ABSTRACT
Background
Paroxysmal nocturnal hemoglobinuria (PNH) clone can be detected in some patients with aplastic anemia (AA) before treatment. But the prognostic value of the presence of pre-treatment PNH clone for intensive immunosuppressive therapy (IIST) is controversial and no consensus on whether the occurrence of PNH/AA-PNH syndrome is related to pre-treatment PNH clone.
Objective
This study aims to summarize the prognostic value of the presence of pre-treatment PNH clone treated with IIST among the AA patients and to elucidate its relationship with the development of PNH / AA-PNH syndrome.
Methods
All published studies on the prognostic value of pre-treatment PNH clone among AA patients were retrieved. Pooled odds ratio (OR) was calculated to compare the rates, along with 95% confidence intervals (CI) and p value to assess whether the results were statistically significant.
Results
The meta-analysis consisted of 15 studies with a combined total of 1349 patients in the cohort. Pre-treatment PNH clone had a positive effect on AA patients 6-month (pooled OR = 1.49,95% Cl: 1.06-2.08, P = 0.020), 12-month (pooled OR = 3.10,95% Cl: 1.89-5.10, P = 0.000), and overall hematological response rate (pooled OR = 1.69,95% Cl: 1.07-2.68, P = 0.024) after IIST. Patients with pre-treatment PNH clone are more likely to develop PNH/AA-PNH syndrome after IIST(pooled OR = 2.78,95%Cl:1.21-6.39, P = 0.016).
Conclusion
Patients with positive pre-treatment PNH clone had better hematological responses to IIST than negative. And, those patients are more likely to develop PNH/AA-PNH syndrome after IIST.
Introduction
Aplastic anemia (AA) is an autoimmune-related nonmalignant bone marrow failure disease mediated by abnormally activated T cells, which leads to the decrease in the quantity and quality of hematopoietic stem/progenitor cells (HSPC), bone marrow three-lineage hematopoiesis disorder, and peripheral blood cytopenia [Citation1]. Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hematopoietic stem cell disease induced by an acquired somatic PIG-A gene mutation resulting in clonal extension of hematopoietic stem cells lacking glycosylphosphatidylinositol (GPI)-anchored protein (GPI-AP) [Citation2]. The overlap between AA and PNH is well-known since the report of aplastic anemia-paroxysmal nocturnal hemoglobinuria (AA-PNH) syndrome in 1967 [Citation3]. With the application of flow cytometry (FCM), more than 70% of the patients with AA PNH clone, PNH clone is caused by HSPCS that acquire PIG-A gene mutations that evade recognition and destruction by abnormally activated T cells in vivo and form dominant clones in vivo to support hematopoiesis. Some patients were found to have PNH clone at the initial diagnosis [Citation4,Citation5].
Currently, the prognostic value of the presence of pre-treatment PNH clone on intensive immunosuppressive therapy (IIST) in AA patients is still controversial. Some studies have reported beneficial effects [Citation6,Citation7], while some studies have considered no effect [Citation8]. Furthermore, patients whose PNH clone were identified pre-treatment did not have clinical PNH-related symptoms, whereas different results were observed after IIST. Sugimori and Zhang found that patients with the presence of PNH clone were more likely to progress to PNH/AA-PNH syndrome [Citation6,Citation9] but the opposite results in studies reported by Li and Sun [Citation8,Citation10]. To further elaborate on these two issues, we performed this meta-analysis.
Material and methods
Literature retrieval and retrieval strategies
We used computer searches of four English databases, Web of Science, PubMed, the Cochrane Library and Embase, and four Chinese databases, Wangfang, Weipu, SinoMed, and China National Knowledge Infrastructure (CNKI) for articles published from inception to September 2022. In addition, we searched online registries clinicaltrials.gov and ChiCTR for studies in which registries had produced results from initial to September 2022, both using the following search terms ‘aplastic anemias’ or ‘AA ‘ and ‘ paroxysmal nocturnal hemoglobinuria ‘ or ‘CD55,CD59 ‘ or ‘PNH’. Searches were limited to human studies, free articles, and English and Chinese literature. Relevant literature was independently obtained by two investigators. Our criteria for inclusion in the literature were as shown below: (1) studies on aplastic anemia; (2) articles published from the inception of each database to September 15, 2022; (3) patients were tested for PNH clone before IIST, and divided into positive PNH clone (PNH+) and negative PNH clone (PNH-) groups according to the test results; (4) treatment regimen: ALG/ATG combined or not combined with CsA; (5) comparison of the efficacy of PNH + and PNH- groups for IIST; (6) sample size >25; (7) reported hematological responses and incidence of PNH/AA-PNH in each group; and (8) for non-English and Chinese, duplicate publications and incomplete data studies were eliminated.
Data extraction
Data were independently collected from the literature by two researchers, including publishing time, journal name, authors, number of patients, number of PNH+ and PNH- patients, gender, median age, disease severity, treatment regimen, 6- and 12-month hematological efficiency, overall response rate (defined as the entire follow-up period), and PNH/AA-PNH incidence. Any disagreement in extracted data was recorded and discussed with a third investigator until a consensus was reached.
Quality evaluation
The Newcastle-Ottawa (NOS) [Citation11] scale was used to evaluate the quality independently by two researchers. The NOS scale has nine entries divided in three main categories as follows: selectivity (four entries), comparability (two entries), and outcome (three entries). Quality was categorized into three categories: low quality (a mark of 1-3), moderate quality (a mark of 4-6), and high quality (a mark of 7-9). Disagreements in the evaluation of quality were recorded and discussed with a third researcher until a consensus was reached.
Statistical analysis
The STATA 15.1 software was used for all of the statistical analyses. Using the I2 statistic and Q test assessment the heterogeneity of the studies (I2 = 0-25% and p > 0.10 for no heterogeneity; I2 = 25-50% and p < 0.10 for moderate heterogeneity; I2 = 50-75% and p < 0.10 for high heterogeneity; and I2 = 75-100% or p < 0.10 for extreme heterogeneity). When heterogeneity between studies was determined (I2 ≤ 50% and p > 0.10), our meta-analysis used the fixed model, instead, using the randomized model. Furthermore, publication bias was assessed using Begg’s and Egger’s tests, P > 0.05, thinking that there is no publication bias [Citation12,Citation13].
Outcome
Study characteristics
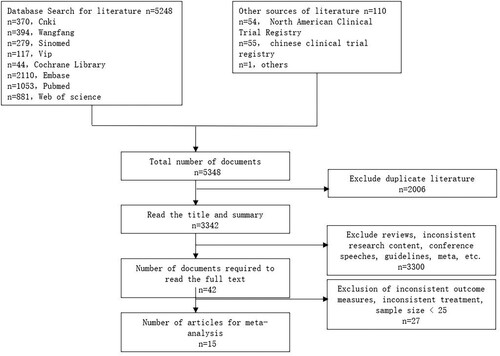
As indicated in , following a systematic literature search, a collection of 5348 studies were collected. Then, we removed 2006 duplicate documents, remaining 3342 documents required further screening. By reading the titles and abstracts, 3,290 studies with inconsistent research objectives were eliminated. Therefore, 42 studies need to be read in full. After scanning the full text, 27 studies were eliminated due to inconsistent observations (n = 6), incomplete data (n = 13), small sample size (n = 4), and overlapping populations (n = 4). Fifteen were finally included [Citation6–10,Citation14–23].
reveals the features of the studies included, one study was prospective [Citation17], the remaining 14 studies were retrospective, 11 studies were from Asia [Citation6–10,Citation15,Citation19–23], 2 studies were from the Americas [Citation14,Citation16], and 2 studies were from Europe [Citation17,Citation18]. There were 1349 patients in the cohort, 535 patients (37.2%) with the presence of pre-treatment PNH clone, with sample sizes ranging from 28 to 180, who were observed and followed up between 1997 and 2020. The median age of the patients was 7–56 years. One study did not mention the use of CsA in the treatment regimen [Citation14], 11 patients with MAA treated with ATG alone [Citation15], ALG/ATG combined with CsA was used in the remaining studies. All studies reported hematologic response rates, and seven of them mentioned the number of patients in each group who developed AA-PNH /PNH [Citation6–9,Citation20,Citation22]. PNH clone in peripheral blood were detected by flow cytometry or combined with fler before IIST in all patients. In the 15 eligible studies, scores of 6–8 were based on the NOS scale ().
Table 1. Characteristics of the included 15 studies.
Table 2. Quality assessment of individual study.
In addition, there were two measures of efficacy. Thirteen studies were based on camitta criteria [Citation6,Citation7,Citation9,Citation14–23]: 1. Complete response (CR): hemoglobin normal for age, neutrophil >1.5 × 10^9/L, and platelet >100 × 10^9/L; 2. Partial response (PR): transfusion independent and no longer meeting criteria for severe AA [Citation24]. The efficacy criteria for the other two studies were: 1. Basically cured: transfusion independent, the symptoms of anemia and bleeding disappeared, HGB > 120 g/L in male or HGB > 110 g/L in female, and ANC > 1.5 × 10^9 /L, PLT > 100 × 10^9 /L, and no recurrence was found after more than 1 year of follow-up. 2. Remission: transfusion independent, the symptoms of anemia and bleeding disappeared. HGB > 120 g/L in male or HGB > 100 g/L in female, WBC is about 3.5 × 10^9, and PLT was elevated to a certain extent, followed up for 3 months, the condition was stable or continued to improve. 3. Obvious progress: anemia and bleeding symptoms were significantly improved, and HGB increased by more than 30 g/L from the common value within 1 month before treatment without blood transfusion, and could be maintained for 3 months [Citation25]. In our meta-analysis, these two efficacy criteria were used to judge the efficacy. Response was defined as: 1. CR + PR 2. Cured basically + Remission + Obvious improvement.
Results of the meta-analysis
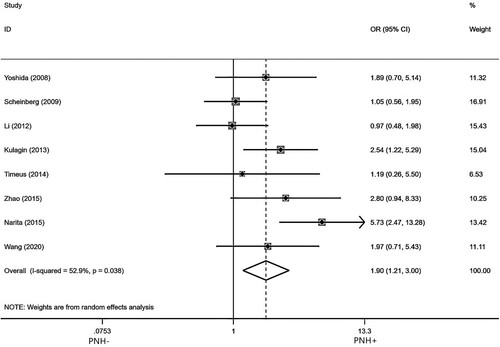
Eight studies [Citation8,Citation10,Citation15–19,Citation23] reported hematologic efficiency in AA patients treated with IIST for 6 months in the presence of pre-treatment PNH clone. After testing for heterogeneity, there was a highly heterogeneity among the studies selected for this meta-analysis (I2 = 52.9%, p = 0.038). So a random model was used to analyze, as indicated in . The pooled OR for the quality of hematologic response at 6 months was 1.90(95%Cl:1.21-3.00). PNH + compared to PNH- (p = 0.006), indicating that pre-treatment PNH clone has a positive effect on hematological efficiency of AA patients treated with IIST for 6 months.
Figure 2. Forest plot of the association between PNH clone and hematologic response rates at 6 months after IIST.
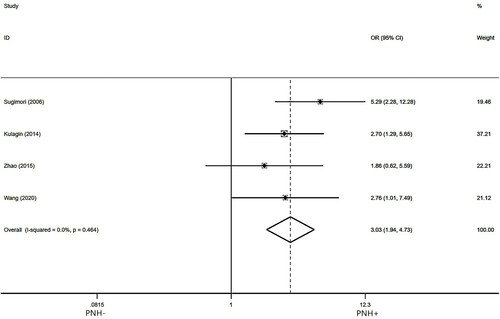
Four studies [Citation8,Citation10,Citation15–19,Citation23] reported hematologic efficiency in AA patients treated with IIST for 12 months in the presence of pre-treatment PNH clone. After testing for heterogeneity, there was no heterogeneity among the studies selected for this meta-analysis(I2 = 0.00%, p = 0.464,). So we used a fixed model to assess the combined OR, as indicated in . The pooled OR for the quality of hematologic response at 12 months was 3.03 (95%Cl:1.94-4.73),PNH + compared to PNH- (p = 0.000), which suggests that pre-treatment PNH clone has a positive effect on the hematological efficiency of AA patients treated with IIST for 12 months.
Figure 3. Forest plot of the association between PNH clone and hematologic response rates at 12 months after IIST.
Seven studies [Citation7,Citation9,Citation14,Citation20–23] reported the overall hematologic efficiency in AA patients treated with IIST in the presence of pre-treatment PNH clone. After testing for heterogeneity, there was no heterogeneity among the studies selected for this meta-analysis (I2 = 22.2%, p = 0.260). So we used a fixed model to assess the combined OR, as indicated in . The pooled OR for the quality of overall hematologic response was 1.65 (95%Cl:1.09-2.49), PNH + compared to PNH- (p = 0.019), indicating that pre-treatment PNH clone has a positive effect on the overall hematologic efficiency of AA patients treated with IIST.
Figure 4. Forest plot for the association between PNH clone and overall hematologic response rates after IIST.
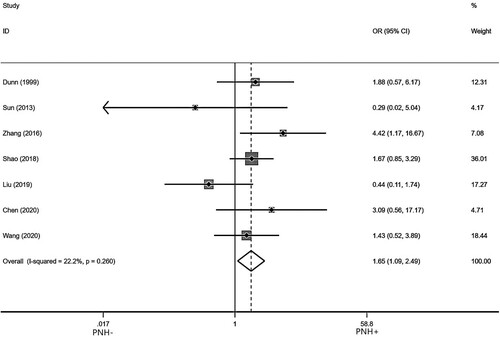
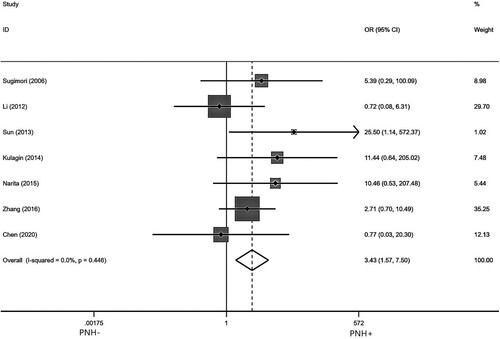
Seven studies [Citation6–10,Citation17,Citation22] reported the incidence of PNH/AA-PNH after IIST in AA patients with pre-treatment PNH clone. After testing for heterogeneity, there was no heterogeneity among the studies selected for this meta-analysis (I2 = 0.00%, p = 0.417). So we used a fixed model to assess the combined OR, as indicated in . The pooled OR value of PNH/AA-PNH syndrome was 2.78 (95% Cl: 1.21–6.39) for PNH+ compared to PNH- (p = 0.016), suggesting that a higher incidence of PNH/AA-PNH after IIST in the presence of pre-treatment PNH clone patients.
Sensitivity and bias in publication
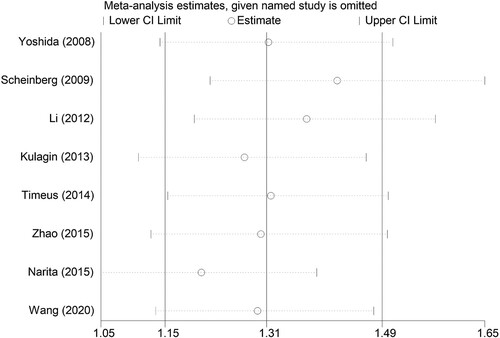
We used relevant 6-month efficiency data to test sensitivity and assess publication bias. As indicated in , when any study was omitted, the results did not show significant differences, indicating that each single study did not affect the stability between pre-treatment PNH clone and hematologic response at 6 months after IIST. Funnel charts show no evidence of dissymmetry ().
Figure 7. Publication bias test of AA on IIST 6-month hematological response: (a) Begg’s funnel plot. (b) Egger’s linear regression test.
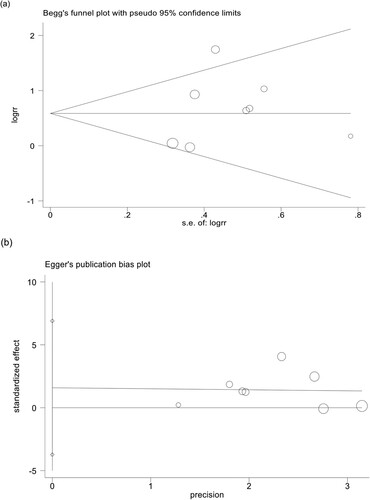
The Begg and Egger test found no evidence of publication bias (Begg’s test p = 0.536, Egger’s test p = 0.492).
Discussion
AA is a benign disease caused by the autoimmune destruction of early hematopoietic cells. Clonal hematopoietic was once considered to be a late complication of AA. With the application of high-throughput sequencing technology, clonal hematopoietic was found in early AA, including 67% of pediatric patients. PNH clonal hematopoiesis mediated by PIG-A gene mutation is one of the most common types of clonal hematopoiesis [Citation26]. GPI-A mutation leads to the loss of GPI-AP expression, and the obtained mutant HSPC has more survival and growth advantages in the bone marrow environment of AA patients. The absence of GPI-connected protein of the membrane ULBP1 and ULBP2 avoids recognition of aberrantly activated T cells. Therefore, GPI-A mutant cells can evade abnormal immune monitoring in vivo through ‘immune escape’ mechanism, and become dominant clones to support hematopoiesis [Citation27,Citation28]. Since the 1990s, the predictive effect of PNH clone on IIST in AA patients has been studied by scientists. However, the influence of the presence of pre-treatment PNH clone on IIST is still controversial. Sugimori demonstrated that the presence of pre-treatment PNH clone in AA patients has a positive effect on hematological response at 12 months (p < 0.01) [Citation6]. This result was also confirmed in a study by Kulagin (p = 0.0097) [Citation17], which included both adult and pediatric patients treated with ATG combined with CsA, and the number of PNH + was 68.0% and 59.2%, respectively. However, a retrospective study of pediatric AA patients showed that the presence of pre-treatment PNH clone predicted a lower overall hematologic efficiency (p = 0.005) [Citation21]. In our reseach, it was verified the presence of pre-treatment PNH clone has a positive effec for the 6 months (pooled OR = 1.49,95% Cl: 1.06-2.08, P = 0.020) and 12 months (pooled OR = 3.10,95% Cl: 1.89-5.10, P = 0.000) hematological response rate and overall hematological response rate (pooled OR = 1.69,95% Cl: 1.07–2.68, P = 0.024) of AA patients after IIST.
Currently, there are two main methods for the detection of PNH clone, traditional FCM and FLAER-based. In a study of 536 samples with suspected PNH, the detection of PNH clone by FCM and FLAER, with a detection rate of 6.2% for FCM and 11.8% for FLAER [Citation29]. Even with the same assay, there were differences in the antigens detected. Two studies both used FLAER to detect PNH clones in granulocytes, Sun used CD24 to label granulocytes but Kulagin used CD11b [Citation10,Citation17]. In our study, granulocytes were used to define a positive clone, assisted by erythrocytes and monocytes. If all are positive, we use the highest as a cut off. In addition, due to different devices and techniques used to detect PNH clone, as well as the number of peripheral blood cells collected from patients, the critical value for determining positive PNH clone was not all the same in each study. For example, Yoshida sets the critical value of PNH clone to >0.0037% [Citation15], and Zhao and Dunn to >1%[Citation14,Citation19]. Then, we used all the studies’ definitions of clone for the definition of PNH clone positive. If the patient’s clone is considered positive by the above studies, we consider it to be positive in this meta-analysis.
HSPCS with PIG-A mutations showed different trends after IIST, with some continuing to support hematopoietic production and some stopping hematopoietic, so that a small number of patients would show PNH-related clinical symptoms after treatment [Citation4,Citation30]. Some studies found that most of the patients who converted to PNH/AA-PNH syndrome during follow-up were found to have the presence of pre-treatment PNH clone [Citation6,Citation9], while Li found that PNH/AA-PNH syndrome occurred in the majority of patients who were negative during follow-up [Citation8]. Our research results indicate that patients with the presence of pre-treatment PNH clone were more likely to develop PNH/AA-PNH syndrome after IIST (pooled OR = 2.78,95%Cl:1.21-6.39, p = 0.016). Typical PNH clone was usually greater than 50%, and larger PNH clone was associated with a higher risk of venous thrombosis [Citation1]. Whereas the PNH clone of AA is smaller, with a median PNH clone of 0.7% (0.4%–1.5%) for monocytes and 0.2% (0.1%–0.7%) for granulocytes in Shao’s study[Citation20]. Hemolysis in AA after IIST is generally subclinical, with infrequent episodes of dark urine, and requires essentially no specific intervention with anticoagulation or eculizumab, and usually requires monitoring instead of treatment.
Our meta-analysis still has some limitations. First, only one prospective study was included [Citation17], and the rest of the studies were retrospective. Second, AA is a rare disease with a limited number of patients included in the study, with two of them having a sample size of <40 [Citation18,Citation22]. Third, it was only in 2017 that the International Clinical Cytometry Society formally proposed a standardized nomenclature regarding PNH clones: 1. PNH cells >1%, it was referred to as PNH clone; 2. PNH clone were called low-volume PNH clone when PNH cells were 0.1% to 1%; and 3. PNH cells < 0.1%, a few GPI-deficient cells or a few PNH phenotype cells are present [Citation31]. There is no uniform definition of PNH clone in the world before this. Therefore, the definition of PNH clone-positive in each study was adopted for analysis in our study. Finally, although six studies included children, only three studies mentioned the overall hematologic response rate of children with AA treated with IIST. And, the remaining studies only reported the hematologic response rate at 6 months or 12 months. Due to the limited amount of literature available for our study, we did not focus on the predictive effect of the presence of pre-treatment PNH clone on pediatric patients. In conclusion, our study found that the presence of pre-treatment PNH clone has a positive effect on the hematological response after IIST. Patients with the presence of pre-treatment PNH clone are more likely to develop PNH/AA-PNH syndrome after IIST.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656.
- Hill A, DeZern AE, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;3:17028.
- Lewis SM, Dacie JV. The aplastic anaemia–paroxysmal nocturnal haemoglobinuria syndrome. Br J Haematol. 1967;13(2):236–251.
- Ren X, Li X, Huo J, et al. Small PNH clones detected by fluorescent aerolysin predict a faster response to immunosuppressive therapy in patients with severe aplastic anaemia. Hematology. 2020;25(1):348–355.
- Wang L, Liu H. Pathogenesis of aplastic anemia. Hematology. 2019;24(1):559–566.
- Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107(4):1308–1314.
- Narita A, Muramatsu H, Sekiya Y, et al. Paroxysmal nocturnal hemoglobinuria and telomere length predicts response to immunosuppressive therapy in pediatric aplastic anemia. Haematologica. 2015;100(12):1546–1552.
- Li YM, Li XX, Ge ML, et al. The clinical significance of evolution of paroxysmal nocturnal haemoglobinuria clones in aplastic anemia patients. Chin J Hematol. 2012;33(2):117–122.
- Zhang J, Li XX, Shi J, et al. Clinical characteristics and evolution of paroxysmal nocturnal hemoglobinuria clones in patients with acquired aplastic anemia. Chin J Hematol. 2016;37(2):124–129.
- Sun YX, Zhu MQ, He GS, et al. The variation and clinical significance of paroxysmal nocturnal hemoglobinuria clone in patients with aplastic anemia before and after immunosuppressive therapy. Chin J Intern Med. 2013;52(7):585–589.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101.
- Egger M, Davey SG, Schneider M. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634.
- Dunn DE, Tanawattanacharoen P, Boccuni P, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131(6):401–408.
- Yoshida N, Yagasaki H, Takahashi Y, et al. Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br J Haematol. 2008;142(3):427–435.
- Scheinberg P, Wu CO, Nunez O, et al. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–216.
- Kulagin A, Lisukov I, Ivanova M, et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. Br J Haematol. 2014;164(4):546–554.
- Timeus F, Crescenzio N, Longoni D, et al. Paroxysmal nocturnal hemoglobinuria clones in children with acquired aplastic anemia: a multicentre study. PLoS One. 2014;9(7):e101948.
- Zhao X, Zhang L, Jing L, et al. The role of paroxysmal nocturnal hemoglobinuria clones in response to immunosuppressive therapy of patients with severe aplastic anemia. Ann Hematol. 2015;94(7):1105–1110.
- Shao HJ, Ji ZH, Miu MH, et al. Analysis of the role of paroxysmal nocturnal hemoglobinuria clones in acquired aplastic anemia in children(in Chinese). J Clin Pediatrics. 2018;36(03):192–196.
- Liu LP, Chen XJ, Yang WY, et al. Predicting response to porcine antilymphocyte globulin plus cyclosporine A in children with acquired severe aplastic anemia. Pediatr Res. 2019;86(3):360–364.
- Chen JJ, Liu KK, Chu JH, et al. Long-term clinical study on efficacy of combined immunosuppressive therapy in aplastic anemia in children(in Chinese). J China Pediatric Blood and Cancer. 2020;25(03):133–137. + 143.
- Wang H, Liu H, Wang T, et al. Relationship between immune status after ATG treatment and PNH clone evolution in patients with severe aplastic anemia. J Clin Lab Anal. 2021;35(3):e23667.
- Camitta BM, Thomas ED, Nathan DG, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53(3):504–514.
- Red Blood Cell Disease (Anemia) Group, Chinese Society of Hematology, Chinese Medical Association. Chinese expert consensus on the diagnosis and treatment of aplastic anemia. Chin J Hematol. 2017;38(1):1–5.
- Babushok DV, Perdigones N, Perin JC, et al. Emergence of clonal hematopoiesis in the majority of patients with acquired aplastic anemia. Cancer Genet. 2015;208(4):115–128.
- Sun L, Babushok DV. Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood. 2020;136(1):36–49.
- Hanaoka N, Kawaguchi T, Horikawa K, et al. Immunoselection by natural killer cells of PIGA mutant cells missing stress-inducible ULBP. Blood. 2006;107(3):1184–1191.
- Sutherland DR, Kuek N, Azcona-Olivera J, et al. Use of a FLAER-based WBC assay in the primary screening of PNH clones. Am J Clin Pathol. 2009;132(4):564–572.
- Luzzatto L. PNH phenotypes and their genesis. Br J Haematol. 2020;189(5):802–805.
- Illingworth A, Marinov I, Sutherland DR, et al. ICCS/ESCCA consensus guidelines to detect GPI-deficient cells in paroxysmal nocturnal hemoglobinuria (PNH) and related disorders part 3 - data analysis, reporting and case studies. Cytometry B Clin Cytom. 2018;94(1):49–66.