ABSTRACT
Background:
Previous studies validated the prognostic significance of lymphocyte to monocyte ratio (LMR) in patients with solid tumors and some hematologic malignancies. However, the correlation between LMR and Myelodysplastic Neoplasms (MDS) was unclear. The study intends to investigate the prognostic impact of LMR on MDS patients.
Methods:
91 newly diagnosed MDS patients were included in this retrospective study. The cut-off of LMR was 3.2 by X-Tile. All patients were divided into the low LMR group (<3.2) and the high LMR group (≥3.2). Clinical characteristics were compared between the two groups.
Results:
Patients in the high LMR group (n = 67) had better OS (P = 0.007) from the Kaplan-Meier survival curves. The results of the univariate analysis demonstrated that LMR was a prognostic factor for OS [hazard ratio (HR) = 2.070, 95%CI 1.201-3.571, P = 0.009]. After multivariate cox analysis, low LMR was confirmed to be an independent predictor of poor OS in MDS patients (HR = 1.872, 95%CI 1.084-3.230, P = 0.024).
Conclusions:
LMR, a representative marker of systematic inflammation and immune response, has potential prognostic significance in MDS patients.
1. Introduction
Myelodysplastic Neoplasms (MDS) is a heterogeneous group of myeloid neoplastic diseases derived from hematopoietic stem cells, characterized by peripheral blood cytopenia, pathological hematopoiesis and risk of transformation to acute myeloid leukemia (AML) [Citation1]. In the past, MDS has been an abbreviation for Myelodysplastic Syndromes. With the increasing understanding of the disease, the fifth edition of the World Health Organization (WHO) classification of myeloid neoplasms changed the name of the disease to Myelodysplastic Neoplasms. Compared to the 2016 classification, the new version emphasizes more on the neoplastic characteristics of MDS and harmonizes terminology with myeloproliferative neoplasms (MPN) [Citation2,Citation3].
The National Cancer Institute reported that the incidence rate of MDS was 4.2 cases per 100,000 people per year in the United States, and the 5-year relative survival rate was only 37.5%. The diagnosis of MDS mainly depends on peripheral blood counts and blood smear, marrow morphology, cytogenetics, immunology and molecular genetics [Citation4]. Nowadays, the Revised International Prognostic Scoring System (IPSS-R) is the most accepted risk stratification system. It consists of five factors: bone marrow cytogenetics, percent of bone marrow blasts, platelet count, hemoglobin level and absolute neutrophil count (ANC) [Citation5,Citation6]. MDS patients received individualized and risk-adapted therapy based on the International Prognostic Scoring System (IPSS) and IPSS-R. However, IPSS-R has some limitations. It is only suitable for predicting the clinical outcomes of untreated MDS patients. Besides, several clinical factors with independent prognostic significance are not integrated, such as red blood cell infusion dependence and gene mutations [Citation7,Citation8]. With this in mind, recently, Bernard et al. [Citation9] developed a clinical molecular prognostic model including somatic gene mutations, named Molecular International Prognostic Scoring System (IPSS-M). It is composed of hematological parameters, cytogenetic abnormalities and genetic mutations that classifies MDS patients into six risk categories. Compared to the IPSS-R, IPSS-M improves prognostic discrimination and is also suitable for prognostic stratification of both primary and treatment-related MDS patients. However, the IPSS-M did not include monocyte and lymphocyte counts, both of which had been demonstrated to have an independent prognostic role in cancer patients [Citation10,Citation11]. Thus, more potential biomarkers should be investigated to precisely predict the prognosis of MDS patients.
Lymphocyte to monocyte ratio (LMR), one of the representative markers of inflammation and immune response, is defined as the absolute lymphocyte count (ALC) divided by absolute monocyte count (AMC). Lymphopenia has been observed in advanced cancer patients and demonstrated to be associated with poor outcomes in patients with various types of cancer. On the other hand, monocytes have been found to be recruited into the tumor microenvironment and promote tumor progression through local immune suppression as well as angiogenesis. Monocytosis has been verified to be a poor prognostic marker in solid tumors [Citation12]. The lower LMR may represent an active inflammatory state. It has been confirmed to be an independent prognostic factor in solid tumors and hematologic malignancies in the past few years, such as colorectal cancer, pancreatic cancer, multiple myeloma (MM) and diffuse large B cell lymphoma (DLBCL) [Citation13–16]. However, there is no consensus on the prognostic role of LMR in patients with MDS. This study aims to investigate the prognostic significance of LMR in MDS.
2. Materials and methods.
2.1. Patients and treatments
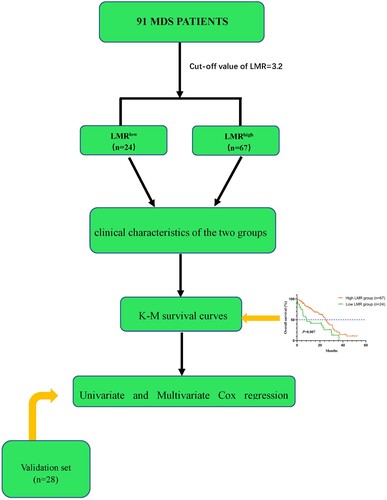
A total of 91 newly diagnosed patients with MDS between March 2010 and January 2021 in the Huaian No.1 People's Hospital were recruited retrospectively. Another 28 MDS patients were collected for external validation. The fifth edition of the WHO classification has revised the categorization of MDS. However, considering that the study was retrospective, the diagnosis of MDS referred to 2016 WHO classification of myeloid neoplasms and acute leukemia [Citation3,Citation17]. The follow-up continued up to April 2021. All patients received individualized treatments based on chemotherapy, hypomethylating agents (HMAs), immunosuppressive drugs, immunomodulatory therapy, allogeneic stem cell transplantation and supportive care according to the NCCN guideline of MDS. This study was approved by the Institutional Review Committee of Huai'an No.1 People's Hospital and was conducted following the Helsinki Declaration.
The inclusion and exclusion criteria of this study were as follows:1) age > 18 years old; 2) diagnosed with MDS according to WHO Definition; 3) available laboratory data for calculating LMR; 4) patients with acquired MDS were excluded.
2.2. LMR and grouping
LMR was defined as the ratio of absolute count of lymphocyte to monocyte. The relevant parameters were acquired from the patient's laboratory test results at the time of initial diagnosis. The median LMR of all 91 patients was 6.57 (range:0.08–213.50). In the study, the cut-off of LMR was derived from X-Tile. All included patients both in the training and validation cohorts were divided into the high and low LMR groups. The clinical characteristics between the two groups were compared, including age, gender, peripheral blood counts, bone marrow blasts, IPSS-R scores, MDS subtypes and treatments.
2.3. Statistical analysis
The cut-off of LMR was determined by X-Tile (version 3.6.1, Yale University, New Haven, CT, United States) [Citation18]. Statistical Package for social sciences (SPSS, version 23, IBM SPSS Statistics 23 software, IBM Corp., Armonk, NY, USA) was used to perform statistical analysis. Mann–Whitney U-test and chi-square test were utilized to evaluate the difference between the two groups and p-value < 0.05 (2-tailed) demonstrated a statistical significance. Kaplan-Meier method has been applied to assess the correlation between LMR and OS. Furthermore, the corresponding p-value was achieved through the log-rank test. Survival curves were graphed by GraphPad Prism (version 8.0.1, GraphPad Software, San Diego, California, USA). Univariate and multivariate Cox regression analyses were conducted to investigate the prognostic factors affecting OS. In univariate analysis, p-value < 0.05 was considered statistically significant, and the corresponding prognostic factors were included in multivariate Cox regression. P-value < 0.05 were considered statistically significant in multivariate analysis.
OS was defined as the period between the first diagnosis and the death of any causes or the last follow-up. The flow chart of the study was displayed in .
3. Results
3.1. Clinical and laboratory characteristics
A total of 91 patients were included in this retrospective study. The clinical and laboratory characteristics of patients were summarized in . The cut-off of LMR was identified as 3.2. The median age of 91 patients was 65 years (range:26–88 years). There were 60 males in the study, 43 of whom were in the high LMR group and the remaining 17 in the low LMR group. There was no statistical difference between the low and high LMR groups (P = 0.555) regarding gender. The median white blood cell (WBC), ALC and AMC were 2.16*109/L, 0.91*109/L and 0.14*109/L, respectively. The median platelet count of all patients was 46*109/L (range:3–765*109/L). Patients in the high LMR group had no significantly higher platelet counts than those in the low LMR group (47*109/L versus 38*109/L, P = 0.829). The median hemoglobin of all patients was 67 g/L (range:37–144 g/L).
Table 1. The clinical characteristics of the enrolled 91 MDS patients.
3.2. Kaplan-Meier survival curves of LMR
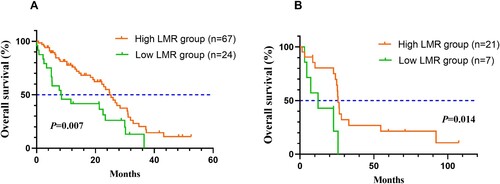
The Kaplan-Meier survival curves were graphed and used to compare OS between the low and high LMR groups by the log-rank test. The survival curves were shown in . Patients with higher LMR experienced better OS than those with lower LMR (P = 0.007). Low LMR was associated with poor OS of MDS patients in the validation set (P = 0.014).
3.3. Univariate and multivariate cox regression analysis
The results of the univariate analysis were displayed in . As shown in the table, patients in the low LMR group had worse OS (HR = 2.070, 95%CI 1.201–3.571, P = 0.009) than those in the high LMR group. Besides, IPSS-R (HR = 3.056, 95%CI 1.639–5.699, P < 0.001) have a prognostic impact on the patients’ OS.
Table 2. The Univariate and Multivariate analysis for OS in MDS patients.
Multivariate Cox regression analysis was performed to explore the potential clinical factors that influence OS in patients with MDS. The results of the multivariate analysis were summarized in . LMR was an independent prognostic factor for OS (HR = 1.872, 95%CI 1.084–3.230, P = 0.024) of patients with MDS. Likewise, IPSS-R was proven to be an independent prognostic factor of MDS patients’ OS (HR = 2.892, 95%CI 1.549–5.402, P = 0.001). The univariate and multivariate analysis for MDS patients’ OS in the validation set were displayed in Supplementary Table 1.
4. Discussion
MDS is a heterogeneous group of clonal hematopoietic stem-cell disorders characterized by ineffective and dysplastic hematopoietic differentiation and variable risk that progressed to acute myeloid leukemia. The fifth edition of the WHO Classification replaced myelodysplastic syndromes with myelodysplastic neoplasms and emphasized MDS as neoplastic disease. According to the National Cancer Institute, MDS patients did not have a significantly higher five-year survival rate than AML patients (36.9% versus 30.5%). The most widely used prognostic scoring systems for MDS today are IPSS-R [Citation19]. However, it has a few limitations. For example, the 5th edition of the WHO Classification emphasized that MDS is a genetically-defined disease type [Citation2]. However, factors associated with gene mutations were not included in the IPSS-R. Recently, a new clinical molecular prognostic model incorporating somatic gene mutations has been developed under the name IPSS-M. However, neither IPSS-R nor IPSS-M included lymphocyte and monocyte counts, which were demonstrated to have predictive prognostic value in cancer patients. LMR has been demonstrated prognostic role in patients with solid tumors in the past few years. Besides, existing research focuses on the predicting value of LMR for hematologic malignancies such as MM and DLBCL [Citation15,Citation16]. However, the prognostic significance of LMR in MDS was unclear. Thus, the retrospective study was conducted to investigate the correlation between LMR and the prognosis of MDS patients.
This study retrospectively collected clinical Information of 91 newly diagnosed MDS patients in our institution. There were 24 patients in the low LMR group and 67 in the high LMR group. Compared to patients with high LMR, those in the low LMR group had an unfavorable influence on OS of MDS patients from the survival curves (P = 0.007). Moreover, this research validated that LMR was an independent prognostic factor in MDS cases after multivariate analysis (HR = 1.872, 95%CI 1.084–3.230, P = 0.024). Additionally, IPSS-R can also independently predict MDS patients’ prognosis (HR = 2.892, 95%CI 1.549–5.402, P = 0.001).
The underlying mechanisms by which LMR correlates with prognosis have not been completely elucidated. Former researchers suggested that systematic inflammation contributes to the development of malignancies and has a vital role in the survival of cancer patients [Citation20]. Specifically, systemic inflammatory and immune response causes tumor-related symptoms, including fever, sweating and weight loss (so-called B symptoms). Additionally, it can mitigate the effectiveness of treatment, increase toxicity and even lead to treatment failure [Citation20,Citation21]. Previous studies have also demonstrated that biochemical indicators and peripheral blood counts or ratios can be used as markers of immunological and inflammatory responses. They include albumin, C-reactive protein (CRP), lactate dehydrogenase (LDH), neutrophils, lymphocytes, monocytes, platelets, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and the systemic immune-inflammation index (SII) [Citation22]. Furthermore, a retrospective study of 503 patients with non-del(5q) MDS verified that lymphocytopenia at diagnosis has an unfavorable influence on the prognosis of MDS patients, as ALC could reflect the host's immune status [Citation23]. Monocytes differentiate into macrophages that participate in tumor infiltration and metastasis in the tumor microenvironment [Citation11]. In solid tumors, increased numbers of monocytes have been shown to be associated with worse prognosis [Citation24,Citation25]. Thus, it can be postulated that lower LMR correlates with the worse prognosis of cancer patients.
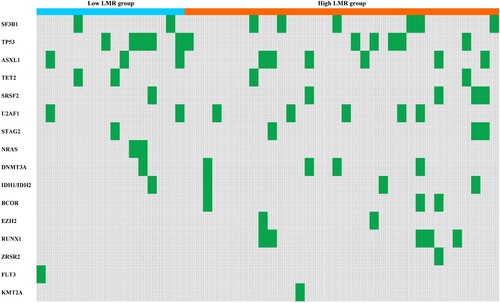
As shown in , the number of patients with SF3B1 mutation was 8, of which 2 were in the low LMR group, and 6 were in the high LMR group. Patients with TP53multihit, FLT3 or KMT2A mutations in the low and high LMR groups were 5 and 5, respectively. Previous studies have demonstrated that SF3B1 was the most common mutant gene in MDS patients and patients with SF3B1 mutation have a relatively good prognosis [Citation26]. MDS patients with TP53 mutation have a high risk of transformation to AML, resistance to conventional therapies and a relatively poor prognosis [Citation27]. Bernard et al. identified TP53multihit, FLT3 and KMT2A mutations as the three predictors most associated with adverse outcomes in MDS. The low LMR group had fewer patients with SF3B1 mutation and the same number of patients with poor prognosis mutations. Although our study confirmed no statistical difference between the two groups of patients in terms of SF3B1 mutations and poor prognosis mutations (P = 0.433), we believe that the sample size was too small to conclude whether there was a difference between the two groups. Other frequent gene mutations in MDS patients were displayed in the heatmap (). This requires physicians to comprehensively evaluate the prognosis of patients by combining various indicators such as LMR and patients’ genetic mutations, select treatment options, and guide clinical practice.
Fig 3. The heatmap of frequent gene mutations in MDS patients (Green represents the occurrence of gene mutation in this patient, gray represents no gene mutation. Patients with missing relevant information have been removed).
The study has a few limitations. It was a retrospective, single-center study with small sample size. In the future, prospective cohort studies with larger samples are required further to explore the prognostic significance of LMR in MDS.
5. Conclusion
In conclusion, LMR, a representative marker of systematic inflammation and immune response, has the potential predicting value for the prognosis of MDS, which deserve to be further studied on a large scale.
Ethics
This study was approved by the Institutional Review Committee of Huai'an No.1 People's Hospital and was conducted following the Helsinki Declaration. All the patients were anonymous. Informed consent was waived because of the retrospective design of the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the results of this study can be obtained from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Cazzola M. Myelodysplastic syndromes. N Engl J Med. 2020;383:1358–1374. doi: 10.1056/NEJMra1904794.
- Khoury JD, Solary E, Abla O, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544.
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:60–87. doi: 10.6004/jnccn.2017.0007.
- Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95:1399–1420. doi: 10.1002/ajh.25950.
- Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489.
- Chinese Society of Hematology CMA, Chinese Society of Lymphoma CA-cA. [Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma(2013)]. Zhonghua Xue Ye Xue Za Zhi. 2013;34:816–819. doi: 10.3760/cma.j.issn.0253-2727.2013.09.019.
- Platzbecker U, Kubasch AS, Homer-Bouthiette C, et al. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia. 2021;35:2182–2198. doi: 10.1038/s41375-021-01265-7.
- de Lange KM, Barrett JC. Understanding inflammatory bowel disease via immunogenetics. J Autoimmun. 2015;64:91–100. doi: 10.1016/j.jaut.2015.07.013.
- Park SJ, Lee J, Kim H, et al. Association between absolute lymphocyte count and overall mortality in patients with surgically resected gastric cancer. Korean J Intern Med. 2021;36:679–688. doi: 10.3904/kjim.2019.358.
- Yamamoto T, Kawada K, Obama K. Inflammation-Related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22; doi: 10.3390/ijms22158002.
- Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:971–978. doi: 10.1016/j.ctrv.2015.10.003.
- Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis. Clin Chim Acta. 2018;481:142–146. doi: 10.1016/j.cca.2018.03.008.
- Tan D, Fu Y, Tong W, et al. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128–138. doi: 10.1016/j.ijsu.2018.05.030.
- Yang J, Guo X, Hao J, et al. The prognostic value of blood-based biomarkers in patients With testicular diffuse large B-cell lymphoma. Front Oncol. 2019;9:1392. doi: 10.3389/fonc.2019.01392.
- Zhang X, Duan J, Wen Z, et al. Are the derived indexes of peripheral whole blood cell counts (NLR, PLR, LMR/MLR) clinically significant prognostic biomarkers in multiple myeloma? A systematic review and meta-analysis. Front Oncol. 2021;11:766672. doi: 10.3389/fonc.2021.766672.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713.
- Scalzulli E, Pepe S, Colafigli G, et al. Therapeutic strategies in low and high-risk MDS: what does the future have to offer? Blood Rev. 2021;45:100689. doi: 10.1016/j.blre.2020.100689.
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3.
- Roxburgh CS, McMillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110:1409–1412. doi: 10.1038/bjc.2014.90.
- Nost TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36:841–848. doi: 10.1007/s10654-021-00752-6.
- Jacobs NL, Holtan SG, Porrata LF, et al. Host immunity affects survival in myelodysplastic syndromes: independent prognostic value of the absolute lymphocyte count. Am J Hematol. 2010;85:160–163. doi: 10.1002/ajh.21618.
- Sasaki A, Kai S, Endo Y, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11:596–602. doi: 10.1007/s11605-007-0140-0.
- Zhang LN, Xiao W, OuYang PY, et al. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumour Biol. 2015;36:8213–8219. doi: 10.1007/s13277-015-3560-6.
- Malcovati L, Stevenson K, Papaemmanuil E, et al. SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS. Blood 2020;136:157–170. doi:10.1182/blood.2020004850.
- Bernard E, Nannya Y, Hasserjian RP, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–1556. doi:10.1038/s41591-020-1008-z.