ABSTRACT
The Philadelphia (Ph) chromosome results from the formation of breakpoint cluster region (BCR)-Abelson 1 (ABL1) fusion gene (BCR-ABL1). The most common type of adult acute lymphoblastic leukaemia (ALL) is Ph chromosome-positive (Ph+); Ph+ ALL has an incidence of 25%∼30%. Several types of BCR-ABL1 fusion transcripts have been reported, including e1a2, e13a2 and e14a2. In addition, some rare BCR-ABL1 transcripts, such as e1a3, have been reported in chronic myeloid leukaemia. However, until now, the presence of e1a3 BCR-ABL1 fusion transcripts has only been reported in a few cases of ALL. In this study, a rare e1a3 BCR-ABL1 fusion transcript was found in a patient diagnosed with Ph+ ALL. However, the patient also suffered from severe agranulocytosis with pulmonary infection and died after being transferred to the intensive care unit before the significance of the presence of e1a3 BCR-ABL1 fusion transcript could be determined. In conclusion, e1a3 BCR-ABL1 fusion transcripts related to Ph+ ALL cases need to be better identified, and appropriate treatment strategies must be designed for such cases.
Introduction
The abnormal Philadelphia (Ph) chromosome is formed by the mutual translocation between chromosomes 9 and 22 (t [9; 22], [q34; q11.2]); the formation of the breakpoint cluster region-Abelson murine leukaemia 1 (BCR-ABL1) fusion gene is its main molecular biological feature [Citation1]. The protein encoded by the BCR-ABL1 fusion gene has abnormally high tyrosine kinase activity [Citation2]. This acquired gene rearrangement is most classically associated with chronic myeloid leukaemia (CML), but it has also been found in acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML) cases [Citation3]. The presence of the BCR-ABL1 transcript in ALL is associated with a relatively poor prognosis and low sensitivity to chemotherapy. The three typical BCR-ABL1 mRNA transcripts are e1a2, e13a2 and e14a2; other rare variants, such as e1a3, b2a3 and e6a2, have also been reported [Citation4,Citation5]. However, these observations were made almost exclusively in CML cases [Citation6], and very little is known about atypical transcripts in Ph chromosome–positive (Ph+) ALL. In this paper, the clinical course of a rare case of Ph+ ALL with an e1a3 BCR-ABL1 fusion transcript is reported.
Case report
A 48-year-old man was admitted to the clinic due to general fatigue in December 2021. Blood tests revealed a marked increase in white blood cells, thrombocytopenia and severe anaemia, which had been treated with component blood transfusion and hydroxyurea therapy in another hospital. The patient was admitted to our institution for further treatment in January 2022. There were no other remarkable findings noted in the patient or his family's medical histories. A physical examination indicated splenomegaly with multiple enlargements of the lymph nodes. Peripheral blood testing established a white blood cell count of 203.0 × 109/L (band neutrophils, 0.5%; segmented neutrophils, 0.5%; lymphocytes’ 9.5%; monocytes, 0.5%; blast cells, 89.0%), haemoglobin levels of 9.3 g/dL, a platelet count of 66 × 109/L and a lactate dehydrogenase level of 540 IU/L.
Three-dimensional colour Doppler echocardiography suggested congenital heart disease, including an atrial septal defect (secondary porous) with an atrial septal bulging tumour. Chest computed tomography showed inflammation of both lungs. A bone marrow examination revealed hypercellularity with 90% myeloperoxidase-negative blast cells. The immunophenotypes of these blast cells from flow cytometry were CD19+, CD10+, CD34+, CD20+, CD13+, CD15−, CD3− and CD56−. Quantitative real-time polymerase chain reaction was negative for the characteristic BCR-ABL1 fusion transcripts p210, p190 and p230.
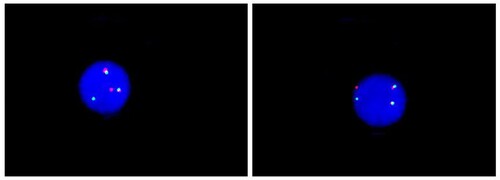
The ALL patient was initially treated with chemotherapy consisting of cyclophosphamide, pirarubicin, vincristine and prednisolone in accordance with the Chinese guidelines for diagnosis and treatment of adult acute lymphoblastic leukemia (2021 Edition). However, a chromosomal analysis of the bone marrow confirmed the presence of Ph chromosome translocation t (9; 22) (q34; q11.2) (). Furthermore, interphase fluorescence in situ hybridisation (iFISH) showed BCR-ABL1 rearrangement, t (9; 22) in 95.5% of the cells analysed (). To clarify whether a BCR-ABL1 fusion (including any rare type) occurred in this patient, next-generation sequencing (NGS) was used for further analysis, and the e1a3 BCR-ABL1 fusion transcript was detected. The BCR-ABL1 fusion gene is the exon 1 of BCR (NM_004327) and the exon 3 of ABL1 (NM_005157) genes and is connected downstream to form an in-frame fusion gene.
Figure 1. Cytogenetic analysis of bone marrow aspiration showing a karyotype 46,xy,t (9;22) (q34;q11). A gene translocation occurred between chromosomes 9 and 22 resulting Philadelphia chromosomes at the sites as indicated with arrows.
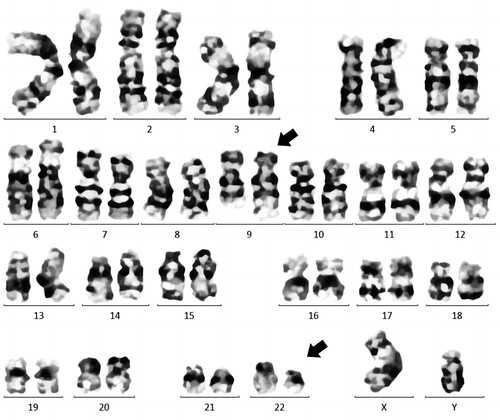
Figure 2. Metaphase FISH image showing BCR-ABL1 fusion gene at minor breakpoint BCR. LSI BCR/ABL1 dual colour translocation probe (Abbott) was used. Green: BCR, Red: ABL1.
Based on these results, the patient was diagnosed with Ph+ ALL. Despite admission to the laminar flow ward immediately after initiation of chemotherapy, the patient developed severe agranulocytosis with pulmonary infection. Tyrosine kinase inhibitor (TKI) was not given at the beginning of the patient's treatment due to persistent severe agranulocytosis associated with infection and financial difficulties.
The patient was not in remission after 15 days of treatment, and bone marrow smears showed that 83.5% of the lymphocytes were naive, and 80.2% of B−ALL cells were detected by bone marrow flow cytometry. Although the patient's care had included timely and broad-spectrum antimicrobial treatment, the patient still presented persistent hypotension, agranulocytosis and septic shock. In addition, the patient's blood culture was positive for Escherichia coli, and he was transferred to the intensive care unit (ICU) for further treatment. However, vasoactive drug treatment was ineffective, and the patient died within a few hours in the ICU.
Discussion
The BCR-ABL1 gene is expressed in 95% of patients with CML, 25–30% of adult ALL and 2–3% of childhood ALL [Citation7]. The most common transcript of the BCR-ABL1 fusion gene in ALL is e1a2, with a molecular weight of 190 kDa (that is, p190); the e13a2 (or b2a2) and e14a2 (or b3a2) transcript variants occur less frequently [Citation7,Citation8]. Other atypical variants, such as the e1a3 transcript reported in this index case, are rarely seen in ALL. This variant is a result of the translocation between exon 1 of BCR on chromosome 22 and exon 3 of ABL1 on chromosome 9. Only a small number of ALL cases with the e1a3-type BCR-ABL1 fusion transcript have been reported, and the clinical significance is still unclear [Citation4,Citation9–13].
The present investigation detected atypical transcripts in roughly 1–2% of all BCR-ABL1-positive patients [Citation14]. Theoretically, the BCR-ABL1 fusion gene should be detected in all samples of the Ph+ chromosome by karyotype analysis. The existence of rare BCR–ABL1 fusion transcripts may be the main reason for Ph+ and real-time PCR (RT–PCR) negative [Citation5,Citation15]. Moreover, iFISH detection uses a probe to directly target both ends of the gene breakpoint, so different break sites of BCR and ABL1 genes can be covered, which has a higher positive detection rate than using RT–PCR.
In the clinical practice of BCR–ABL1 gene screening, multiple diagnostic modalities can improve the detection rate of the rare fusion gene. The e1a3 BCR-ABL1 variant fusion is an atypical transcript in adult ALL; one of the main features of this type of translocation is the absence of the exon a2, normally present in the other translocations [Citation16]. The ABL1 exon a2 is generally selected as the primer binding region for the detection of the BCR-ABL1 fusion transcript by RT–PCR, especially for detecting the major BCR-ABL1 fusions [Citation9]. Therefore, BCR-ABL1 fusions lacking the ABL1 exon a2 region may have gone undetected by conventional RT–PCR methods in some Ph+ ALL cases [Citation13].
The deletion of ABL1 exon a2 from the BCR-ABL1 fusion transcript results in a protein that lacks two-thirds of the Src homology 3 (SH3) domain [Citation6]. The SH3 domain is considered to play a negative regulatory role in the kinase domain (SH1); the lack of an SH3 domain might result in a more aggressive form of Ph+ leukaemia [Citation13,Citation15]. In contrast, the SH3 domain is required as a signal transducer and activator of transcription 5 activation by BCR–ABL protein, leading to full leukemogenesis [Citation17]. Therefore, deletion of the SH3 domain might induce a less progressive clinical course.
López-Andrade et al. [Citation18] analysed 20 reports of B−ALL with e1a3 fusion; 44% of the cases were refractory or relapsed after therapy, and in 55% of cases, patients had died before the last follow up. Patients treated with conventional chemotherapy and TKI-targeted therapy are reported to have a higher survival rate than patients not treated with TKI [Citation4]. In this case, the patient progressed rapidly after diagnosis and eventually died. Considering this case and the others previously described, Ph+ ALL patients expressing e1a3 BCR-ABL1 transcripts are associated with an overall poor prognosis, suggesting that TKI and allogeneic stem cell transplantation should be considered for eligible patients in the early stages of the disease [Citation3,Citation7,Citation19,Citation20].
The patient's shorter duration of survival reported in this case might be due to severe infection, which complicated the treatment, cardiac insufficiency associated with congenital heart disease and delayed TKI treatment due to low granulocyte and financial difficulties. Infections are a common cause of mortality in ALL patients. In the index patient, an increased risk of severe infection could be linked to impaired cellular and humoral immunity from the cumulative effects of ALL and cancer chemotherapy.
Conclusion
A case of Ph + ALL with the rare e1a3 BCR-ABL1 fusion transcript is reported. If a BCR-ABL1 negative result is obtained by conventional screening, then RT–PCR, iFISH and NGS analysis should be carried out to avoid misdiagnosis. The correct diagnosis will allow physicians to make the right decisions. Stem cell transplantation and TKI treatment should be made available to patients as early as possible to improve survival.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are included in this published article.
References
- Ma B, Feng H, Feng C, et al. Kill two birds with one stone: a multifunctional dual-targeting protein drug to overcome imatinib resistance in Philadelphia chromosome-positive leukemia. Adv Sci (Weinh). 2022;9(13):2104850. doi:10.1002/advs.202104850
- Deininger MW, Vieira S, Mendiola R, et al. BCR-ABL tyrosine kinase activity regulates the expression of multiple genes implicated in the pathogenesis of chronic myeloid leukemia. Cancer Res. 2000;60(7):2049–2055.
- Kurita D, Hatta Y, Hojo A, et al. Adult acute lymphoblastic leukemia with a rare b3a3 type BCR/ABL1 fusion transcript. Cancer Genet. 2016;209(4):161–165. doi:10.1016/j.cancergen.2015.12.014
- Burmeister T, Schwartz S, Taubald A, et al. Atypical BCR-ABL mRNA transcripts in adult acute lymphoblastic leukemia. Haematologica. 2007;92(12):1699–1702. doi:10.3324/haematol.11737
- Demehri S, Paschka P, Schultheis B, et al. E8a2 BCR-ABL: more frequent than other atypical BCR-ABL variants? Leukemia. 2005;19(4):681–684. doi:10.1038/sj.leu.2403604
- Martinez-Serra J, Del Campo R, Gutierrez A, et al. Chronic myeloid leukemia with an e1a3 BCR-ABL fusion protein: transformation to lymphoid blast crisis. Biomark Res. 2014;2:14. doi:10.1186/2050-7771-2-14
- Langabeer SE. The e1a3 BCR-ABL1 fusion transcript in Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Lab Med. 2015;35(5):540–541. doi:10.3343/alm.2015.35.5.540
- Lyu X, Yang J, Wang X, et al. A novel BCR-ABL1 fusion gene identified by next-generation sequencing in chronic myeloid leukemia. Mol Cytogenet. 2016;9:47. doi:10.1186/s13039-016-0257-5.
- Fujisawa S, Nakamura S, Naito K, et al. A variant transcript, e1a3, of the minor BCR-ABL fusion gene in acute lymphoblastic leukemia: case report and review of the literature. Int J Hematol. 2008;87(2):184–188. doi:10.1007/s12185-008-0031-5
- Soekarman D, van Denderen J, Hoefsloot L, et al. A novel variant of the bcr-abl fusion product in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 1990;4(6):397–403.
- Iwata S, Mizutani S, Nakazawa S, et al. Heterogeneity of the breakpoint in the ABL gene in cases with BCR/ABL transcript lacking ABL exon a2. Leukemia. 1994;8(10):1696–1702.
- Wilson GA, Vandenberghe EA, Pollitt RC, et al. Are aberrant BCR-ABL transcripts more common than previously thought? Br J Haematol. 2000;111(4):1109–1111. doi:10.1046/j.1365-2141.2000.02471.x
- Shin SY, Cho JH, Kim HJ, et al. Two cases of acute lymphoblastic leukemia with an e1a3 BCR-ABL1 fusion transcript. Ann Lab Med. 2015;35(1):159–161. Erratum in: Ann Lab Med. 2015;35(4):477. doi:10.3343/alm.2015.35.1.159
- Crisà E, Nicolosi M, Ferri V, et al. Atypical chronic myeloid leukemia: where are we now? Int J Mol Sci. 2020;21(18):6862. doi:10.3390/ijms21186862
- Sheets JW, Eulitt P, He R, et al. Philadelphia chromosome-positive acute myeloid leukemia With e1a3 BCR-ABL1 fusion transcript. Hemasphere. 2020;4(6):e484. doi:10.1097/HS9.0000000000000484
- Al-Ali HK, Leiblein S, Kovacs I, et al. CML with an e1a3 BCR-ABL fusion: rare, benign, and a potential diagnostic pitfall. Blood. 2002;100(3):1092–1093. doi:10.1182/blood-2002-03-0930
- Roman J, Jimenez A, Barrios M, et al. E1a3 as a unique, naturally occurring BCR-ABL transcript in an indolent case of chronic myeloid leukaemia. Br J Haematol. 2001;114(3):635–637. doi:10.1046/j.1365-2141.2001.02971.x
- López-Andrade B, Sartori F, Gutiérrez A, et al. Acute lymphoblastic leukemia with e1a3 BCR/ABL fusion protein. A report of two cases. Exp Hematol Oncol. 2015;5:21. doi:10.1186/s40164-016-0049-y
- Langabeer SE, Haslam K, Kelly J, et al. Acute lymphoblastic leukaemia with an e1a3 BCR-ABL1 fusion. Acta Haematol. 2011;126(4):214–215. doi:10.1159/000330956
- Ahmed IO, Ochogwu LO, Owojuyigbe TO, et al. Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia with e1a3 BCR-ABL1 transcript in a Nigerian with sickle cell anemia: a case report. J Med Case Rep. 2021;15(1):504. doi:10.1186/s13256-021-03060-5
