?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective:
Use red blood cell stabilizer to store the antibody screening and antibody identification reagent red blood cells (RBCs) treated with 0.01 mol/L DTT and investigate its value in the pre-transfusion examinations of patients treated with daratumumab.
Method:
Determined the optimal incubation time for the 0.01 mol/L DTT-treated RBCs method by evaluating the effect of treatment at different time points. Added ID-CellStab to store DTT-treated RBCs, determined the maximum shelf life of reagent RBCs by monitoring the hemolysis index, and assessed changes in the antigenicity of blood group antigens on the surface of RBCs during storage with antibody reagents.
Result:
A protocol for long-term storage of reagent red blood cells treated with the 0.01 mol/L DTT method was established. The optimal incubation time was 40-50 min. RBCs could be stored stably for 18 days after adding ID-CellStab. The protocol was able to eliminate pan-agglutination caused by daratumumab, with no significant changes in the antigens of most blood group systems, except for some attenuation of K antigen and Duffy blood group system antigens during the storage period.
Conclusion:
The storage protocol of reagent RBCs based on the 0.01 mol/L DTT method does not affect the detection of most blood group antibodies and retains a certain degree of detection ability for anti-K antibodies, allowing patients treated with daratumumab to quickly perform pre-transfusion examinations, making up for the shortcomings of currently commercial reagent RBCs.
Introduction
Multiple myeloma (MM) is a malignant plasma cell disease in which the tumor cells originate from plasma cells in the bone marrow, and its incidence accounts for about 10% of hematologic tumors [Citation1]. Because CD38 molecules are highly expressed on the surface of myeloma cells and only minimally expressed on red blood cells (RBCs), normal lymphocytes, and bone marrow cells [Citation2, Citation3], daratumumab, a monoclonal antibody targeting the CD38 antigen, is a new approach to MM therapy [Citation4, Citation5]. However, a disadvantage of daratumumab is its interference in pre-transfusion tests caused when the anti-CD38 antibodies in the patients’ plasma bind to the CD38 cell surface antigens of the screening reagent RBCs resulting in pan-agglutination in the indirect anti-human globulin tests (IATs), thus interfering with antibody screening/identification and cross-matching tests. According to current studies, the strength of the pan-agglutination is approximately 1 + to 2+, and it persists even after 6 months of drug withdrawal [Citation6].
0.2 mol/L dithiothreitol (DTT) is the most commonly used method to eliminate daratumumab interference internationally [Citation7]. DTT cleaves the disulfide bond in the extracellular region of the CD38 molecule, denaturing the CD38 antigen and thus eliminating the phenomenon of pan-agglutination. However, some clinically important antigens (e.g. K antigen) are also destroyed after the treatment [Citation8]. Since the destruction of K antigen is related to the concentration of DTT solution [Citation9], Hosokawa et al. [Citation10] proposed a new treatment protocol of 0.01 mol/L low concentration of DTT to preserve K antigenicity. But experimental details such as incubation time and the effect on other blood group antigens had not been explored. Moreover, DTT-treated reagent RBCs can’t be reused and DTT treatment is time-consuming. It takes 1–2 h to prepare the reagents for each patient to be tested [Citation11, Citation12], delaying the patients’ transfusion therapy. Therefore, our group established a storage protocol of reagent red blood cells based on 0.01 mol/L DTT treatment and explored its value in the pre-transfusion examinations of patients treated with daratumumab by systematically investigating the extended shelf life of reagent red blood cells and the changes of antigen intensity during the storage period.
Materials and methods
Research subjects
In this study, EDTA anticoagulated blood specimens from 20 patients treated in the Department of Hematology of the First People's Hospital of Foshan from 2019–2022 were selected as the research subjects. These patients were diagnosed with multiple myeloma and were receiving daratumumab at a dose of 16 mg/kg, administered weekly for the first and second months, biweekly for the next four months, and monthly after six months. All patients had been excluded from diseases such as autoimmune diseases and cold agglutinin syndrome that cause pan-agglutination phenomenon.
Reagents and instruments
ABO-Rh/Reverse grouping cassette, anti-IgG C3b/C3d cassette, Bliss, centrifuge, antibody screening reagent red blood cells, VITROS 5600 integrated system (Ortho Clinical Diagnostics (OCD), USA), antibody identification panel cells (Reagens, Hungary), antibody identification panel cells (Changchun Bioxun, China), 1M DTT (Solarbio, Beijing, China), pH 7.3 PBS (Wanjia, Henan, China), anti-K (Diagast, France), anti-D (Baso, Zhuhai, China), anti-Jka (Immucor, USA), anti-Lea, anti-Leb, anti-M (Millipore, USA), anti-C, anti-E, anti-e, antibody screening reagent red blood cells (Shanghai PBC, China), anti-c, anti-Fya, anti-Fyb, anti-S, anti-N, anti-P1 (CE-Immundiagnostika GmbH, Germany), incubator, ID-CellStab (Bio-rad, USA).
Pre-transfusion routine serological examinations
All specimens before and after treatment with daratumumab were subjected to pre-transfusion examinations such as ABO blood group identification, RhD blood group identification, irregular antibody screening tests, irregular antibody identification tests, and Coomb’s tests in accordance with the AABB Technical Manual [Citation13] and the manufacturers’ operating instructions. Patient plasma was stored at – 20°C and thawed at 37°C before use.
Incubation time of 0.01 mol/L DTT-treated RBCs method
Our group treated OCD antibody screening reagent RBCs using 0.01 mol/L DTT, based on a modification of the standard DTT method of the AABB Technical Manual by Hosokawa [Citation10] et al. A brief summary is as follows: ①Diluted 1 mol/L DTT with pH 7.3 PBS to 0.01 mol/L. ②2 ml of 0.01 mol/L DTT was mixed with 100 µl of packed red blood cells and divided into 6 aliquots using test tubes. ③Incubated at 37 °C for 10, 20, 30, 40, 50, and 65 min, respectively. ④Used Ph 7.3 PBS to wash the treated RBCs 4 times at room temperature. The DTT-treated RBCs were resuspended in pH 7.3 PBS and the concentration was adjusted to 3%. Then, indirect anti-human globulin tests were performed with DTT-treated RBCs and plasma from 20 patients treated with daratumumab in microcolumn gel cards to assess the effect of treatment with different incubation times. The AB type plasma of normal humans was used as the negative control. The configuration scheme is shown in .
Table 1. Configuration of the 0.01 mol/L DTT methods.
Determination of hemolysis index during storage
Using the spectrophotometric technique, the hemolysis index of four different brands of untreated reagent RBCs on the last day of the storage period was measured by VITROS 5600 integrated system to set the threshold for the degree of hemolysis of reagent RBCs in this study. Antibody screening/identification reagent RBCs from OCD and Reagens manufacturers were treated with 0.01 mol/L DTT method according to the optimal incubation time, after which ID-CellStab was added and the concentration was adjusted to 3%, stored in 4°C. The hemolysis index of the supernatant was measured every 3 days. Used DTT-treated RBCs added to pH 7.3 PBS as the control to evaluate the shelf life of reagent RBCs after the addition of cell stabilizers.
The hemolysis index is calculated from the absorbance of the sample at the primary wavelength of 522nM and the secondary wavelength of 750nM. The formula is as follows:
HI: hemolysis index, A: absorbance, λ: wavelength, [dA/dλ]: derivative absorbance vector, [aH]: hemolysis coefficient vector (522-750nM)
Assessment of changes in blood group antigens
Standardization of reagent antibodies
Diluted all antibodies in multiple ratios. The homozygous cells of the corresponding antigens in OCD reagent RBCs were selected for the standardization of reagent antibodies by tube tests or IATs according to the manuals. Four consecutive dilutions of each antibody with agglutination strength between 1 + and 4 + were selected for subsequent antigen intensity tests. Diluted antibodies were stored at – 20°C and thawed at 37°C before use.
Determination of antigen intensity
The agglutination strength of each antigen (including CD38) of reagent RBCs treated with 0.01 mol/L DTT method was tested on day 0, day 7, day 14, and day 18, using diluted antibody reagents, plasma from one of the patients treated with daratumumab, and corresponding antigen-positive cells by tube tests or IATs. Untreated reagent RBCs of the same period were used as controls. The intensity of RBC antigens was expressed as the total score of agglutination strength.
Result
Pre-transfusion routine serological tests
ABO blood group identification and RhD blood group identification in 20 patients treated with daratumumab were not interfered with by the drug. Direct anti-human globulin test results were positive in two patients (1+) and negative in the rest. Antibody screening and antibody identification tests showed pan-agglutination (1 + to 2+) in all patients.
Effect of treatment with different incubation times
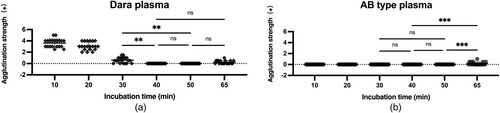
Treatment of reagent RBCs with 0.01 mol/L DTT for 40-50 min was able to eliminate the pan-agglutination phenomenon caused by daratumumab. When the incubation time was less than 30 min, some specimens still showed weakly positive agglutination ((a)). When the incubation reached 65 min, weakly positive reaction results were observed in some patients’ plasma and normal AB-type plasma of the control group ((b)).
Figure 1. Effects of different incubation times in 0.01 mol/L DTT-treated reagent RBCs. (a) plasma with daratumumab. (b) AB type plasma. (The values are the total score of agglutination strength of 3 antibody screening cells. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001, NS = No statistical significance). Dara: daratumumab
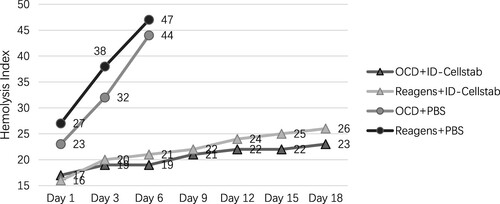
Shelf life of 0.01 mol/L DTT-treated reagent RBCs
The hemolysis index of commercial reagent RBCs from four manufacturers on the last day of the shelf life is shown in . Based on the lowest value of the hemolysis index among all reagent RBCs on the last day, the hemolysis index 26 was used as the threshold for the degree of hemolysis of reagent RBCs.
Table 2. Hemolysis index of reagent red blood cells of different manufacturers.
The hemolysis index of the DTT-treated reagent RBCs reached a set threshold on day 18 of the storage period after the addition of ID-CellStab. In contrast, reagent RBCs without the addition of cell stabilizers had reached the threshold of the hemolysis index on day 3 (not tested after day 6) ().
Dilution multiples of antibody reagents
The dilution multiple of the 16 antibodies is shown in , and the following four dilutions were selected for each antibody (Leb had only two) for subsequent antigen agglutination strength tests.
Table 3. The choice of antibodies dilutions for assessment of blood group antigen intensity.
Changes in the antigenicity of DTT-treated RBCs
After treatment of 0.01 mol/L DTT, plasma with daratumumab was tested for CD38 antigen on the surface of RBCs, and the results showed that agglutination had decreased to negative. Detection of other antigens of the blood group system using diluted antibody reagents showed varying degrees of strength reduction of K antigen, Fya antigen and Fyb antigen. But at the end of the storage period, all these antigens were still detected on some of the cells. No significant alterations were found in all antigens including Rh system, MNSs system, Lewis system, Kidd system, and P1 antigen ().
Table 4. Changes in the total score of antigen agglutination strength.
The number of each agglutination grades of CD38, K antigen, Fya antigen, and Fyb antigen are shown in . CD38 antigen of all RBCs was not detected after 0.01 mol/L DTT treatment. All K-positive RBCs (including one homozygous and three heterozygous cells) showed a decrease in agglutination strength after the 0.01 mol/L DTT treatment. Of the nine Fya-positive RBCs, three cells (two homozygous and one heterozygous) showed diminished agglutination strength, and the other six had no change. Of the eight Fyb-positive RBCs, one heterozygous cell showed diminished agglutination strength, and the other seven had no change.
Table 5. Agglutination strength of CD38, K antigen, Fya antigen, and Fyb antigen during the storage period.
Discussion
Patients with MM often require blood transfusion therapy during the course of their disease management [Citation14]. It has been reported that MM patients treated with daratumumab are rarely immunized to develop new alloantibodies due to factors such as the use of daratumumab, receiving chemotherapy, or underlying pathophysiology [Citation14, Citation15] (Table S1). But the phenomenon of pan-agglutination on pre-transfusion examinations is often mistaken for the presence of high-frequency antibodies or auto-antibodies and tends to mask the detection of pre-existing alloantibodies, which leads to the misdiagnosis and the occurrence of adverse transfusion reactions from transfusing incompatible blood products. Several articles stated that umbilical cord blood RBCs [Citation16] and Lu(a-b-) RBCs [Citation17] do not present pan-agglutination after administration of daratumumab, but there are no commercially available reagents based on the production of these two RBCs. Therefore, our group focused on the reagent RBCs of 0.01 mol/L DTT treatment including the extension of storage time and the assessment of changes in antigenic intensity.
In routine pre-transfusion examinations, all patients treated with daratumumab showed around 1 + to 2 + pan-agglutination in the antibody screening tests. Some articles reported that the pan-agglutination can persist even up to 6 months after withdrawal from the drug [Citation6]. In contrast, ABO blood grouping and RhD blood grouping are not affected by the drug because daratumumab, an IgG1 monoclonal antibody [Citation18], does not interfere with blood grouping serology experiments in saline media. The direct anti-human globulin test was also unaffected by the drug in most patients, which is related to the fact that the binding of CD38 antigen and antibody leads to the loss of CD38 antigen on the surface of RBCs, which can occur even after 6 h of drug administration [Citation19].
Although Hosokawa used 30 min as the incubation time for DTT-treated RBCs, this study found that the 0.01 mol/L DTT method was more effective in treating RBCs for 40-50 min, at which time the interference of daratumumab could be completely eliminated. And when the incubation time was less than 30 min, some reagent RBCs still had agglutination results in reaction with patient plasma, suggesting that CD38 antigen was not completely destroyed, while after 65 min of incubation, some reagent RBCs of the control group showed the phenomenon of auto-agglutination, indicating that the treatment time was too long and might have caused damage to the RBC membranes.
Hemolysis is a direct manifestation of disruption of the structural integrity of the RBCs. Hemolysis produces RBC debris, causing smearing effects that can interfere with the interpretation of results [Citation20]. The hemolysis index in automated analyzers is an efficient method for detecting hemolysis and there is a linear relationship between the hemolysis index and the amount of free hemoglobin [Citation21]. Therefore, the hemolysis index was added to this study to determine the storage time of reagent RBCs. ID-CellStab is a specially formulated, glycine buffered saline to stabilize RBCs. According to the product manual, both the antibody reagents and the microcolumn gel cards in our laboratory require 3% RBC suspension for experiments. Thus, our group added ID-Cellstab to the DTT-treated RBCs and adjusted the concentration to 3% so that the subsequent experiments with the tube tests and IATs would not require reconfiguration of the RBC suspension. The addition of the ID-CellStab enabled the stable storage of DTT-treated RBCs for 18 days, substantially delaying the rate of red blood cell destruction compared to those without stabilizers. This may be related to the preservatives, antibiotics, and nutrients in ID-CellStab, which serve to stabilize the red blood cell membrane structure, inhibit bacterial growth and maintain metabolism.
The agglutination strength is not only affected by the number of antigens on the RBC surface but also related to the concentration of detection antibodies. Too high or too low antibody activity can mask changes in antigen quantity. Therefore, in this study, the antibody reagents were standardized before the assessment of antigen intensity. Previous studies have shown that the K antigen is very sensitive to DTT treatment [Citation9]. But some of the K-antigen positive RBCs in this study were still able to show positive results at the end of the storage period, indicating that treatment with 0.01 mol/L DTT does not completely destroy the activity of K antigen and retains some ability to detect the strong antibodies (all 4+, some of 3+, ). Fya and Fyb antigens are usually considered to be resistant to DTT treatment [Citation22]. In this study, the attenuation of Fya and Fyb antigen intensity occurred in some RBCs after DTT treatment, which is consistent with the results of Sigle [Citation23] and Hugan’s [Citation24] studies. The reason for this needs further experimental investigation. Therefore, for these three antigens, especially the K antigen, it is recommended to select reagent RBCs containing the corresponding homozygous antigen for the DTT treatment to enhance the ability to detect the irregular antibodies. The antigens of Rh system, MNSs system, Lewis system, Kidd system, and P1 antigen did not show significant changes in agglutination strength before and after treatment, indicating that the 0.01M DTT treatment method did not affect the detection of antibodies to these blood group systems.
Compared with Hosokawa’s [Citation10] study, the present study provides a more in-depth evaluation of experimental details such as incubation time and antigen intensity, and extends the shelf life of DTT-treated RBCs to 18 days, solving the ‘pain point’ that DTT-treated RBCs can be used only once. Compared to the traditional high-concentration DTT treatment protocol of Disbro [Citation11] and Sigle [Citation23], the present protocol does not completely destroy the activity of the K antigen. Since anti-K antibodies can cause severe hemolytic transfusion reactions and hemolytic disease of the newborn, this protocol is more advantageous in ensuring the safety of blood transfusion, especially when applied to populations with a high frequency of K antigens such as Caucasians (9%) and Arabs (25%) [Citation22]. Besides, some studies pointed out that after 0.2M DTT treatment, the red blood cells would become sticky, and some RBCs even showed stronger pan-agglutination results in the microcolumn gel cards than before treatment [Citation25]. But this phenomenon was not observed in the 0.01M DTT treatment of our study. This is another advantage over traditional methods.
The shortcoming of this study is that the k antigen and antigens of blood group systems such as JMH, LW, and Lutheran are also sensitive [Citation6] to DTT treatment, but our laboratory lacks rare antibody reagents such as anti-k, anti-JMH, anti-LWa, anti-Lua, and anti-Lub. The effect of the 0.01 mol/L DTT treatment on these antigens has not been evaluated. It is worth noting that there are three anti-CD38 monoclonal antibodies including daratumumab, isatuximab and MOR202 nowadays. Our group used plasma from patients treated with daratumumab for the experiment in this study. Whether other anti-CD38 monoclonal antibodies are suitable for this protocol requires further validation, although a study has reported that pan-agglutination caused by isatuximab was weaker than daratumumab and could also be eliminated by the DTT solution [Citation26].
In conclusion, daratumumab is increasingly being used in the therapy of patients with relapsed and refractory multiple myeloma [Citation18]. This study provides transfusion departments and blood banks with a storage protocol of reagent RBCs for the pre-transfusion examinations of patients treated with daratumumab. This protocol enables stable storage of DTT-treated reagent RBCs for 18 days without affecting the detection of most antibodies, which is significantly better than other current treatment methods. In clinical applications, it can guarantee that patients receive safe, timely, and effective blood transfusion treatment. And as alloantibodies can be rapidly detected, current expert recommendations such as phenotyping and genotyping of patients’ RBCs prior to the daratumumab therapy and the provision of phenotype-matching or K-negative RBCs may be unnecessary [Citation6–8, Citation17]. Hosokawa named his research protocol the ‘Osaka method’, and similarly, our group named the described protocol the ‘Foshan method’.
Statement of ethics
The study was approved by the ethics committee of the participating institutions. Written informed consent was obtained from the patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nijhof IS, van de Donk NWCJ, Zweegman S, et al. Current and new therapeutic strategies for relapsed and refractory multiple myeloma: an update. Drugs. 2018;78(1):19–37.
- Albeniz I, Demir Ö, Türker-Şener L, et al. Erythrocyte CD38 as a prognostic marker in cancer. Hematology. 2007;12(5):409–414.
- Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. Faseb J. 1996;10(12):1408–1417.
- Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin Proc. 2016;91(1):101–119.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766.
- Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion. 2015;55(6. Pt 2):1545–1554.
- Chapuy CI, Aguad MD, Nicholson RT, et al. International validation of a dithiothreitol (DTT)-based method to resolve the daratumumab interference with blood compatibility testing. Transfusion. 2016;56(12):2964–2972.
- Lancman G, Arinsburg S, Jhang J, et al. Blood transfusion management for patients treated with anti-CD38 monoclonal antibodies. Front Immunol. 2018;9:2616.
- Branch DR, Muensch HA, Hian ALSS, et al. Disulfide bonds are a requirement for kell and cartwright (Yta) blood group antigen integrity. Br J Haematol 1983;54(4):573–578.
- Hosokawa M, Kashiwagi H, Nakayama K, et al. Distinct effects of daratumumab on indirect and direct antiglobulin tests: a new method employing 0.01 mol/L dithiothreitol for negating the daratumumab interference with preserving K antigenicity (Osaka method). Transfusion. 2018;58(12):3003–3013.
- Disbro WL. Stability guidelines for dithiothreitol-treated red blood cell reagents used for antibody detection methods in patients treated with daratumumab. Immunohematol. 2017;33(3):33–105.
- Izaguirre EC, del Mar Luis-Hidalgo M, González LL, et al. New method for overcoming the interference produced by anti-CD38 monoclonal antibodies in compatibility testing. Blood Transfus. 2020;18(4):290–294.
- Fung MK, Eder A, Spitalnik SL, et al. AABB technical manual. 19th ed. Bethesda (MD): American Association of Blood Banks; 2017.
- Phou S, Costello C, Kopko PM, et al. Optimizing transfusion management of multiple myeloma patients receiving daratumumab-based regimens. Transfusion. 2021;61(7):2054–2063.
- Ye Z, Wolf LA, Mettman D, et al. Risk of RBC alloimmunization in multiple myeloma patients treated by daratumumab. Vox Sang 2020;115(2):207–212.
- Schmidt AE, Kirkley S, Patel N, et al. An alternative method to dithiothreitol treatment for antibody screening in patients receiving daratumumab. Transfusion. 2015;55(9):2292–2293.
- Anani WQ, Duffer K, Kaufman RM, et al. How do I work up pretransfusion samples containing anti-CD38? Transfusion. 2017;57(6):1337–1342.
- Tzogani K, Penninga E, Schougaard Christiansen ML, et al. EMA review of daratumumab for the treatment of adult patients with multiple myeloma. Oncologist. 2018;23(5):594–602.
- Sullivan HC, Gerner-Smidt C, Nooka AK, et al. Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood. 2017;129(22):3033–3037.
- Yu Y, Sun X, Guan X, et al. Effects of hydroformylation treatment on the storage time and blood group antigen expressions of reagent red blood cells. Transfus Apher Sci. 2014;50(3):462–466.
- Söderberg J, Jonsson PA, Wallin O, et al. Haemolysis index – an estimate of preanalytical quality in primary health care. Clin Chem Lab Med 2009;47(8):940–944.
- Reid ME L-FC, Olsson ML. The blood group antigen facts book. 3rd ed. Waltham (MA): Academic Press; 2012.
- Sigle J-P, Mihm B, Suna R, et al. Extending shelf life of dithiothreitol-treated panel RBCs to 28 days. Vox Sang 2018;113(4):397–399.
- Hugan SL, Cooling L, Larsson VM. An evaluation of storage time for dithiothreitol-treated reagent cells. Transfusion. 2017;57(10):2545–2546.
- Wagner FF. Antibody testing in patients treated with anti-CD38: there is still room for improvement. Blood Transfus. 2020;18(4):244–246.
- Chami B, Okuda M, Moayeri M, et al. Anti-CD38 monoclonal antibody interference with blood compatibility testing: differentiating isatuximab and daratumumab via functional epitope mapping. Transfusion. 2022;62(11):2334–2348.