myeloma Working Group (IWMG) Criteria [Citation30].
The NICHE-MM study was reviewed and approved by the Human Genetic Resource Administration of China and this study was approved by the institutional ethics committees.
Interphase fluorescence in situ hybridization (iFISH) studies
iFISH was performed on purified CD138 + plasma cells as previously reported [Citation8]. iFISH panel included 13q14 deletion, Del(17P) and 1q21 gain/amplification, t(4;14), t(11;14), t(14;16). High-risk cytogenetic abnormality (HRCA) referred to t(4;14), t(14;16), and Del(17P). The cut-off level for translocation, deletions, and amplifications was 10% [Citation8].
Re-staging and stage migration
We employed the ISS [Citation22] and RISS [Citation25] for staging at diagnosis and re-staging at progression. Patients who had ISS stage II disease but lacked iFISH and LDH analysis at diagnosis or progression, were also classified to be at RISS stage II according to the RISS definition. Re-staging by ISS and RISS was uniformly done according to clinical information at first indication of disease progression to ensure that all patients were re-stratified at relatively consistent time points.
Patterns of stage migration from diagnosis were defined as follows: improved ISS/RISS stage migration – patients who migrated to lower ISS/RISS stage at progression; maintained ISS/RISS stage – patients who maintained their original ISS/RISS stage at progression; deteriorated ISS/RISS stage migration – patients who migrated to higher ISS/RISS stage at progression.
Statistical analyses
The chi-square or Fisher exact test was used to compare categorical variables. The t-test was used to compare continuous variables. Correlation was assessed using Spearman's test and two-tailed P-values were represented. Multivariable-adjusted Cox proportional hazards survival models were developed to assess the prognostic utility of staging at diagnosis, first-line treatment options, and re-staging at progression on PPS based on the univariable analysis including ISS staging, upfront ASCT, progression-free survival (PFS), and ISS re-staging in model 1 and RISS staging, upfront ASCT, PFS, and RISS re-staging in model 2. Additionally, multivariate analyses were also performed to evaluate the impact of stage migration on PPS: variables include ISS stage migration, PFS, and ASCT in model 1 and RISS stage migration with PFS and ASCT in model 2. Variables with a P-value < 0.1 in the univariable analysis were included in multivariable models and all results were reported as hazard ratios with 95% confidence intervals (CIs). PFS was calculated as the time from diagnosis to any form of disease progression or death; PPS was defined as the time from disease progression to death or last follow-up [Citation31–33]. Overall survival (OS) was calculated as the time from diagnosis to death or last follow-up. Survival analyses were performed using the Kaplan–Meier method, followed by inter-group comparisons using the log-rank test. The median follow-up duration was analysed using the reverse Kaplan–Meier method. Statistical significance was set at a two-sided P-value < 0.05. Statistical analyses were performed using Statistical Package for Social Sciences (SPSS version 25.0, IBM, Chicago, IL) and R language (version R 4.1.2 Foundation for Statistical Computing, Tsinghua, China).
Results
Characteristics at diagnosis and progression
Among the 263 included patients, the median age at diagnosis was 57 years, and 161 (61.2%) patients were male; 213 (80.9%) patients received PI-based induction treatment and 129 (49%) were alive.
Comparison of the characteristics of all included patients at diagnosis was done based on whether or not they had received first-line ASCT. There was no difference in sex distribution, types of M-protein, and ISS/RISS distribution, while the ASCT group patients were younger, had a longer duration of PFS, and a higher rate of PI-based induction regimen (Supporting Information, Table S1). Baseline characteristics at progression and second-line treatment options are shown in . The rates of renal insufficiency were lower at progression (6.4%) compared to those at diagnosis (11%), (P = 0.085). Regarding the HRCA at progression, the rates of t(4;14) and t(14;16) were comparable to those at diagnosis. However, the rate of Del(17P) at progression was 26.9%, which was significantly higher than 12.7% at diagnosis (P = 0.001). When we grouped the HRCA of relapsed patients according to different stage migration patterns, we further found that the incidence of t(4;14) and t(14;16) were also comparable among different migration patterns; however, compared to patients at maintained or improved original stage, the incidence of Del(17P) was significantly higher in the deteriorated stage migration for both ISS and RISS (P < 0.001, ). Besides, there were no obvious difference in length of PFS and incidence of renal insufficiency between the three patterns of stage migration at progression for both ISS and RISS. Second-line treatment mainly consists of the following regimens: PI-based, IMiD-based, and VRD (bortezomib plus lenalidomide and dexamethasone)- or IRD (ixazomib plus lenalidomide and dexamethasone)-based; others include high-dose chemotherapy followed by second ASCT, CD38 monoclonal antibody, and chemotherapy. Under the three patterns of stage migration, the second-line treatment regimens among the different groups of relapsed patients were roughly comparable.
Table 1. Characteristics of patients at diagnosis and progression based on different patterns of stage migration.
OS and PFS according to staging at diagnosis
At diagnosis, ISS data were available for all included patients. There were 51 (19.4%), 94 (35.7%), and 118 (44.9%) patients at ISS stage I, II, and III, respectively. The mOS of patients with ISS stage I, II, and III disease was 72.4 (95% CI: 56.1–88.5), 68.4 (95% CI: 63.4–73.5), and 43.3 (95% CI: 34.8–51.9) months, respectively (P = 0.001; Supporting Information Figure S1). The mPFS in patients with ISS stage I, II, and III was 24.1 (95% CI: 17.0–31.2), 22.3 (95% CI: 17.1–27.5), and 16.9 (95% CI: 14.3–19.5) months, respectively (P = 0.0049; Supporting Information Figure S1). RISS staging data were available for 257 patients. There were 40 (15.6%), 155 (60.3%), and 62 (24.1%) patients with stage RISS I, II, and III, respectively (3 patients with ISS II but lacking iFISH detection, were classified as RISS II). The mOS in patients with RISS stage I, II, and III disease was 72.3 (95% CI: 57.6–87.1), 63.8 (95% CI: 55.6–71.9), and 35.2 (95% CI: 28.5–42.0) months, respectively (P < 0.001; Supporting Information Figure S2). The mPFS in patients with RISS stage I, II, and III disease was 23.8 (95% CI: 15.8–31.7), 20.6 (95% CI: 16.9–24.2), and 14.3 (95% CI: 10.9– 17.6) months, respectively (P = 0.021; Supporting Information Figure S2).
PPS according to re-staging at progression
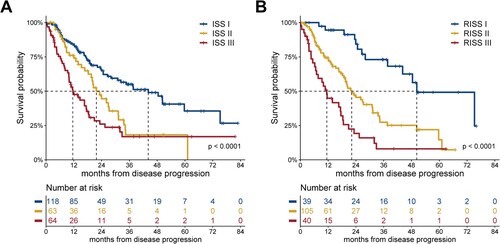
At the time of disease progression, ISS re-staging data were available for 245 patients. There were 118 (48.2%), 63 (25.7%), and 64 (26.1%) patients with ISS stage I, II, and III, respectively. RISS re-staging was available for 184 patients with 39 (21.2%), 105 (57.1%), and 40 (21.7%) patients with RISS stage I, II, and III, respectively (3 patients with ISS II lacking both iFISH and LDH analysis at progression, were categorized as RISS II). The mPPS in patients with ISS stage I, II, and III disease was 44.2 (95% CI: 28.5–59.8), 21.7 (95% CI: 16.5–27.0), and 11.6 (95% CI: 7.0–16.1) months, respectively (P < 0.0001; A). The mPPS in patients with RISS stage I, II, and III disease was 50.3 (95% CI: 31.3–69.3), 22.2 (95% CI: 16.1–28.2), and 11.4 (95% CI: 8.3–14.5) months, respectively (P < 0.0001; B).
Prognostic impact of stage migration on PPS
Regarding the ISS stage at progression, 107 patients migrated to a lower stage (by one stage, 74 patients; by two stages, 33 patients), 119 patients maintained the original stage, and 19 patients migrated to a higher stage (by 1 stage, 16 patients; by 2 stages, 3 patients). The mPPS in patients with improved, maintained, and deteriorated ISS stage was 33.6 (95% CI: 23.5–43.7), 20.9 (95% CI: 15.7–26.0), and 16 (95% CI: 2.4–30.8) months, respectively (P = 0.0051; A).
Figure 2. PPS according to stage migration at progression. (A) PPS according to ISS stage migration at progression, (B) PPS according to RISS stage migration at progression. ISS: International Staging System; PPS: post-progression survival; RISS: Revised International Staging System.
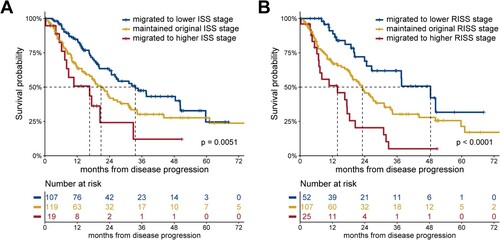
Regarding the RISS stage at disease progression, 52 patients migrated to a lower stage (by one stage, 50 patients; by two stages, 2 patients), 107 patients maintained the original stage, and 25 patients migrated to a higher stage (by one stage, 23 patients; by two stages, 2 patients). The mPPS in patients with improved, maintained, and deteriorated RISS stage was 48.4 (95% CI: 31.4–65.5), 23.1 (95% CI: 17.5–28.7), and 13.9 (95% CI: 6.2–25.9) months, respectively (P < 0.001; B).
Moreover, as the stage migration can be further separated into different circumstances (for example, both ISS/RISS stage 3 migration to stage 1 or stage 2 were classified as improved stage migration), we conducted a more thorough survival analysis and PPS according to each category of ISS/RISS stage migration, and the results are shown in Figure S3.
Prognostic re-staging univariate and multivariate models on PPS
In the univariate analysis for PPS, higher ISS/RISS stage at diagnosis and progression predicted worse outcomes (). Besides, patients without ASCT therapy or those who showed rapid progression of PFS <20 months also related to worse PPS. Subsequently, to assess the relationships among staging at diagnosis, prior ASCT, and re-staging at progression, we formed a separate multivariable-adjusted Cox proportional hazards model () using each ISS and RISS data. Regarding PPS adjusted by PFS, re-staging using ISS/RISS re-staging at progression showed superior prognostic utility over ISS/RISS staging at diagnosis. Moreover, re-staging by ISS and RISS at progression predicted PPS regardless of whether the patient had previously received first-line ASCT or had experienced early progression.
Table 2. Prognostic re-staging univariate and multivariate models on post progression survival
Prognostic stage migration univariate and multivariate models on PPS
Spearman’s analysis between re-staging at progression and stage migration showed that ISS re-staging is moderately correlated with ISS stage migration (r = 0.588, P = 0.01), while RISS re-staging is moderately correlated with RISS stage migration (r = 0.560, P = 0.01). Thus, dependent Cox models were prepared including ISS stage migration and RISS stage migration separately to evaluate the significance of stage migration in PPS prediction (). Univariate analysis demonstrated that both non-first-line ASCT, early progression, and deteriorated ISS/RISS stage migration predicted worse PPS. In multivariate analysis on PPS adjusted by PFS, maintained and deteriorated ISS/RISS migration showed worse PPS compared to improved ISS/RISS stage migration during progression, regardless of whether the patient was treated with first-line ASCT or had experienced early progression.
Table 3. Stage migration univariate and multivariate models on post progression survival.
Discussion
In this retrospective real-world study, we performed staging at diagnosis and re-staging at progression using both ISS and RISS in our consecutive, treated MM cohort, to investigate whether re-staging and stage migration from diagnosis can predict PPS in relapsed MM patients.
Initially, we employed survival analysis and then demonstrated that re-staging using ISS/RISS had the ability to discriminate the three groups of patients having significantly different PPS. More specifically, our study showed the mPPS of patients with ISS/RISS staging I, II and III at progression is approximately 4, 2 years, and 1 year respectively; the prognostic discrimination ability according to RISS re-staging at relapse/progression is consistent with a previously published report [Citation29]. Furthermore, as expected, re-staging at progression not only showed superior prognostic utility over stratification at diagnosis for both models, but was also an independent prognostic factor for PPS, regardless of whether patients had previously received ASCT or had early progression.
Migration to a higher ISS/RISS stage at progression was associated with poor PPS, which could be attributed to aggressive progression with higher disease burden and genetically high-risk abnormalities [Citation3,Citation8], as we found significantly higher incidence of Del(17P) in deteriorated ISS/RISS migration group compared to stage improved or maintained group. In contrast, migration to a lower stage at progression was associated with significant PPS, which could be attributed to the indolent biological behaviour of recurrent plasma cells with monoclonal gammopathy of undetermined significance-like features [Citation34,Citation35]. To further explore the impact of stage migration on PPS, we performed the correlation analysis between re-staging at progression and staging migration at first diagnosis. Given that a moderately moderate intensity of correlation was observed between them in the analysis (patients with advanced stage at progression are more likely to be classified as the deteriorated or maintained stratification group, while patients with lower stage are more likely to be classified as the improved or maintained stratification group), dependent Cox hazard ratio survival models were established with stage migration, first-line ASCT, and PFS. Not surprisingly, both stage migration by ISS and RISS at progression are independent prognostic impactors in PPS, regardless of whether patients had previously received ASCT or had early progression. To the best of our knowledge, this is the first study to demonstrate the prognostic utility of stage migration for MM.
This study has several limitations. First, this was a retrospective single-centre study involving heterogeneous treatment regimens. Second, our findings are only applicable to patients with relatively complete staging system parameters at relapse/progression but not to those with sustained remission or missing staging system parameters. Moreover, stage deviation can occur during re-staging, as the stage criteria based on biochemical indicators can be affected by medical intervention. Third, 57.1% (105/184) of the patients were re-staged to the RISS II at progression and such a large group may stand a good chance of having different cytogenetic architecture and anticipated PPS; thus, applying R2-ISS re-staging may help overcome this limitation. Fourth, compared to the previously established risk stratification algorithm [Citation18] for patients with relapsed myeloma that takes both patient frailty and disease aggressiveness into consideration, the ISS and RISS does not consider patient frailty (age and Eastern Cooperative Oncology Group performance status). Fifth, owing to the study design, stage migration could only occur in two ways in two of the three cases (for patients with stage I, it could only migrate to a higher stage or be maintained at the original stage, and for patients with stage III, it could only migrate to a lower stage or be maintained at the same stage). However, when the analysis was restricted to patients with ISS II/RISS II at diagnosis, stage migration can also discriminate relapsed patients into three groups with different PPS.
Although ISS/RISS re-staging is not perfect, our findings demonstrated the prognostic value of simple re-staging methods at progression using the ISS or RISS. This is particularly important for patients maintained at ISS/RISS III disease or those that migrated to advanced ISS/RISS stage at progression as it suggests that prior therapy was not sufficient to overcome the poor prognostic factors carried by patients; thus, post-progression treatment options with new drug regimens involving different mechanisms of action are needed. Previous studies showed that these patients may further benefit from consolidation therapy of high-dose chemotherapy followed by salvage ASCT, CD38-targeting monoclonal antibody therapy, or anti-BCMA CAR-T immunotherapy after progression [Citation36–40].
Owing to the lack of a direct comparison between the prognostic assessment values of ISS/RISS/R2-ISS and previously established models [Citation18,Citation41] in unified research, and as miscellaneous parameters or calculation methodologies cannot be easily applied in clinical implementation, further explorations on more practical score-weighted re-staging models like the R2-ISS, or a model combining patient frailty with cytogenetics, progression kinetics [Citation42,Citation43], and minimal residual disease [Citation44–46] may better predict PPS and guide clinical second-line treatment strategies in relapse setting. We expect that this model can be established using a large-scale, multicentre, prospective controlled trial in the future.
Conclusion
Our study shows that risk stratification of patients with MM at relapse/progression may change from that at initial diagnosis altering the patient's original prognostic trajectory.Thus, it is critical to perform dynamic risk stratification and develop an individualized treatment strategy at the time of relapse/progression in patients with MM.
Author contributions
G.A. and L.Q. designed the study. H.F. analysed data, prepared figures, and wrote the manuscript. H.F., W.Y., L.L.,J.L and J.X. collected data and performed follow-up. G.A. conceived the project and provided leadership. H.F., W.S., Y.X, S.Y., S.D., C.D, D.Z., and G.A. managed patients. G.A. suggested revisions. All authors reviewed the manuscript and provided final approval for submission.
Clinical trial notation
This trial was registered at www.clincialtrials.gov (NCT04645199).
Ethics statement
The NICHE-MM study was reviewed and approved by the Human Genetic Resource Administration of China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data of this study are available from the corresponding author by reasonable request.
Additional information
Funding
References
- Chakraborty R, Liu HD, Rybicki L, et al. Progression with clinical features is associated with worse subsequent survival in multiple myeloma. Am J Hematol. 2019;94(4):439–445.
- Goldman-Mazur S, Visram A, Kapoor P, et al. Outcomes following biochemical or clinical progression in patients with multiple myeloma. Blood Adv. 2022 Apr 12.
- Bygrave C, Pawlyn C, Davies F, et al. Early relapse after high-dose melphalan autologous stem cell transplant predicts inferior survival and is associated with high disease burden and genetically high-risk disease in multiple myeloma. Br J Haematol. 2021;193(3):551–555.
- Corre J, Montes L, Martin E, et al. Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk. Haematologica. 2020;105(9):e480–e483.
- Kastritis E, Roussou M, Eleutherakis-Papaiakovou E, et al. Early relapse after autologous transplant is associated with very poor survival and identifies an ultra-high-risk group of patients With myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(7):445–452.
- Kumar S, Mahmood ST, Lacy MQ, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42(6):413–420.
- Lee H, Duggan P, Chaudhry A, et al. Early relapse for multiple myeloma patients undergoing single autologous stem cell therapy: a single-center experience. Clin Lymphoma Myeloma Leuk. 2018;18(1):e69–e75.
- An G, Yan Y, Xu Y, et al. Monitoring the cytogenetic architecture of minimal residual plasma cells indicates therapy-induced clonal selection in multiple myeloma. Leukemia. 2020;34(2):578–588.
- Corre J, Cleynen A, du Pont S R, et al. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia. 2018;32(12):2636–2647.
- Croft J, Ellis S, Sherborne AL, et al. Copy number evolution and its relationship with patient outcome-an analysis of 178 matched presentation-relapse tumor pairs from the Myeloma XI trial. Leukemia. 2021;35(7):2043–2053.
- Dutta AK, Alberge JB, Sklavenitis-Pistofidis R, et al. Single-cell profiling of tumour evolution in multiple myeloma - opportunities for precision medicine. Nat Rev Clin Oncol. 2022;19(4):223–236.
- Goldman-Mazur S, Kumar SK. Current approaches to management of high-risk multiple myeloma. Am J Hematol. 2021;96(7):854–871.
- Jones JR, Weinhold N, Ashby C, et al. Clonal evolution in myeloma: the impact of maintenance lenalidomide and depth of response on the genetics and sub-clonal structure of relapsed disease in uniformly treated newly diagnosed patients. Haematologica. 2019;104(7):1440–1450.
- Misund K, Hofste O, Bruinink D, et al. Clonal evolution after treatment pressure in multiple myeloma: heterogenous genomic aberrations and transcriptomic convergence. Leukemia. 2022 Jul;36(7):1887–1897.
- Shen YJ, Mishima Y, Shi J, et al. Progression signature underlies clonal evolution and dissemination of multiple myeloma. Blood. 2021;137(17):2360–2372.
- Yan Y, Qin X, Liu J, et al. Clonal phylogeny and evolution of critical cytogenetic aberrations in multiple myeloma at single-cell level by QM-FISH. Blood Adv. 2022;6(2):441–451.
- Zamagni E, Barbato S, Cavo M. How I treat high-risk multiple myeloma. Blood. 2022;139(19):2889–2903.
- Hájek R, Delforge M, Raab MS, et al. Development and validation of a novel risk stratification algorithm for relapsed multiple myeloma. Br J Haematol. 2019 Nov;187(4):447–458.
- Hájek R, Gonzalez-McQuire S, Szabo Z, et al. Novel risk stratification algorithm for estimating the risk of death in patients with relapsed multiple myeloma: external validation in a retrospective chart review. BMJ Open. 2020 Jul 14;10(7):e034209.
- Du J, Lu J, Gao W, et al. Serum-free light chains combined with the revised international staging system could further distinguish the superior and inferior clinical outcome of multiple myeloma patients. Ann Hematol. 2020 Aug;99(8):1779–1791. doi:10.1007/s00277-020-04162-8. Epub 2020 Jun 27. PMID: 32594218.
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854.
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420.
- Abdallah NH, Binder M, Rajkumar SV, et al. A simple additive staging system for newly diagnosed multiple myeloma. Blood Cancer J. 2022;12(1):21.
- D'Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: a European myeloma network (EMN) report within the HARMONY project. J Clin Oncol. 2022 Oct 10;40(29):3406–3418.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869.
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012 Jan;26(1):149–157.
- Dimopoulos MA, Schjesvold F, Doronin V, et al. Oral ixazomib-dexamethasone vs oral pomalidomide-dexamethasone for lenalidomide-refractory, proteasome inhibitor-exposed multiple myeloma: a randomized phase 2 trial. Blood Cancer J. 2022 Jan 24;12(1):9.
- Hou J, Jin J, Xu Y, et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China continuation study. J Hematol Oncol. 2017 Jul 6;10(1):137.
- Tandon N, Rajkumar SV, LaPlant B, et al. Clinical utility of the revised international staging system in unselected patients with newly diagnosed and relapsed multiple myeloma. Blood Cancer J. 2017;7(2):e528.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548.
- Chiang CL, Huang HC, Shen CI, et al. Post-progression survival in secondary EGFR T790M-mutated non-small-cell lung cancer patients with and without osimertinib after failure of a previous EGFR TKI. Target Oncol. 2020;15(4):503–512.
- Imai H, Kaira K, Minato K. Clinical significance of post-progression survival in lung cancer. Thorac Cancer. 2017;8(5):379–386.
- Imai H, Kishikawa T, Minemura H, et al. Post-progression survival influences overall survival among patients with advanced non-small cell lung cancer undergoing first-line pembrolizumab monotherapy. Oncology. 2021;99(9):562–570.
- Paiva B, Vidriales MB, Rosinol L, et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia. 2013;27(10):2056–2061.
- Zhan F, Barlogie B, Arzoumanian V, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109(4):1692–1700.
- Cook G, Ashcroft AJ, Cairns DA, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3(7):e340–e351.
- Cook G, Williams C, Brown JM, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(8):874–885.
- Ramasamy K, Gay F, Weisel K, et al. Improving outcomes for patients with relapsed multiple myeloma: challenges and considerations of current and emerging treatment options. Blood Rev. 2021;49:100808.
- van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
- van de Donk N, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8(6):e446–e461.
- Chavda SJ, Maciocia PM, Mesiri P, et al. A new prognostic model for myeloma patients relapsing from upfront autologous transplantation based on ISS and PFS1. Br J Haematol. 2019 Apr;185(2):350–353.
- D'Agostino M, Zaccaria GM, Ziccheddu B, et al. Early relapse risk in patients with newly diagnosed multiple myeloma characterized by next-generation sequencing. Clin Cancer Res. 2020;26(18):4832–4841.
- Ravichandran S, Law S, Mahmood S, et al. Early relapse is an adverse prognostic marker in systemic immunoglobulin light chain (AL) amyloidosis. Leukemia. 2022 Apr;36(4):1180–1184.
- Cavo M, San-Miguel J, Usmani SZ, et al. Prognostic value of minimal residual disease negativity in myeloma: combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood. 2022;139(6):835–844.
- de Tute RM, Pawlyn C, Cairns DA, et al. Minimal residual disease after autologous stem-cell transplant for patients with myeloma: prognostic significance and the impact of lenalidomide maintenance and molecular risk. J Clin Oncol. 2022 Sep 1;40(25):2889–2900.
- Diamond B, Korde N, Lesokhin AM, et al. Dynamics of minimal residual disease in patients with multiple myeloma on continuous lenalidomide maintenance: a single-arm, single-centre, phase 2 trial. Lancet Haematol. 2021;8(6):e422–e432.