ABSTRACT
Relapsed/Refractory Diffuse Large B Cell Lymphoma have a dismal prognosis in need of innovative treatments. This prospective phase 2 study enrolled 32 patients between 2013 and 2017 with Relapsed/Refractory Diffuse Large B Cell Lymphoma treated with Rituximab and Lenalidomide (R2). Median age was 69 years (40–86), 90.1% had received at least 2 prior lines of treatment, 81% were defined as having High Risk disease according to our criteria and ECOG performance status was > 2 in 51.6%. Patients received a median number of 2 cycles of R2 (1–12). With a median follow up of 22.6 months, the objective response rate was 12.5%. Median progression free survival was 2.6 months (95% CI, [1.7–2.9]) and median overall survival was 9.3 months (95% CI, [5.1–Not estimable]). This study therefore did not achieve its primary endpoint and the R2 regimen cannot be recommended in Relapsed/Refractory Diffuse Large B Cell Lymphoma patients with High Risk features.
Introduction
Diffuse large B cell lymphomas (DLBCL) account for 30% of all Non Hodgkin Lymphoma (NHL) with approximately 150,000 new cases diagnosed yearly in the world. Despite an advanced stage at diagnosis among the vast majority of patients, around 60% will be cured with a R-CHOP treatment [Citation1]. Nevertheless, patients who fail frontline R-CHOP therapy have a poor outcome, especially those who are refractory or relapse shortly after first line treatment. Even though some patients can be cured with a second line of treatment entailing autologous stem cell transplantation (ASCT), most of these patients turn out to be refractory to subsequent chemo based treatments and eventually only 30%–40% of patients will respond to a salvage treatment according to the SCHOLAR-1 study [Citation2].
Lenalidomide (LEN) is an oral immunomodulatory drug (Cereblon E3 ligase modulator; CelMod) with direct antitumor effects as well as indirect effects on microenvironnement. In vitro studies have shown that exposure of lymphoid cells to lenalidomide was responsible for a down-regulation of MYC and its genic targets through cereblon and IRF4 as well as up-regulation of tumor suppressor genes.
A phase 2 study [Citation3] has shown overall response rates of 28% in DLBCL and 45% in transformed lymphoma. Complete responses (CR) ranged from 7% to 21% in those two situations. Due to the observed single-agent activity of both rituximab and lenalidomide, the potential to increase antibody-dependent cell-mediated cytotoxicity and the lack of overlapping toxicity profiles, we hypothesized that the combination might prove to be an effective therapeutic regimen for patients with relapsed/refractory NHL.
We therefore conducted a single-stage phase 2 study that enrolled patients with R/R DLCBL. High-risk patients in this study were defined as having a refractory disease or patients relapsing within 12 months after frontline treatment and not eligible to high dose chemotherapy (HDC) and ASCT. Other high risk features were patients who had received ≥3 prior therapy lines (HDC and ASCT included) and patients with high adjusted age international prognostic index (aaIPI), patients with histological transformation (HT) or patients with a non-germinal center (non-GC) COO signature at relapse.
Materials and methods
Patients
Eligible patients aged ≥18 years had histologically confirmed CD20+ B-cell relapsed DLBCL after at least 2 prior regimens, or refractory disease to first-line therapy (and not eligible to HDC and ASCT). Cell of origin (COO) was assessed at time of entry in the study. High-risk patients were defined as refractory patients or patients relapsing within 12 months and not eligible to high dose chemotherapy and ASCT. Other high risk features were patients who had received ≥3 prior therapy lines (HDC and ASCT included) and patients with high adjusted age international prognostic index (aaIPI), patients with histological transformation (HT) or patients with a non-germinal center (non-GC) COO signature at relapse. COO signature was defined using immunohistochemical staining according to the Hans classification.
Patients were treated at Institut Paoli-Calmettes, Marseille, France.
The study was approved by the Institutional Review Board at Institut Paoli Calmettes, Marseille, France, and was compliant with institutional guidelines and the Declaration of Helsinki.
Informed written consent was obtained from all participants before enrollment. The study was registered as NCT01939327.
Additional inclusion criteria include the following: patients with bidimensional measurable disease; Eastern Cooperative Oncology Group performance status of 0,1, 2 or 3; 1 prior line of therapy (prior autologous stem cell transplant was allowed); a serum bilirubin concentration <2 mg/dl (except in case of hemolytic anemia) and calculated creatinine clearance (Cockcroft-Gault formula) of > 50 mL/min; platelet counts >60,000/mm3 and an absolute neutrophil count >1500/mm3; and aspartate amino transferase and alanine amino transferase concentrations of less than five times the upper limit of normal. Candidates for autologous Stem Cell Transplantation (ASCT) or patients who had received a prior allogenic stem cell transplantation were excluded from this trial as well as patients with active central nervous system lymphoma history, HIV infection or prior use of lenalidomide.
Study design
All patients received 25 mg oral lenalidomide daily on days 1–21 of each 28-day cycle and 375 mg/m2 of intravenous rituximab on day 8 of each cycle and up to a maximum of 12 cycles.
The study design required a reduction of the lenalidomide dose from 25 to 20, 15, 10, and 5 mg in a de-escalating fashion if there were grade 3 or 4 non-hematological toxicities or hematological toxicities.
Adverse events (AE) were assessed weekly during the first month of treatment and twice a month thereafter based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Anticoagulants, antithrombotic medication and prophylaxis for tumor lysis syndrome with allopurinol were not required by the protocol. Patients were treated until disease progression or for up to 12 cycles of treatment or withdrawal for toxicity.
The primary efficacy end point was the overall response rate (complete response (CR) or partial response (PR) or stable disease (SD)). Response to treatment was assessed according to the Cheson 2007 criteria every 3 cycles using positron emission tomography (PET) or PET/CT. Secondary objectives were duration of response (DOR), progression free survival (PFS), time to progression (TTP), overall survival (OS) and safety. The study was supported by Celgene (Bristol Myers Squibb company).
Statistical analysis
Statistical analyses were performed at the significance level α = 0.05 by using SAS® 9.4 software. Patients’ characteristics were described with counts (frequencies) for categorical endpoints and medians (ranges) for quantitative variables. Percentages were calculated based on available data.
According to East software calculation, 38 eligible patients were planned to be enrolled to demonstrate that the true overall response rate (complete response or partial response or stable disease) was no less than p0 = 20% (considered unacceptable) with an alpha risk of 5% and a power of 90% under the opposite alternative p1 = 40% (desirable rate). This sample size was taking into account a rate of 10% non-evaluable patients. Overall response rate was tested to the theoretical value of 20% by a right-sided exact binomial test. Associated 90%-level exact binomial bilateral confidence intervals (CI) were estimated for overall and objective (complete response or partial response) response rates. These analyses were performed on all subjects with at least one post-baseline efficacy assessment (CT/MRI scan) performed at least 56 days (+/−7days) after Day 1 Cycle 1.
Overall Survival (OS) was defined as the time from inclusion to death. Progression Free Survival (PFS) was defined as the time from inclusion to progression or death. Time to Progression (TTP) was defined as the time from inclusion to progression. Patients without considered events were right-censored at the last follow-up. Time-to-event criteria and associated bilateral confidence intervals were estimated by using the classic Kaplan-Meier's method. Follow-up were estimated by using the reverse Kaplan-Meier's method.
Results
From October 2013 to October 2017, 34 patients were enrolled. Patients’ main characteristics are summarized in . The median age was 69 years and 51.6% of the population had an ECOG performance status over 2. Most patients had a stage III–IV disease at the time of enrollment and the cohort entailed mostly high-risk patients as described above (81.25%). About fifty-three percent of patients had been highly treated with at least 3 lines of prior therapies. All patients had received prior Rituximab-containing therapies.
Table 1. Baseline patients characteristics.
Patients received a median number of 2 cycles of Rituximab-Lenalidomide (1–12) and the median total dose of Lenalidomide received was 1050 mg (200–6200 mg).
Dose adjustment related to toxicity occurred 16 times in 13 patients. The main reason for dose adjustment of Lenalidomide was hematological toxicity in 50% of the cases.
Safety
In regards to safety, most frequent adverse events occurring in more than 15% of patients were neutropenia in 28% (16 events in 9 patients), anemia in 24%, fatigue in 38%, fever in 26%, nausea in 18%, diarrhea in 18% and constipation in 15% with the majority being of grade 1 or 2.
Thirty-seven grade >2 treatment-emergent adverse events (TEAE) occurred in 28 patients (82% of the cohort). Most common AE were neutropenia (32.4%), febrile neutropenia (13.5%), infections (10.8%), thrombopenia and anemia (8.1% each), hematuria (5.4%) and cutaneous rash, acute kidney injury, nausea, diarrhea, pulmonary embolism, deep thrombosis, fatigue and myocardial infarction (2.7% each). Those are summarized in .
Table 2. Treatment-emergent adverse events (TEAE) > 2.
Efficacy
Thirty-two out of 34 patients were evaluated for response (2 patients interrupted treatment before completing first cycle because of premature death for 1 patient and 1 for protocol deviation). Median follow-up was 22.6 months (95% CI, [9.6–54.8]) at the moment of the final analysis. Six patients responded (18.8%, 90% exact binomial CI, [8.5%–33.7%]). This response rate was not significantly greater than the unacceptable rate of 20% (p = 0.64, exact binomial right-sided test), and primary objective was not reached.
Two patients obtained a CR, two a PR and two achieved a SD as best response.
The overall response rate was 18.8% and the objective response rate (CR + PR) was therefore 12.5% (90% exact binomial CI, [4.4%–26.4%]). All the responders (4) had a high risk aaIPI score of 2-3.
For those patients, median duration of response (mDOR) was 9.3 months (95% CI, [3–32.7]). Only 2 out of 32 patients (both in CR) completed the 12 cycles of treatment. Most patients (27/32; 84%) discontinued treatment because of disease progression (missing data 3/32).
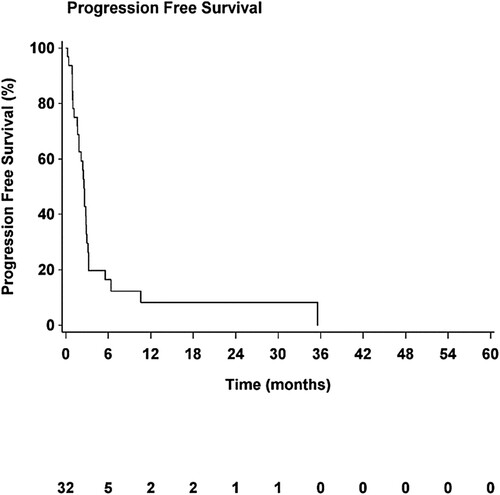
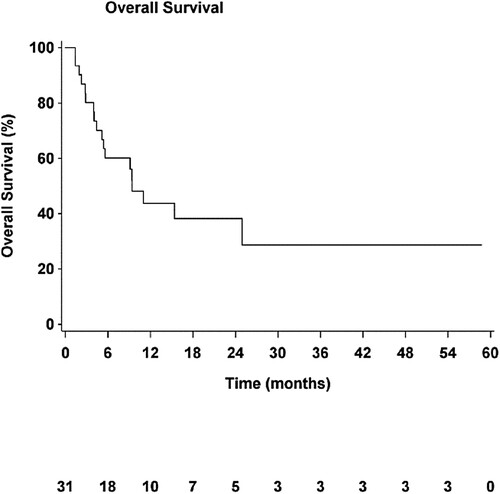
The median PFS (mPFS) of the cohort was 2.6 months (95% CI, [1.7–2.9]; ) and median OS (mOS) was 9.4 months (95% CI, [5.1–Not estimable], ). Median TTP (mTTP) was 2.5 months (95% CI, [1.7–2.9]).
After failing treatment, 20 patients (64.5%) received subsequent line of treatment.
At the time of last follow-up 18 patients (56%) had died from lymphoma.
Discussion
The results of both the responses and survival rates in this study were disappointing compared to what had previously been published.
Therefore, this study did not meet its primary endpoint.
Lenalidomide has since been studied many times as a single agent [Citation3–9] or in combination with anti CD20 monoclonal antibody [Citation10–13] in different types of aggressive B cell lymphomas. This has been done in prospective trials [Citation3,Citation4,Citation10–12] or in a retrospective way [Citation5,Citation7–9,Citation13].
Some studies included different subtypes such as mantle cell lymphoma, DLBCL, histological transformation or grade 3 follicular lymphomas [Citation3,Citation4,Citation10,Citation12] while others only addressed DLBCL [Citation5,Citation7–9,Citation11,Citation13]. Drawing clear conclusions from such various studies is difficult.
Overall, the use of LEN monotherapy provided ORRs ranging from 27.5 to 43.5% (CRs 12 to 23.5%). Median DOR and mPFS ranged from 6.2 to 12 months and 2.4 to 34 months respectively.
When combined to an antiCD20 monoclonal antibody, LEN demonstrated ORRs of 33.3%–41.2% (CRs 18.3%–35.3%). Some studies seemed to demonstrate a significant better outcome in patients with non GCB subtypes [Citation5,Citation7] but mostly in retrospective reports.
As an example, the same year we started enrolling patients, Wang et al. [Citation12] published the results of a phase 2 study entailing 45 patients with mostly R/R DLBCL or HT lymphoma or FL grade 3. When it comes to analyzing baseline characteristics of the cohort, there was no significant difference with their patients except for being younger (median age 66yo). Patients received Lenalidomide 20 mg combined with Rituximab (administered weekly over the first cycle). The ORR in this study was 33.3% with a CR rate of 22.2%. Median PFS was 3.7 months and median OS was 10.7 months. The major difference with our study is that the Rituximab-Lenalidomide regimen was not a stand-alone treatment since 9 in 15 patients who achieved a response, proceeded to ASCT with 8 patients out of 9 being still alive at the time of analysis (median follow up 29.1 months). The authors also performed an analysis without censoring at the time of ASCT, and the median PFS was still 3.7 months but the mDOR extended from 10.2 to 30.9 months.
Another prospective study published by the LYSA in 2018 [Citation10] showed similar results by combining Obinutuzumab with Lenalidomide in 71 patients with R/R DLBCL. ORR was 35.2% and CR/CRu was estimated at 18.3%. Median PFS and OS were of 4.1 and 10.6 months respectively. There was again a trend of a better activity in non-GCB subtypes as well as among non-refractory patients. In that study, patients had received a median of 2 prior therapies but 68% of them were refractory to Rituximab and/or prior line of therapy.
To explain such a discrepancy with our results is difficult since direct comparison of cohorts of patients turns out to be challenging. Some of the baseline characteristics may nevertheless have contributed to those poor results. Indeed, our patients had a median age of 69yo, a high-risk aaIPI in 81.25%, almost half had a history of histological transformation, 53.1% had received at least 3 prior lines of therapy and finally 51.8% of them had an ECOG status of 3 or more which are common poor prognosis factors throughout all lines of treatments. Therefore, 81.25% of our patients met our predefined High-Risk DLBCL features. Important information missing in our study is the number of patients who had received prior autologous stem cell transplant, which could also have worsened prognosis. Nevertheless, one study didn't seem to show any difference with or without prior ASCT [Citation6].
The very few responders precluded any analysis of the response according to the COO. It is very likely that the high-risk features of our patients erased the possible better prognosis of non-GCB subtype.
From a safety perspective, there was no new signal. In most cases, there were significantly less AE in our cohort than in previous studies especially the one published by Wang et al. This is unfortunately probably reflecting the shorter exposure to treatment (patients in our study received only a median number of 2 cycles before discontinuation when 185 cycles were delivered in 45 patients).
Explorations on the role of lenalidomide in 2022
Even if the prognosis of the patients with R/R DLBCL remains poor (especially in those patients failing or ineligible to autologous stem cell transplant), it has improved with the advent of Chimeric Antigen Receptor (CAR) T cell therapies (CART) as third line treatment.
Whether bridging therapy is useful or not is still debatable. In the different studies published so far, many different bridging regimen have been used and no data has arisen showing the superiority of one over another [Citation14,Citation15]. Some of these patients could therefore benefit from LEN +/− anti CD20 monoclonal antibody (mAb) as a bridging therapy while awaiting CAR-T cells reinjection (especially those with a non-GCB subtype) since its safety compares favorably to other chemotherapy combination and would help preserve a good performance status prior to reinfusion.
Furthermore, based on preclinical studies [Citation16] it has been suggested that LEN could improve CAR-T cells efficiency by enhancing antitumor functions via T cells. In a recent retrospective study published by Thieblemont et al. [Citation17] patients relapsing after CART received different subsequent therapies at different times. Among those 59 patients, 41 patients received LEN +/− antiCD20 mAb. Eleven patients started treatment before day 15 post CAR-T infusion and had a higher ORR and CR than the rest of the cohort. Interestingly this was associated (in 6 assessable patients) with a higher CAR-T cells expansion in blood during the first 28 days as compared to other patients (relapsing or not). Whether this translated into better long-term outcomes was not discussed.
In another recent study [Citation18], a group of 7 patients was exposed to LEN as soon as day 15 until progression and compared to a group of 9 patients with similar characteristics without administration of LEN. Noticeably, 94% of those patients had non-GCB subtype. The whole cohort had an ORR of 81.3%. With a median follow up of 9 months, even if there was no difference in PFS, there was a significant improvement in OS in favor of the group of patients treated with LEN (1yOS 100% vs 33.3% in the control group).
Nevertheless, some patients are not eligible to such treatment. In those patients, a combination of LEN with Tafasitamab, a Fc-modified, humanized, anti-CD19 monoclonal antibody, has been recently approved in the R/R setting, according to the results of the L-MIND study [Citation19]. In this study, even if the patients’ characteristics were more favorable, results showed an outstanding ORR of 57.5% (CR 40%) and a median duration of response of 43.9 months in responders (mPFS and mOS of 11.6 and 33.5 months respectively) with a subset of patients likely experiencing cure. In comparison, the phase 2 study [Citation20] using Tafasitamab as a single-agent in 35 R/R DLBCL had shown a median DOR of 20.1 months and a mPFS of 2.7 months (even if the median number of prior therapies was higher in this study compared to the L-MIND study: 3 vs 2).
More recently, a phase 1 study [Citation21] combining Brentuximab-Vedotin and Lenalidomide in R/R DLBCL has shown a promising 57% ORR (CR 35%) and 10.2 months of mPFS in 37 patients. Higher response rates were seen in CD30+ DLBCL. This led to exploring this combination with Rituximab in the ongoing phase 3 ECHELON 3 trial [Citation22].
Conclusion
To summarize, even if Lenalidomide remains an important drug in management of R/R DLBCL, this study failed to achieve its objectives. The contradictory results observed here compared to other studies might be explained by the enrollment of frailer patients and the higher number of poor prognostic factors. Therefore, the R2 regimen cannot be recommended as a standalone treatment after second line treatment. Other combinations seem more promising.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions
Reda Bouabdallah and Jean Marc Schiano contributed to the study conception and design. Material preparation, data collection and analysis were performed by Christophe Zemmour, Leonor Lopez Almeida and Robin Noel. The first draft of the manuscript was written by Robin Noel. Catalina Montes de Oca, Reda Bouabdallah and Jean Marc Schiano commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). Norbert Vey has received honoraria payment from Roche®, BMS® and Celgene®.
Additional information
Funding
References
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi:10.1056/NEJMra2027612.
- Crump M, Neelapu S, Farooq U et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi:10.1182/blood-2017-03-769620.
- Witzig TE, Vose JM, Zinzani PL et al . An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol Off J Eur Soc Med Oncol. 2011;22(7):1622–1627. doi:10.1093/annonc/mdq626.
- Wiernik PH, Lossos I, Tuscano JM et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(30):4952–4957. doi:10.1200/JCO.2007.15.3429.
- Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117(22):5058–5066. doi:10.1002/cncr.26135.
- Vose JM, Habermann T, Czuczman MS et al. Single-agent lenalidomide is active in patients with relapsed or refractory aggressive non-Hodgkin lymphoma who received prior stem cell transplantation. Br J Haematol. 2013;162(5):639–647. doi:10.1111/bjh.12449.
- Mondello P, Steiner N, Willenbacher W et al. Lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: is it a valid treatment option? Oncologist. 2016;21(9):1107–1112. doi:10.1634/theoncologist.2016-0103.
- Broccoli A, Casadei B, Chiappella A et al. Lenalidomide in pretreated patients with diffuse large B-cell lymphoma: an Italian observational multicenter retrospective study in daily clinical practice. Oncologist. 2019;24(9):1246–1252. doi:10.1634/theoncologist.2018-0603.
- Rodgers TD, Baran A, Reagan PM et al. Efficacy of lenalidomide in high-risk diffuse large B-cell lymphoma. Br J Haematol. 2020;188(4):e33–e36. doi:10.1111/bjh.16302.
- Houot R, Cartron G, Bijou F et al. Obinutuzumab plus lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study. Leukemia. 2019;33(3):776–780. doi:10.1038/s41375-018-0282-y.
- Zinzani PL, Pellegrini C, Gandolfi L et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk. 2011;11(6):462–466. doi:10.1016/j.clml.2011.02.001.
- Wang M, Fowler N, Wagner-Bartak N et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902–1909. doi:10.1038/leu.2013.95.
- Ivanov V, Coso D, Chetaille B et al. Efficacy and safety of lenalinomide combined with rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(11):2508–2513. doi:10.3109/10428194.2014.889822.
- Paillassa J, Vercellino L, Di Blasi R et al. Impact of bridging chemotherapy on clinical outcomes of CD19 CAR T therapy in relapse/refractory diffuse large B-cell lymphoma in real world experience. Blood. 2019;134(Suppl_1):2886–2886. doi:10.1182/blood-2019-129421.
- Dwivedy Nasta S, Hughes ME, Namoglu EC et al. A characterization of bridging therapies leading up to commercial CAR T-cell therapy. Blood. 2019;134(Suppl_1):4108–4108. doi:10.1182/blood-2019-131399.
- Otáhal P, Prukova D, Kral V et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology. 2016;5(4):e1115940. doi:10.1080/2162402X.2015.1115940.
- Thieblemont C, Chevret S, Allain V et al. Lenalidomide enhance CAR T-cells response in patients with refractory/relapsed large B cell lymphoma experiencing progression after infusion. Blood. 2020;136(Suppl_1):16–17. doi:10.1182/blood-2020-136279.
- Ping N, Qu C, Li M et al. Overall survival benefits provided by lenalidomide maintenance after chimeric antigen receptor T cell therapy in patients with refractory/relapsed diffuse large B-cell lymphoma. Ann Transl Med. 2022;10(6):298. doi:10.21037/atm-22-20.
- Duell J, Maddocks KJ, Gonzalez-Barca E et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417–2426. doi:10.3324/haematol.2020.275958.
- Jurczak W, Zinzani PL, Hess G et al. A phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood. 2019;134(Suppl_1):4078–4078. doi:10.1182/blood-2019-124297.
- Ward JP, Berrien-Elliot M, Gomez F et al. Phase 1/dose expansion trial of brentuximab vedotin and lenalidomide in relapsed or refractory diffuse large B-cell lymphoma. Blood. 2022;139(13):1999–2010. doi:10.1182/blood.2021011894.
- Bartlett NL, Yasenchak CA, Ashraf K, et al. Brentuximab vedotin in combination with lenalidomide and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) (ECHELON-3, trial in progress). Blood. 2021;138(Suppl_1):3564–3564. doi:10.1182/blood-2021-151583.