ABSTRACT
Objectives:
The meta-analysis sought to evaluate the efficacy and safety of a combination of venetoclax (Ven) and azacitidine (AZA) in the treatment of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).
Methods
We searched PubMed, Excerpta Medica Database (EMBASE), Cochrane Library, and Web of Science for eligible studies from inception to June 2022. We used the Cochrane Risk of Bias 2.0 (RoB 2.0) and Methodological Index for Non-Randomized Studies (MINORS) to evaluate the quality of the included literature. The inverse variance method was used to calculate the pooled proportion and 95% confidence interval (CI).
Results
The meta-analysis included nineteen studies with a total of 1615 patients. The pooled overall CR/CRi (complete response (CR)/complete response with incomplete blood count recovery (CRi)) rate for AML and MDS was 57.9% (95% CI 49.5-65.9%, I2 = 83%). Subgroup analyses showed that the rate of pooled CR/CRi was 67.5% (95% CI 61.1-73.3%, I2 = 54%) for the new-diagnosed (ND) AML group, 30% (95% CI 20-44.1%, I2 = 66%) for relapsed/refractory (R/R) AML, and 67.6% (95% CI 52.6-79.8%, I2 = 65%) for MDS, respectively. One randomized controlled trial (RCT) showed that CR/CRi was 64.7% in ND-AML patients. A total of 9 studies reported adverse events, with neutropenia being the most common of grade 3–4 adverse events, with a rate of 53.7% (95% CI 61.1-73.3%, I2 = 54%).
Conclusion
The present meta-analysis demonstrated that the Ven + AZA regimen is efficacious for the treatment of AML and MDS, with it being more effective for ND-AML than R/R AML. The most common adverse effects of this regimen are grade 3–4 neutropenia and neutropenia with fever.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of hematopoietic malignancies characterized by a decrease in normal blood cells due to blocked proliferation and apoptosis of primitive cell clones, with the median age of diagnosis being 68 years [Citation1,Citation2]. Older patients are often unable to tolerate intensive chemotherapy due to having more comorbidities and lower physical status at the time of diagnosis. Even though a few elderly patients are evaluated as fit patients (eligible for intense chemotherapy) by the geriatric assessment (GA) tools, owing to the higher incidence of adverse risk cytogenetic abnormalities in older patients with AML, which leads to lower rates of complete remission and long-term survival with intense chemotherapy [Citation3,Citation4]. Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem/progenitor cell-derived clonal disorders with a high risk of conversion to AML, where the median age of diagnosis is approximately 70 years [Citation5,Citation6]. The primary therapy for MDS, especially for high-risk MDS, is hypomethylating agents, but the overall results are poor, with a 5-year survival rate of approximately 31% [Citation7]. In recent years, there has been a greater understanding of the molecular mechanisms of AML/MDS, which has promoted the application of targeted agents such as: FLT3 inhibitors, IDH inhibitors, and selective BCL-2 inhibitors in elderly AML/MDS patients, particularly selective BCL-2 inhibitors have shown promising results in AML/MDS clinical studies [Citation8–10].
Anti-apoptotic BCL-2 family proteins are widely expressed in myeloid malignancies. Venetoclax (Ven) is a BH3 mimetic that selectively binds to BCL-2 and activates cell apoptosis via mitochondrial outer membrane permeability [Citation11]. A combination of Ven and azacitidine (AZA) is recommended for the treatment of elderly or unfit AML patients. In recent years, several retrospective analyses or clinical trials have suggested the efficacy of Ven + AZA in AML treatment. However, the evidence from these studies was limited because the efficacy varied widely among studies and some studies were small in size [Citation12–14]. Given the efficacy of Ven + AZA in AML, the investigation of this combination has been promoted in MDS. A phase Ib clinical trial of Ven + AZA for de novo high-risk MDS showed an effective response time of 30 days and an overall response rate of 74% [Citation15]. However, the studies on Ven + AZA for MDS that have been reported so far are small-scale and exploratory.
Therefore, the objective of this study is to conduct a systematic review and meta-analysis to evaluate the efficacy and adverse events of Ven + AZA in patients with AML/MDS.
Materials and methods
Data sources and literature search strategy
This study has been conducted in the International Prospective System for Registration Review (Registration No. CRD42022347624). Two researchers jointly participated in the research process. Relevant articles published before June 2022 were searched in Pubmed, Cochrane, Web of Science, and EMBASE respectively. The keywords were ‘venetoclax or GDC-0199 or RG7601 or Venclexta or ABT-199’, ‘Azacitidine or Azacytidine or Vidaza or NSC102816 or 5 azacytidine’ ‘leukemia, myeloid, acute or leukemia or acute myeloid leukemia or ANLL or AML’, ‘Myelodysplastic syndrome or MDS’. The full search strategy is shown in Table S1.
Inclusion and exclusion criteria
Inclusion criteria: (1) Participants: Adult patients with AML and MDS treated with Ven + AZA (including newly diagnosed, refractory/relapsed patients); (2) Intervention: Ven + AZA; (3) Comparator: Intensive chemotherapy group, reduced-intensity chemotherapy group, supportive therapy group or no control group; (4) Outcome: The study provides the primary outcome on CR/CRi rates, secondary outcomes on ORR and median OS, and the rate of 3–4 grade hematologic adverse events; (5) Study design: Cohort study, randomized controlled trials (RCTs), or non-randomized controlled trials (NRCTs).
Exclusion criteria: (1) Case reports, reviews, meta-analyses, comments, conference abstracts, and non-human studies. (2) Treatment regimens that included drugs other than Ven in combination with AZA. (3) The study results did not include one of the primary outcome measures. (4) Original data could not be obtained. (5) Duplicate publications.
Two investigators (YFD and CHL) independently read the title, abstract and full text to decide whether the literature was included. Any discrepancy in the inclusion literature was resolved by consensus.
Definition of treatment response
CR/CRi consisted of complete response (CR) and CR with incomplete blood count recovery (CRi). ORR included CR/CRi, morphologic leukemia-free status (MLFS), and partial remission (PR). The criteria for response refer to the 2003 International Working Group, 2017 European Leukemia Net, and the International Response Unified Criteria revised by the MDS International Working Group in 2006 [Citation16–18]. Overall Survival (OS) was defined as the time between treatment initiation and death or was censored at the last follow-up.
Adverse events of different grades: Adverse events were graded according to the National Cancer Institute Common Terminology Standard for Adverse Events, VERSION 4.03 [Citation19].
Article quality assessment
In this meta-analysis, the risk of bias was assessed with the RoB 2.0 for RCTs, and NRCTs were assessed using the methodological index for non-randomized studies (MINORS) guidelines [Citation20,Citation21]. MINORS has 12 items, the first 8 were related to non-comparative studies, while the remaining 12 items were related to comparative studies. The items are scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The assessment of methodological quality is presented in Figure S1 and Table S2. Two investigators (YFD and CHL) independently evaluated each study for risk bias, and any discrepancy was resolved through discussion.
Data extraction and statistical analysis
Two investigators (YFD and CHL) independently collected the data using a pre-designed format. Discrepancies were resolved through consensus. Review Manager (version 5.4.1) was used for this meta-analysis. For dichotomous data CR/CRi, ORR, and adverse events, the generic inverse variance was used for dichotomous variable analysis without a control group, and a 95% confidence interval (CI) was reported for each measurement. This method uses the calculation method of ratio type data, where the odds ratio (OR) value calculated by Revman software is converted into the following method to obtain the final result: 95% CI: Pf = OR/ (1 + OR); 95% CI lower limit conversion: LLOR = LLOR/(1 + LLOR); 95% CI upper limit conversion: ULOR = ULOR/(1 + ULOR). Q test and I2 test (I2 ≤ 25%: no heterogeneity; 26-50%: low heterogeneity; 51-75%: intermediate heterogeneity; >75%: high heterogeneity) for heterogeneity analysis. For p <0.1 or I2 >50%, there was statistical heterogeneity among the references, and the random effects model was used. When p > 0.1 or I² < 50%, the fixed effects model was used for analysis [Citation22]. To determine the causes of heterogeneity, subgroup and sensitivity analysis were conducted on the results with high heterogeneity. Sensitivity analysis is performed by sequentially removing individual studies to assess the impact of each study on the statistical results (p <0.05 was considered statistically significant).
Results
Literature search results
A total of 2070 articles were retrieved. From these results, 647 duplicates and 1335 irrelevant papers were excluded. After reading the full text of the remaining 88 papers, 69 were excluded because of unavailable data or a lack of primary outcome. Finally, 1 RCT and 18 NRCTs met the inclusion criteria for this meta-analysis. Using the Revman software, a flow chart of literature screening was prepared (). A total of 1477 patients with AML and 138 patients with MDS were included in the study.
Characteristics of included studies
A total of 1 RCT and 18 NRCTs involving 1615 patients were eligible for this meta-analysis [Citation23–41], as shown in . The included studies were published between 2017 and 2022. The sample size of the trials ranged from 6 to 439 patients (median number 33) with ages ranging between 19 and 91 years, and a male proportion of 59%. Eleven of these studies originated from North America, five from Asia, and two from Europe. The last study was a multicenter RCT involving multiple countries. The use of this regimen (Ven + AZA) refers to . Seven studies published the number of cycles (from 1 to 12) of the Ven + AZA therapy.
Table 1. Clinical characteristics of patients included in the studies.
Table 2. The treatment regimen of Included Studies in the meta-analysis.
Assessment of article quality
The Cochrane Collaboration determined that one of the RCTs in this meta-analysis was not at a high risk of bias. The remaining 18 NRCTs were assessed using MINORS, two of which had a control group we assessed with 12 items, and the remaining 16 were single-arm studies that we could only assess with the first 8 items.
Efficacy of overall study
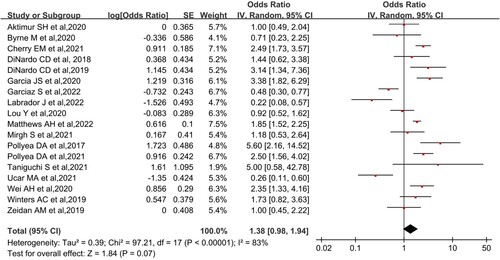
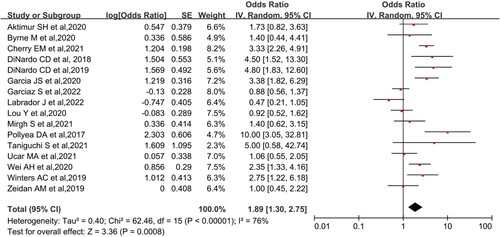
It mainly included the primary outcome on CR/CRi, as well as secondary outcomes on ORR and OS. Analysis of 18 NRCTs using random-effects models showed that the overall CR/CRi after Ven + AZA treatment for AML or MDS was 57.9% (95% CI, 49.5-65.9%, I2 = 83%; ) and overall ORR was 65.4% (95% CI, 56.5-73.3%, I2 = 76%; ). Both results showed high heterogeneity. A phase III RCT from DiNardo et al. suggested that CR/CRi was 64.7% and ORR achieved 66.4% [Citation25]. Because more than half of the patients in some studies included in this meta-analysis survived during the follow-up period, the median overall survival could not be reached. The longest follow-up in this meta-analysis was from a retrospective analysis of primary AML, with a median survival of 29.3 months (95% CI, 12.8-NR) [Citation37]. Another study involving relapsed/refractory (R/R) AML reported a median follow-up of 5.1 months (1.7-15.8 months) and median overall survival of 5.3 months (not reported 95% CI) [Citation40]. The included MDS groups did not obtain a median survival during the follow-up period.
Subgroup analysis
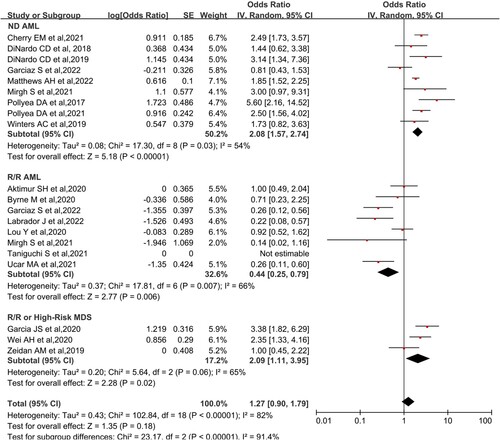
According to their disease characteristics, the patients were divided into three different study subgroups: newly diagnosed AML (ND-AML), R/R AML, and MDS. The results were as follows: ND-AML pooled CR/CRi 67.5% (95% CI,61.1-73.3%, I2 = 54%), R/R AML pooled CR/CRi 30.1% (95%CI, 20.0-44.1%, I2 = 66%), high-risk MDS 67.6% (95% CI, 52.6-79.8%. I2 = 65%) (). The results of ORR were as follows: ND-AML pooled ORR 77.0% (95% CI, 68.7-83.7%, I2 = 48%), R/R AML pooled ORR 45.7% (95% CI, 35.9-55.9%, I2 = 46%), R/R or High-risk MDS ORR 67.6% (95%CI, 52.6-79.8%, I2 = 65%) (Figure S2). AML was classified into favorable/intermediate and poor prognosis groups according to cytogenetic risk stratification. The CR/CRi rates for the favorable/intermediate and poor groups were 59% (95% CI, 39.4-76.1%. I2 = 65%) and 62.1% (95% CI, 48.4-73.1%. I2 = 0%), respectively (Figure S3). The results of the subgroup analysis according to race were as follows: North America pooled CR/CRi 63.8% (95% CI,63.1-73.0%, I2 = 44%), Asia pooled CR/CRi 45.9% (95%CI, 31.0-61.7%, I2 = 65%), and Europe pooled CR/CRi 26.5% (95% CI, 14.5-43.2%. I2 = 52%) (Figure S4).
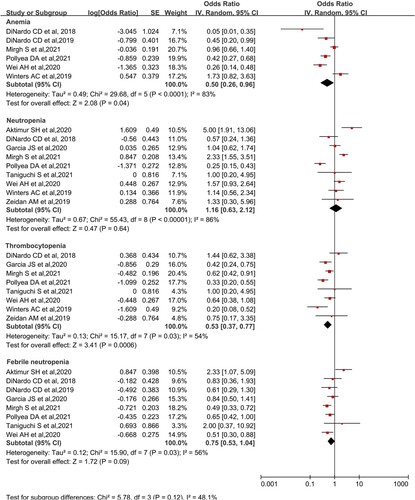
Adverse effects
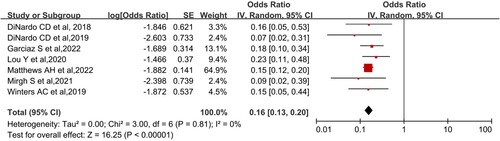
The analysis of grade 3–4 adverse reactions and mortality within 60 days was performed. Grade 3–4 adverse reactions were analyzed for anemia, neutropenia, thrombocytopenia, and febrile neutropenia. A total of 9 studies reported grade 3–4 adverse reactions. Seven studies reported mortality within 60 days. The combined results were as follows: Anemia was 33.3% (95% CI, 20.6-48.9%, I2 = 83%), neutropenia was 53.7% (95% CI, 38.7-67.9%, I2 = 86%), thrombocytopenia was 34.6% (95% CI, 27.0-43.5%, I2 = 54%), Febrile neutropenia was 42.9% (95% CI, 34.6-51.0%, I2 = 56%) (). The pooled mortality within 60 days was 13.8% (95%CI, 11.5-16.7%, I² = 0; ). A study DiNardo et al. reported that its grade 3–4 hematologic adverse effects were 26% for anemia, 45% for thrombocytopenia, and 42% for both neutropenia and neutropenia with fever, respectively [Citation25]. The study, however, did not report 60 days mortality (7% mortality at 30 days).
Sensitivity analysis
Sensitivity analysis was used to test the robustness of our results. We performed sensitivity analysis by removing one piece of literature at a time, which showed that the pooled rate of the remaining literature was similar to the overall meta-results (Figure S5). In addition, we found that Labrador J. et al. had the greatest impact on CR/CRi, with a CR/CRi changed to 0.60 (0.52-0.68) after removing that study [Citation32]. We also divided the studies into 3 subgroups based on disease characteristics, which showed a decrease from high to moderate heterogeneity.
Discussion
This meta-analysis included 19 papers with a total of 1615 patients and systematically evaluated the efficacy and safety of Ven + AZA in the treatment of AML and MDS. We conducted a systematic review on a phase Ⅲ RCT from DiNardo et al. study, in which the CR/CRi and ORR were 64.7% and 66.4% respectively [Citation25]. We combined the 18 NRCTs in this study and found that the CR/CRi and ORR rates were 57.9% and 65.4% respectively, and the combined I2 values were 83% and 76% respectively, indicating a high heterogeneity of the combined data. We excluded the literature one by one to perform the sensitivity analysis showing results were stable and found that the study by Labrador J. et al. had a significant impact on the CR/CRi rate [Citation32]. It could be related to that study cohort being refractory/relapsed AML patients and carrying more adverse molecular biological variations (e.g. P53/FLT3). Our subgroup analysis revealed that heterogeneity was reduced across all subgroups. We hypothesized that the source of heterogeneity was related to cytogenetics and mutations in each patient subgroup. Subgroup analysis showed that the CR/CRi and ORR rates were higher in the ND-AML and MDS groups than in the R/R-AML group, while the CR/CRi and ORR rates in the ND-AML group were comparable to the MDS group. The AML groups were classified into favorable/intermediate and unfavorable prognostic groups according to cytogenetic risk stratification. According to our findings, the favorable/intermediate and poor prognosis groups had similar CR/CRi rates of 59% and 62.1% for Ven + AZA, respectively. The similarity in remission rates between the two groups could be explained by a small sample size in the poor prognosis group, which reduced statistical validity. We analyzed grade 3–4 hematologic adverse events and found that neutropenia and neutropenia with fever were the most common, with incidences of 53.7% and 42.9%, respectively, which were higher than anemia and thrombocytopenia. We further found that the early mortality rate (< 60 days) of the regimen was 13.8%. The results of the adverse reaction analysis showed heterogeneity, which could be attributed to the age of the population included in each center, the cycles of the treatment regimen, and differences in treatment interventions.
Ven was approved in 2018 for the treatment of AML, and studies of Ven in combination with hypomethylating agents for AML/MDS have followed. A previous meta-analysis of Ven combined with hypomethylating agents for AML and MDS showed CR and ORR rates of 56% and 68%, respectively [Citation42]. This is consistent with the findings of our study. It appears that either AZA or decitabine (DEC) in combination with Ven for MDS and AML have similar efficacies. A network meta-analysis comparing single-agent AZA and DEC in AML and MDS revealed that DEC was more likely to achieve better CR and ORR, but AZA could achieve better OS in patients ≥75 years of age or with high-risk-MDS. DEC was likely to achieve better CR, ORR, and OS in elderly AML patients, but at relatively high toxicity [Citation43]. This meta-analysis suggests that the Ven + AZA regimen for AML had a CR/CRi of 60.4%, which is slightly lower than that reported in the DiNardo et al. phase Ⅲ clinical trial, which may be related to the fact that some of the literature we included were from real-world studies. Our study also suggests that the Ven + AZA regimen is more effective than single-agent AZA and conventional chemotherapy regimens in elderly patients with AML [Citation25, Citation44]. According to geographical subgroups, we observed higher efficacy in the North American group than in the Asian and European groups. This could be due to the different mutations backgrounds races, as well as the inclusion of fewer studies in Asia and Europe [Citation45]. However, more clinical evidence is needed to confirm this in the future. This meta-analysis showed that the remission rate and median OS of Ven + AZA for ND-AML were superior to R/R-AML efficacy. This could be because R/R-AML is more likely to carry an unfavorable karyotype and poor prognostic mutations which leads to Ven + AZA resistance [Citation46,Citation47]. The mechanism of resistance to Ven is still unclear, however recently it has been found that RAS/MAPK/MCL-1 may be one of the resistance mechanisms [Citation48]. Due to the different follow-up times for each study, a quantitative analysis of median overall survival was not possible. The longest follow-up in this study was from a retrospective analysis of ND-AML reported by Winters. et al., with a median survival of 29.3 months (95% CI, 12.8-NR) [Citation37]. Another study on R/R AML by Labrador. et al reported a median overall survival of 4 months (2.6-5.8 months) [Citation28]. We found that the CR/CRi of Ven + AZA for high-risk MDS was 67.6%, which is higher than the efficacy of MDS treated with hypomethylating agents alone [Citation49,Citation50]. A randomized, double-blind, phase Ⅲ clinical trial of Ven + AZA versus AZA + placebo for high-risk MDS is currently underway (NCT04401748). In the future, Ven + AZA may become one of the effective treatments for high-risk MDS. This study looked at the adverse effects of this regimen and found that the common adverse effects were neutropenia (53.7%) and neutropenia with fever (42.9%). This is similar to the results of the meta-analysis by Liu. et al describing a combination of Ven and hypomethylating agents in the treatment of AML/MDS [Citation42]. According to this study, AZA does not increase the incidence of adverse reactions as compared to decitabine. We calculated the early mortality (<60 days) rate of the regimen as 13.8%. A phase III clinical trial comparing AZA monotherapy with Semi-Intensive Fludarabine and Cytarabine in the treatment of elderly AML was reported by Vives et al, which showed a 60 days mortality rate of 20% in the AZA monotherapy group [Citation51]. This study and the other reported studies suggest that Ven combined with AZA does not increase early mortality.
The current study has several advantages. Firstly, we were the first to pool data focusing solely on Ven in combination with a single hypomethylating agent (AZA), making the study highly specific. Second, to ensure the reliability of our results, we used a comprehensive search strategy, clearly defined selection criteria, performed a strict quality assessment, and reported in accordance with the PRIMSA statement. Third, we investigated the efficacy of Ven + AZA in patients with AML/MDS for different geographic regions, which has seldom been reported in the past.
Every meta-analysis has flaws, and our study also has several limitations. First, this meta-analysis included a single RCT and lacked prospective studies, which may affect the reliability of the results. As a result, Therefore, more RCTs and prospective clinical studies are required to confirm our findings. Second, because more than half of the participants in the meta-analysis were from the United States, it is unclear whether the evidence from this study can be extended to other populations. Third, the data in this meta-analysis is highly heterogeneous. We used sensitivity analysis to obtain a few factors that caused heterogeneity, but identifying every cause of heterogeneity is difficult. Fourth, despite a comprehensive search of several databases, available literature on MDS is scarce (3 articles), which may limit statistical power and affect the robustness of the results. We hope to update our meta-analysis by adding more studies in the future. Finally, it is difficult to quantitatively synthesize data because of the small sample sizes of some analyzed subgroups, and more sample sizes must be accumulated for analysis.
Conclusion
The present meta-analysis demonstrated that the Ven + AZA regimen is efficacious for the treatment of AML and MDS, with it being more effective for ND AML than R/R AML. The most common adverse effects of this regimen are grade 3–4 neutropenia and neutropenia with fever, but it does not increase early mortality in patients. Additional data from prospective clinical studies are needed to further evaluate the efficacy of Ven + AZA in different genetic prognostic stratification of AML/MDS.
Ethics approval and consent to participate
This study did not directly involve human subjects and therefore did not require ethical approval for institutional board approval.
Availability of data and material
The original manuscripts contained in the study are contained in the article/supplementary material and inquiries can be directed to the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–52.
- Acute Myeloid Leukemia (AML) SEER 22 2015–2019, All Races, Both Sexes.
- Burnett AK, Hills RK, Russell N. Twenty five years of UK trials in acute myeloid leukaemia: what have we learned? Br J Haematol. 2020;188(1):86–100.
- Urbino I, Secreto C, Olivi M, et al. Evolving therapeutic approaches for older patients with acute myeloid leukemia in 2021. Cancers (Basel). 2021;13(20):5075.
- Zeidan AM, Shallis RM, Wang R, et al. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1–15.
- Surveillance Research Program, National Cancer Institute. All cancer sites combined: recent trends in SEER age-adjusted incidence rates, 2000-2019 by sex, delay-adjusted SEER incidence rate, all races, all ages. Accessed June 22, 2022.
- Zeidan AM, Shallis RM, Wang R, et al. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1–15.
- Daver N, Wei AH, Pollyea DA, et al. New directions for emerging therapies in acute myeloid leukemia: the next chapter. Blood Cancer J. 2020;10(10):107.
- Bazinet A, Bravo GM. New approaches to myelodysplastic syndrome treatment. Curr Treat Options Oncol. 2022;23(5):668–687.
- Palmieri R, Paterno G, De Bellis E, et al. Therapeutic choice in older patients with acute myeloid leukemia: A matter of fitness. Cancers (Basel). 2020;12(1):120.
- Lever JR, Fergason-Cantrell EA. Allosteric modulation of sigma receptors by BH3 mimetics ABT-737, ABT-263 (Navitoclax) and ABT-199 (Venetoclax). Pharmacol Res. 2019;142:87–100.
- Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253–E255.
- De Bellis E, Imbergamo S, Candoni A, et al. Venetoclax in combination with hypomethylating agents in previously untreated patients with acute myeloid leukemia ineligible for intensive treatment: a real-life multicenter experience. Leuk Res. 2022;114:106803.
- Yamamoto K, Shinagawa A, DiNardo CD, et al. Venetoclax plus azacitidine in Japanese patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Jpn J Clin Oncol. 2022;52(1):29–38.
- Saygin C, Carraway HE. Current and emerging strategies for management of myelodysplastic syndromes. Blood Rev. 2021;48:100791.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Cheson BD, Bennett JM, Kopecky KJ, et al. International working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003 Dec 15;21(24):4642–9.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25.
- Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03 (2010) Us Department of Health and Human Services. National Institutes of Health National Cancer Institute. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5 ( 7.pdf.
- Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane bias methods group; cochrane statistical methods group. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–60.
- Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: Long term follow-up from a phase 1b study. Am J Hematol 2021;96(2):208–217.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Cherry EM, Abbott D, Amaya M, et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv 2021; 5(24): 5565–5573.
- Byrne M, Danielson N, Sengsayadeth S, et al. The use of venetoclax-based salvage therapy for post-hematopoietic cell transplantation relapse of acute myeloid leukemia. Am J Hematol 2020;95(9):1006–1014.
- Labrador J, Saiz-Rodriguez M, de Miguel D, et al. Use of venetoclax in patients with relapsed or refractory acute myeloid leukemia: The PETHEMA registry experience. Cancers (Basel). 2022;14(7):1734.
- Jacqueline S, Safety GM. Efficacy, and patient-reported outcomes of venetoclax in combination With azacitidine for the treatment of patients With higher-risk myelodysplastic syndrome: A phase 1b study. Clin Adv Hematol Oncol. 2020;19(1):14–16.
- DiNardo CL, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228.
- Matthews AH, Perl AE, Luger SM, et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022;Jul 12;6(13):3997–4005.
- Ucar MA, Ozet G, Koyuncu MB, et al. Real world results of venetoclax combined with hypomethylating agents in relapsed/refractory AML. Eur Rev Med Pharmacol Sci. 2021;25(21):6557–6565.
- Zeidan AM, Pollyea DA, Garcia JS, et al. A phase 1b study evaluating the safety and efficacy of venetoclax As monotherapy or in combination with azacitidine for the treatment of relapsed/refractory myelodysplastic syndrome. Blood. 2019;134:565.
- Mirgh S, Sharma A, Shaikh M, et al. Hypomethylating agents + venetoclax induction therapy in acute myeloid leukemia unfit for intensive chemotherapy - novel avenues for lesser venetoclax duration and patients with baseline infections from a developing country. Am J Blood Res. 2021;11(3):290–302.
- Taniguchi S, Yamauchi T, Choi I, et al. Venetoclax in combination with azacitidine in Japanese patients with acute myeloid leukaemia: phase 1 trial findings. Jpn J Clin Oncol 2021;51(6):857–864.
- Pollyea DA, Stevens BM, Winters A, et al. Venetoclax (Ven) with azacitidine (Aza) for untreated elderly acute myeloid leukemia (AML) patients (Pts) unfit for induction chemotherapy: single center clinical experience and mechanistic insights from correlative studies. Blood. 2017;130:181.
- Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv 2019; 3(20): 2911–2919.
- Wei AH, Garcia JS, Borate U, et al. A phase 1b study evaluating the safety and efficacy of venetoclax in combination with azacitidine in treatment-naive patients with higher-risk myelodysplastic syndrome. Blood. 2019;134:568.
- Aktimur SH, Gunes AK, Akidan O, et al. Efficacy of the Combination of Venetoclax and Azacitidine in Elderly of Frail Relapsed/Refractory Patients with Acute Myeloid Leukemia, First Multi-Institutional Real World Experience from Turkey. UHOD - Uluslar Hematol-Onkol Derg. 2020;30(4):213–221.
- Lou YJ, Shao L, Mao LP, et al. Efficacy and predictive factors of venetoclax combined with azacitidine as salvage therapy in advanced acute myeloid leukemia patients: A multicenter retrospective study. Leuk Res 2020 Apr;91:106317.
- Labrador J, Saiz-Rodríguez M, de Miguel D, et al. Use of Venetoclax in Patients with Relapsed or Refractory Acute Myeloid Leukemia: The PETHEMA Registry Experience. Cancers (Basel). 2022;14(7):1734.
- Liu B, Guo Y, Deng L, et al. The efficacy and adverse events of venetoclax in combination with hypomethylating agents treatment for patients with acute myeloid leukemia and myelodysplastic syndrome: a systematic review and meta-analysis. Hematology. 2020;25(1):414–423.
- Liu W, Zhou Z, Chen L, et al. Comparison of azacitidine and decitabine in myelodysplastic syndromes and acute myeloid leukemia: A network meta-analysis. Clin Lymphoma Myeloma Leuk. 2021;21(6):e530–e544.
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–9.
- Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood. 2017;130(15):1699–1705.
- Tsai CH, Hou HA, Tang JL, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia. 2016;30(7):1485–92.
- Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10(4):536–551.
- Zhang Q, Riley-Gillis B, Han L, et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct Target Ther. 2022;7(1):51.
- Sekeres MA, Othus M, List AF, et al. Randomized phase II study of azacitidine alone or in combination With lenalidomide or With vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American intergroup study SWOG S1117. J Clin Oncol. 2017;35(24):2745–2753.
- Prebet T, Sun Z, Figueroa ME, et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. J Clin Oncol. 2014;32(12):1242–8.
- Vives S, Martinez-Cuadron D, Bergua Burgues J, et al. A phase 3 trial of azacitidine versus a semi-intensive fludarabine and cytarabine schedule in older patients with untreated acute myeloid leukemia. Cancer. 2021;127(12):2003–2014.